Xylogenesis Responses to a Mediterranean Climate in Holm Oak (Quercus ilex L.)
Abstract
1. Introduction
2. Materials and Methods
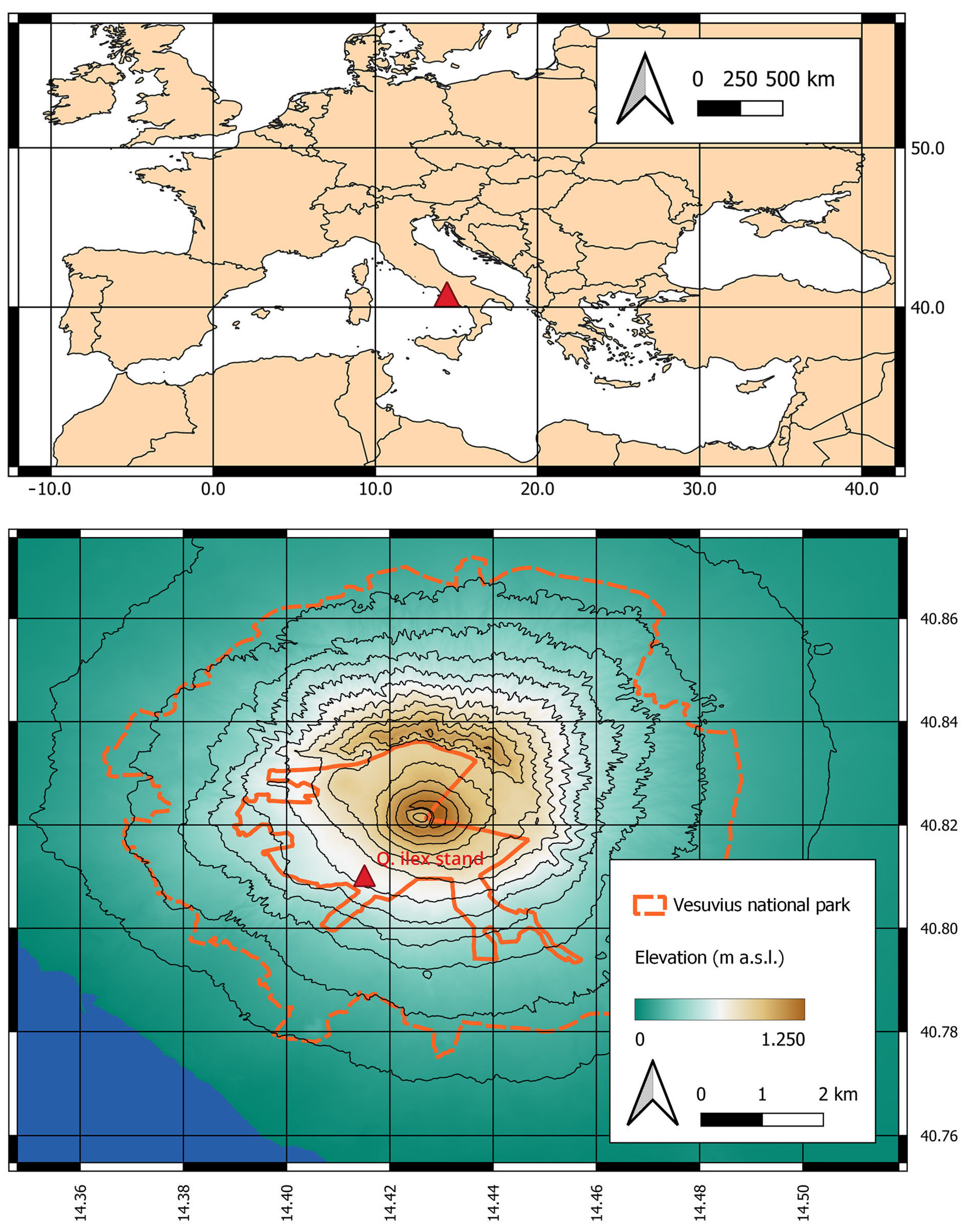
2.1. The Study Area
2.2. The Sampling Microcores
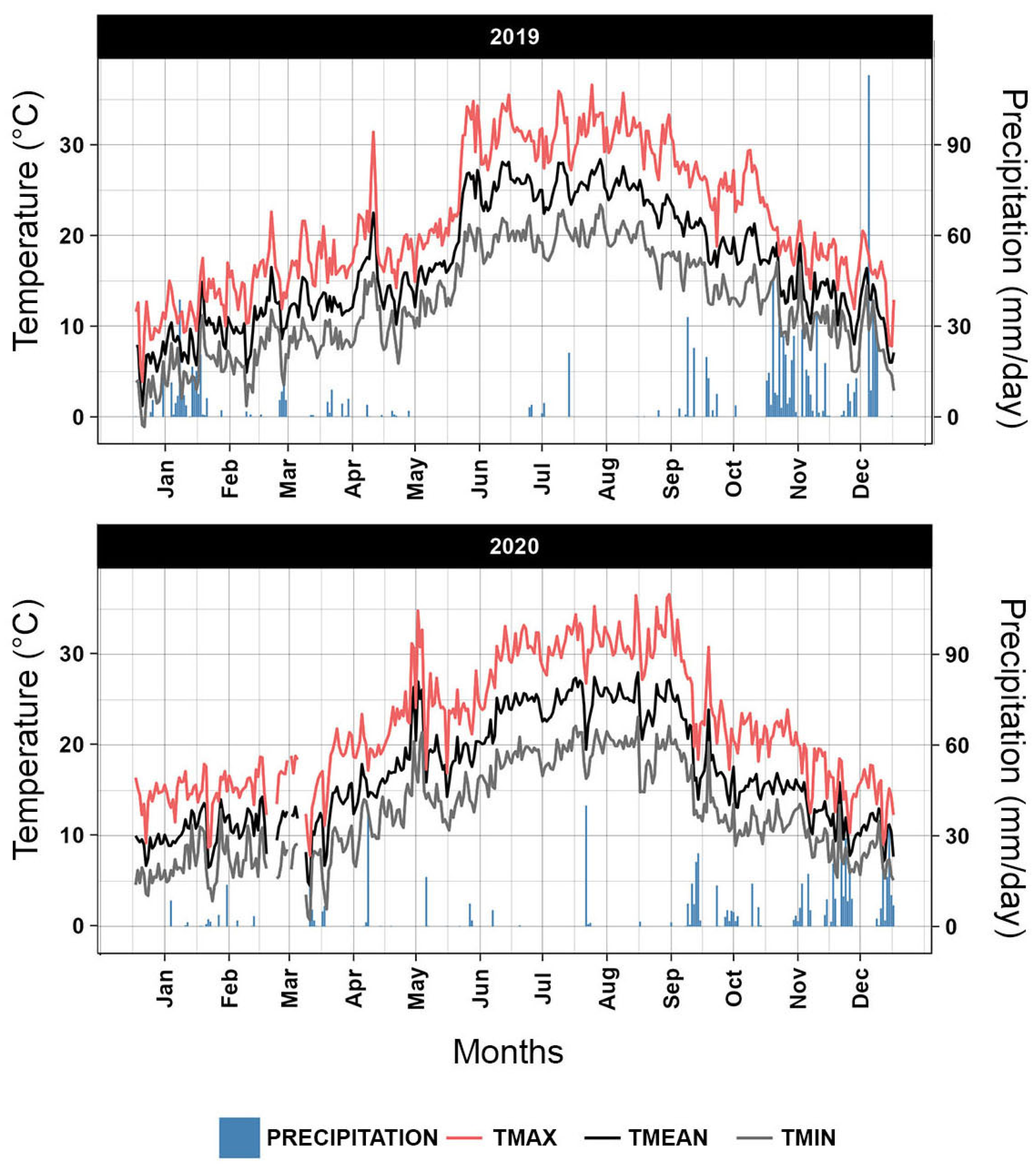
2.3. Meteorological Data
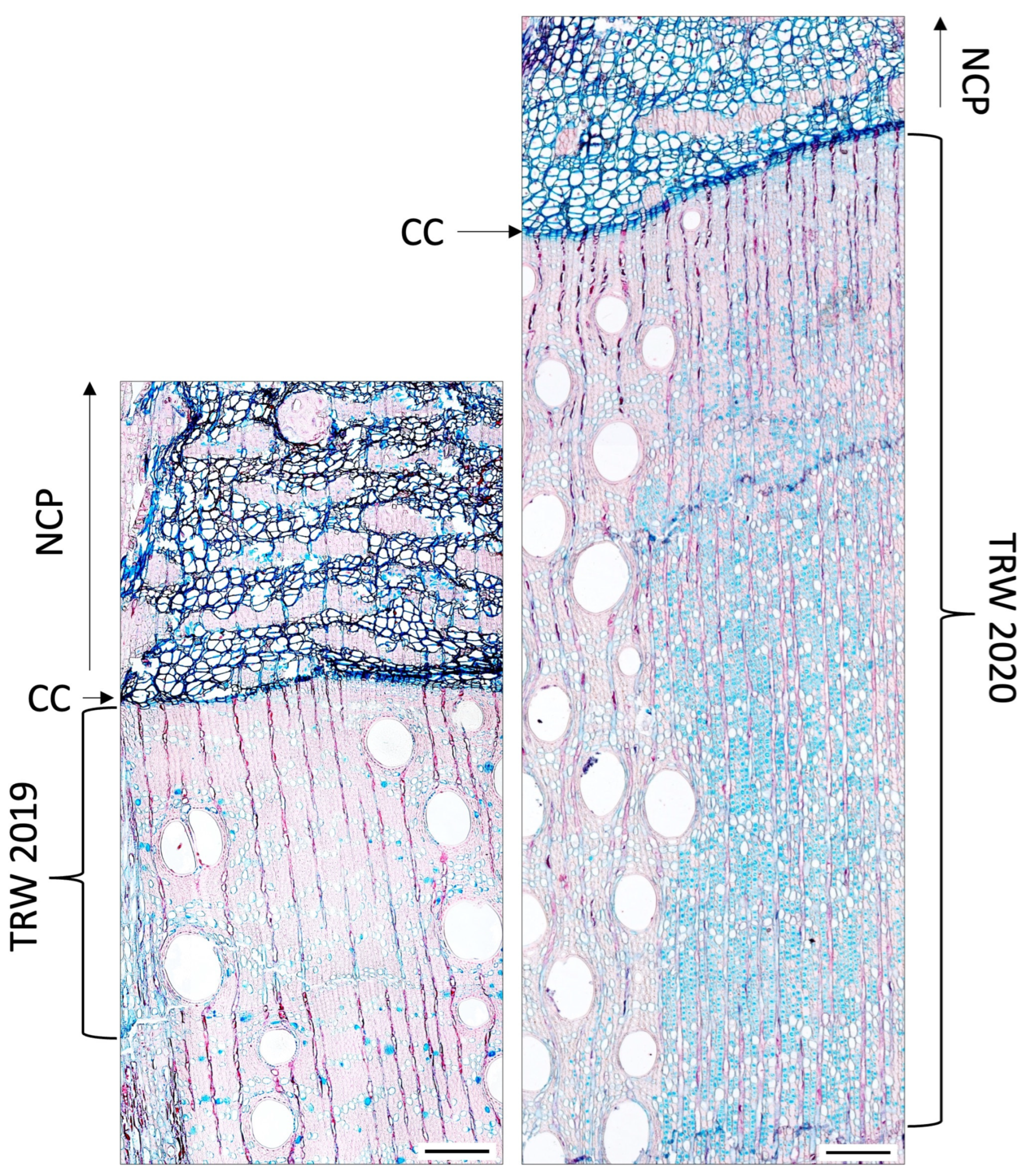
2.4. Xylogenesis, Microscopy and Anatomical Criteria
2.5. The Rolling Window Correlation Analysis
3. Results
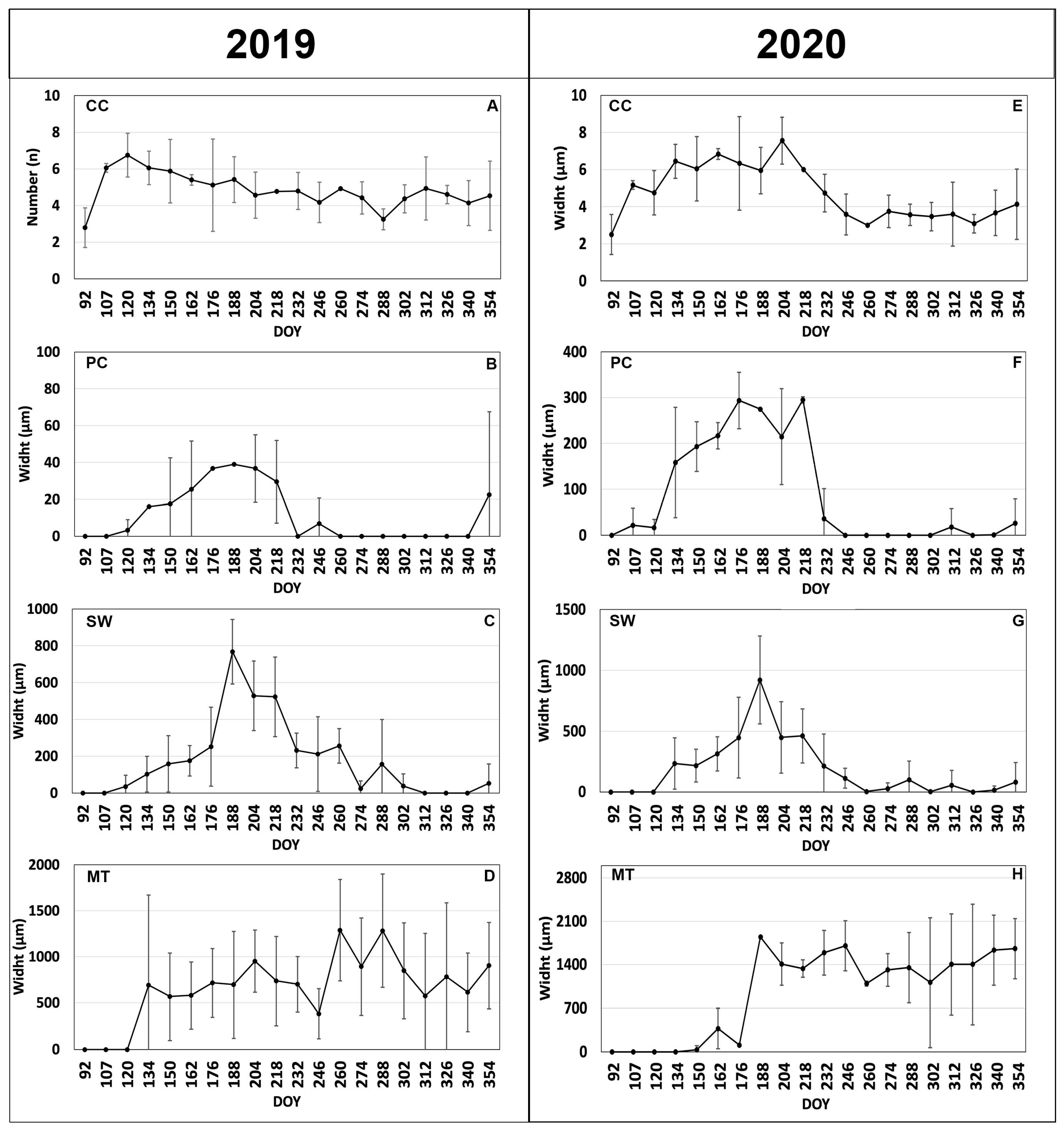
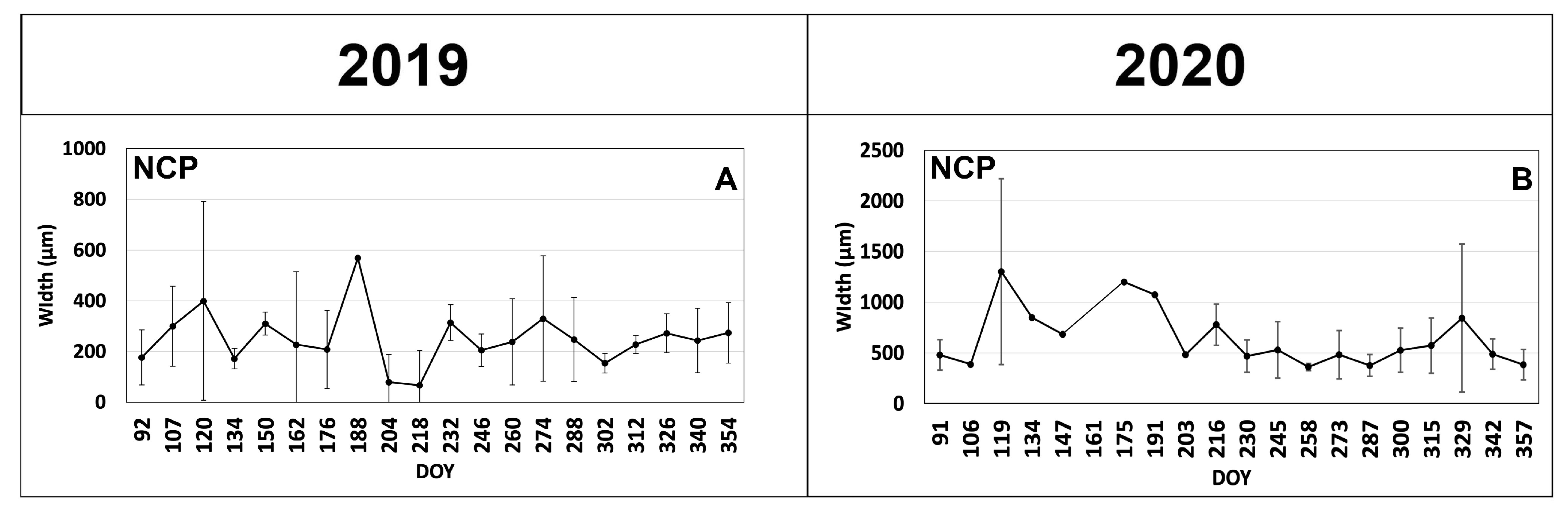
3.1. Cambial Activity and Xylem Formation
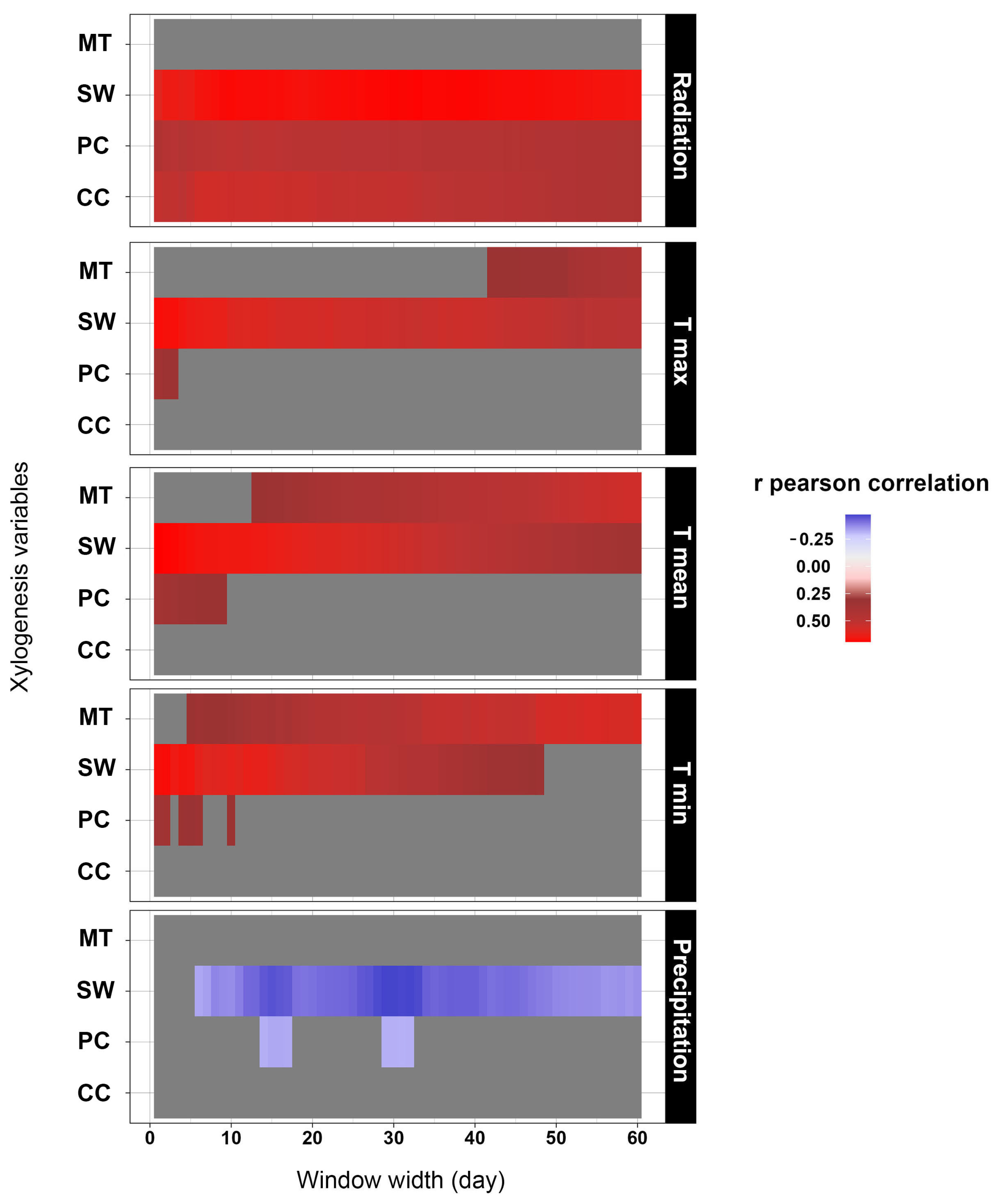
3.2. Rolling Window Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Barreda, S.; Valeriano, C.; Camarero, J.J. Drought Constrains Acorn Production and Tree Growth in the Mediterranean Holm Oak and Triggers Weak Legacy Effects. Agric. For. Meteorol. 2023, 334, 109435. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Bolte, A.; Dorado-Liñán, I.; Etzold, S.; Fonti, P.; et al. Climate Sensitivity and Drought Seasonality Determine Post-Drought Growth Recovery of Quercus Petraea and Quercus Robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef]
- Liu, D.; Ogaya, R.; Barbeta, A.; Yang, X.; Peñuelas, J. Long-Term Experimental Drought Combined with Natural Extremes Accelerate Vegetation Shift in a Mediterranean Holm Oak Forest. Environ. Exp. Bot. 2018, 151, 1–11. [Google Scholar] [CrossRef]
- Ogaya, R.; Escolà, A.; Liu, D.; Barbeta, A.; Peñuelas, J. Effects of Thinning in a Water-Limited Holm Oak Forest. J. Sustain. For. 2020, 39, 365–378. [Google Scholar] [CrossRef]
- Natalini, F.; Alejano, R.; Vázquez-Piqué, J.; Cañellas, I.; Gea-Izquierdo, G. The Role of Climate Change in the Widespread Mortality of Holm Oak in Open Woodlands of Southwestern Spain. Dendrochronologia 2016, 38, 51–60. [Google Scholar] [CrossRef]
- Quinto, L.; Navarro-Cerrillo, R.M.; Palacios-Rodriguez, G.; Ruiz-Gómez, F.; Duque-Lazo, J. The Current Situation and Future Perspectives of Quercus ilex and Pinus Halepensis Afforestation on Agricultural Land in Spain under Climate Change Scenarios. New For. 2021, 52, 145–166. [Google Scholar] [CrossRef]
- Greiser, C.; Hederová, L.; Vico, G.; Wild, J.; Macek, M.; Kopecký, M. Higher Soil Moisture Increases Microclimate Temperature Buffering in Temperate Broadleaf Forests. Agric. For. Meteorol. 2024, 345, 109828. [Google Scholar] [CrossRef]
- Kašpar, J.; Krůček, M.; Král, K. The Effects of Solar Radiation on Daily and Seasonal Stem Increment of Canopy Trees in European Temperate Old-growth Forests. New Phytol. 2024, 243, 662–673. [Google Scholar] [CrossRef]
- López-Ballesteros, A.; Rodríguez-Caballero, E.; Moreno, G.; Escribano, P.; Hereş, A.; Yuste, J.C. Topography Modulates Climate Sensitivity of Multidecadal Trends of Holm Oak Decline. Glob. Chang. Biol. 2023, 29, 6336–6349. [Google Scholar] [CrossRef]
- Zalloni, E.; Battipaglia, G.; Cherubini, P.; Saurer, M.; De Micco, V. Wood Growth in Pure and Mixed Quercus ilex L. Forests: Drought Influence Depends on Site Conditions. Front. Plant Sci. 2019, 10, 397. [Google Scholar] [CrossRef]
- Meeussen, C.; De Pauw, K.; Sanczuk, P.; Brunet, J.; Cousins, S.A.O.; Gasperini, C.; Hedwall, P.-O.; Iacopetti, G.; Lenoir, J.; Plue, J.; et al. Initial Oak Regeneration Responses to Experimental Warming along Microclimatic and Macroclimatic Gradients. Plant Biol. 2022, 24, 745–757. [Google Scholar] [CrossRef]
- Attorre, F.; Alfò, M.; De Sanctis, M.; Francesconi, F.; Valenti, R.; Vitale, M.; Bruno, F. Evaluating the Effects of Climate Change on Tree Species Abundance and Distribution in the Italian Peninsula. Appl. Veg. Sci. 2011, 14, 242–255. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Climate Change Effects in a Mediterranean Forest Following 21 Consecutive Years of Experimental Drought. Forests 2021, 12, 306. [Google Scholar] [CrossRef]
- Martín-Sánchez, R.; Peguero-Pina, J.J.; Alonso-Forn, D.; Ferrio, J.P.; Sancho-Knapik, D.; Gil-Pelegrín, E. Summer and Winter Can Equally Stress Holm Oak (Quercus ilex L.) in Mediterranean Areas: A Physiological View. Flora 2022, 290, 152058. [Google Scholar] [CrossRef]
- Diez, J.M.; Ibáñez, I.; Miller-Rushing, A.J.; Mazer, S.J.; Crimmins, T.M.; Crimmins, M.A.; Bertelsen, C.D.; Inouye, D.W. Forecasting Phenology: From Species Variability to Community Patterns. Ecol. Lett. 2012, 15, 545–553. [Google Scholar] [CrossRef]
- Guada, G.; Vázquez-Ruiz, R.A.; García-González, I. Meteorological Conditions Control the Cessation Rather than the Beginning of Wood Formation in a Sub-Mediterranean Ring-Porous Oak. Agric. For. Meteorol. 2020, 281, 107833. [Google Scholar] [CrossRef]
- Aguilar-Romero, R.; Pineda-Garcia, F.; Paz, H.; González-Rodríguez, A.; Oyama, K. Differentiation in the Water-Use Strategies among Oak Species from Central Mexico. Tree Physiol. 2017, 37, 915–925. [Google Scholar] [CrossRef]
- Fallon, B.; Cavender-Bares, J. Leaf-level Trade-offs between Drought Avoidance and Desiccation Recovery Drive Elevation Stratification in Arid Oaks. Ecosphere 2018, 9, e02149. [Google Scholar] [CrossRef]
- del Castillo, J.; Comas, C.; Voltas, J.; Ferrio, J.P. Dynamics of Competition over Water in a Mixed Oak-Pine Mediterranean Forest: Spatio-Temporal and Physiological Components. For. Ecol. Manag. 2016, 382, 214–224. [Google Scholar] [CrossRef]
- Camarero, J.J.; Rubio-Cuadrado, Á.; Gazol, A. Climate Windows of Intra-Annual Growth and Post-Drought Recovery in Mediterranean Trees. Agric. For. Meteorol. 2021, 308, 108606. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Camarero, J.J.; Cueva, E.; Espinosa, C.I.; Escudero, A. Climate Seasonality and Tree Growth Strategies in a Tropical Dry Forest. J. Veg. Sci. 2020, 31, 266–280. [Google Scholar] [CrossRef]
- Battipaglia, G.; Campelo, F.; Vieira, J.; Grabner, M.; De Micco, V.; Nabais, C.; Cherubin, P.; Carrer, M.; Bräuning, A.; Čufar, K.; et al. Structure and Function of Intra–Annual Density Fluctuations: Mind the Gaps. Front. Plant Sci. 2016, 7, 595. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Morin, H.; Deslauriers, A. Causes and Correlations in Cambium Phenology: Towards an Integrated Framework of Xylogenesis. J. Exp. Bot. 2012, 63, 2117–2126. [Google Scholar] [CrossRef]
- Vieira, J.; Carvalho, A.; Campelo, F. Tree Growth under Climate Change: Evidence from Xylogenesis Timings and Kinetics. Front. Plant Sci. 2020, 11, 90. [Google Scholar] [CrossRef]
- Niccoli, F.; Kabala, J.P.; Pacheco-Solana, A.; Battipaglia, G. Impact of Intra-Annual Wood Density Fluctuation on Tree Hydraulic Function: Insights from a Continuous Monitoring Approach. Tree Physiol. 2024, 44, tpad145. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, F.; Pacheco-Solana, A.; De Micco, V.; Battipaglia, G. Fire Affects Wood Formation Dynamics and Ecophysiology of Pinus Pinaster Aiton Growing in a Dry Mediterranean Area. Dendrochronologia 2023, 77, 126044. [Google Scholar] [CrossRef]
- Morino, K.; Minor, R.L.; Barron-Gafford, G.A.; Brown, P.M.; Hughes, M.K. Bimodal Cambial Activity and False-Ring Formation in Conifers under a Monsoon Climate. Tree Physiol. 2021, 41, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Rossi, S.; Yang, B.; Qin, C.; Li, G. Environmental Drivers for Cambial Reactivation of Qilian Junipers (Juniperus Przewalskii) in a Semi-Arid Region of Northwestern China. Atmosphere 2020, 11, 232. [Google Scholar] [CrossRef]
- Griar, J. Cambial Cell Production and Structure of Xylem and Phloem as an Indicator of Tree Vitality: A Review. In Sustainable Forest Management—Current Research; InTech: Milton, Australia, 2012. [Google Scholar]
- Balducci, L.; Cuny, H.E.; Rathgeber, C.B.K.; Deslauriers, A.; Giovannelli, A.; Rossi, S. Compensatory Mechanisms Mitigate the Effect of Warming and Drought on Wood Formation. Plant Cell Environ. 2016, 39, 1338–1352. [Google Scholar] [CrossRef]
- Gryc, V.; Hacura, J.; Vavrcík, H.; Urban, J.; Gebauer, R. Monitoring of Xylem Formation in Picea Abies under Drought Stress Influence. Dendrobiology 2012, 67, 15–24. [Google Scholar]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; Mcdowell, N.; et al. Tree Mortality from Drought, Insects, and Their Interactions in a Changing Climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Campelo, F.; De Luis, M.; Bräuning, A.; Grabner, M.; Battipaglia, G.; Cherubini, P. Intra-Annual Density Fluctuations in Tree Rings: How, When, Where, and Why? IAWA J. 2016, 37, 232–259. [Google Scholar] [CrossRef]
- Campelo, F.; Gutiérrez, E.; Ribas, M.; Sánchez-Salguero, R.; Nabais, C.; Camarero, J.J. The Facultative Bimodal Growth Pattern in Quercus ilex—A Simple Model to Predict Sub-Seasonal and Inter-Annual Growth. Dendrochronologia 2018, 49, 77–88. [Google Scholar] [CrossRef]
- Balzano, A.; Čufar, K.; De Micco, V. Xylem and Phloem Formation Dynamics in Quercus ilex L. at a Dry Site in Southern Italy. Forests 2021, 12, 188. [Google Scholar] [CrossRef]
- Altieri, S.; Niccoli, F.; Kabala, J.P.; Liyaqat, I.; Battipaglia, G. Influence of Drought and Minimum Temperature on Tree Growth and Water Use Efficiency of Mediterranean Species. Dendrochronologia 2024, 83, 126162. [Google Scholar] [CrossRef]
- Niccoli, F.; Esposito, A.; Altieri, S.; Battipaglia, G. Fire Severity Influences Ecophysiological Responses of Pinus Pinaster Ait. Front. Plant Sci. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Stinca, A. Vegetation Features of Two Vascular Plant Species Presumed Extinct and Recently Rediscovered in the Natural Habitat of Community Interest 8320 from Mt. Vesuvius, Italy. Plant Sociol. 2023, 60, 13–23. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A New Tool for Sampling Microcores from Tree Stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Balzano, A.; Čufar, K.; Battipaglia, G.; Merela, M.; Prislan, P.; Aronne, G.; De Micco, V. Xylogenesis Reveals the Genesis and Ecological Signal of IADFs in Pinus pinea L. and Arbutus unedo L. Ann. Bot. 2018, 121, 1231–1242. [Google Scholar] [CrossRef]
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A State-of-the-Art Global Reanalysis Dataset for Land Applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Pompa-García, M.; Camarero, J.J.; Colangelo, M. Different Xylogenesis Responses to Atmospheric Water Demand Contribute to Species Coexistence in a Mixed Pine–Oak Forest. J. For. Res. 2023, 34, 51–62. [Google Scholar] [CrossRef]
- Balzano, A.; Čufar, K.; De Micco, V. Cell-Wall Fluorescence Highlights the Phases of Xylogenesis. IAWA J. 2021, 43, 80–91. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought Events Determine Performance of Quercus ilex Seedlings and Increase Their Susceptibility to Phytophthora Cinnamomi. Agric. For. Meteorol. 2014, 192–193, 1–8. [Google Scholar] [CrossRef]
- Niccoli, F.; Kabala, J.P.; Altieri, S.; Faugno, S.; Battipaglia, G. Impact of Toumeyella Parvicornis Outbreak in Pinus pinea L. Forest of Southern Italy: First Detection Using a Dendrochronological, Isotopic and Remote Sensing Analysis. For. Ecol. Manag. 2024, 566, 122086. [Google Scholar] [CrossRef]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic Bimodal Xylogenesis in Conifers from Continental Mediterranean Climates. New Phytol. 2010, 185, 417–480. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; Ribas, M.; Gutiérrez, E. Plastic Bimodal Growth in a Mediterranean Mixed-Forest of Quercus ilex and Pinus halepensis. Dendrochronologia 2021, 67, 125836. [Google Scholar] [CrossRef]
- Battipaglia, G.; Kabala, J.P.; Pacheco-Solana, A.; Niccoli, F.; Bräuning, A.; Campelo, F.; Cufar, K.; de Luis, M.; De Micco, V.; Klisz, M.; et al. Intra-Annual Density Fluctuations in Tree Rings Are Proxies of Air Temperature across Europe. Sci. Rep. 2023, 13, 12294. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-Annual Radial Growth and Water Relations of Trees: Implications towards a Growth Mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef]
- Battipaglia, G.; De Micco, V.; Brand, W.A.; Saurer, M.; Aronne, G.; Linke, P.; Cherubini, P. Drought Impact on Water Use Efficiency and Intra-Annual Density Fluctuations in Erica Arborea on Elba (Italy). Plant Cell Environ. 2014, 37, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Rozas, V.; García-González, I.; Zas, R. Climatic Control of Intra-Annual Wood Density Fluctuations of Pinus Pinaster in NW Spain. Trees Struct. Funct. 2011, 25, 443–453. [Google Scholar] [CrossRef]
- De Luis, M.; Novak, K.; Raventós, J.; Gričar, J.; Prislan, P.; Čufar, K. Climate Factors Promoting Intra-Annual Density Fluctuations in Aleppo Pine (Pinus halepensis) from Semiarid Sites. Dendrochronologia 2011, 262, 1630–1638. [Google Scholar] [CrossRef]
- Balzano, A.; Battipaglia, G.; Cherubini, P.; De Micco, V. Xylem Plasticity in Pinus Pinaster and Quercus ilex Growing at Sites with Different Water Availability in the Mediterranean Region: Relations between Intra-Annual Density Fluctuations and Environmental Conditions. Forests 2020, 11, 379. [Google Scholar] [CrossRef]
- Campelo, F.; Gutiérrez, E.; Ribas, M.; Nabais, C.; Freitas, H. Relationships between Climate and Double Rings in Quercus ilex from Northeast Spain. Can. J. For. Res. 2007, 37, 1915–1923. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gričar, J.; Gruber, A.; Huang, J.; et al. Pattern of Xylem Phenology in Conifers of Cold Ecosystems at the Northern Hemisphere. Glob. Chang. Biol. 2016, 22, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- De Luis, M.; Novak, K.; Raventós, J.; Gričar, J.; Prislan, P.; Čufar, K. Cambial Activity, Wood Formation and Sapling Survival of Pinus Halepensis Exposed to Different Irrigation Regimes. For. Ecol. Manag. 2011, 262, 1630–1638. [Google Scholar] [CrossRef]
- Swidrak, I.; Gruber, A.; Kofler, W.; Oberhuber, W. Effects of Environmental Conditions on Onset of Xylem Growth in Pinus Sylvestris under Drought. Tree Physiol. 2011, 31, 483–493. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Manes, F.; Seufert, G.; Vitale, M. Ecophysiological Studies of Mediterranean Plant Species at the Castelporziano Estate. Atmos. Environ. 1997, 31, 51–60. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; De Santo, A.V. Photosynthetic Response of Quercus ilex L. Plants Grown on Compost and Exposed to Increasing Photon Flux Densities and Elevated CO2. Photosynthetica 2005, 43, 615–619. [Google Scholar] [CrossRef]
- Buttò, V.; Rozenberg, P.; Deslauriers, A.; Rossi, S.; Morin, H. Environmental and Developmental Factors Driving Xylem Anatomy and Micro-density in Black Spruce. New Phytol. 2021, 230, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Balzano, A.; De Micco, V.; Čufar, K.; De Luis, M.; Gričar, J. Intra-Seasonal Trends in Phloem Traits in Pinus Spp. from Drought-Prone Environments. IAWA J. 2020, 41, 219–235. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental Factors Influence Plant Vascular System and Water Regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyaqat, I.; Balzano, A.; Niccoli, F.; Kabala, J.P.; Merela, M.; Battipaglia, G. Xylogenesis Responses to a Mediterranean Climate in Holm Oak (Quercus ilex L.). Forests 2024, 15, 1386. https://doi.org/10.3390/f15081386
Liyaqat I, Balzano A, Niccoli F, Kabala JP, Merela M, Battipaglia G. Xylogenesis Responses to a Mediterranean Climate in Holm Oak (Quercus ilex L.). Forests. 2024; 15(8):1386. https://doi.org/10.3390/f15081386
Chicago/Turabian StyleLiyaqat, Iqra, Angela Balzano, Francesco Niccoli, Jerzy Piotr Kabala, Maks Merela, and Giovanna Battipaglia. 2024. "Xylogenesis Responses to a Mediterranean Climate in Holm Oak (Quercus ilex L.)" Forests 15, no. 8: 1386. https://doi.org/10.3390/f15081386
APA StyleLiyaqat, I., Balzano, A., Niccoli, F., Kabala, J. P., Merela, M., & Battipaglia, G. (2024). Xylogenesis Responses to a Mediterranean Climate in Holm Oak (Quercus ilex L.). Forests, 15(8), 1386. https://doi.org/10.3390/f15081386








