The Characteristics and Influential Factors of Earthworm and Vermicompost under Different Land Use in a Temperate Area, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sample Preparation
2.3. Data and Statistical Analysis
3. Results
3.1. Earthworm Community Composition and Vermicompost Characteristics
3.2. C, N, and C/N of Vermicompost and Soil in Different Land-Use Types
3.3. Comparison of the Microbial Community Diversity between Soil and Vermicompost
3.4. The Environmental Factors of Bacterial Communities in Vermicompost
4. Discussion
4.1. Effects of Different Land-Use Types on Earthworm Communities
4.2. Functional Role of Earthworm Communities on Bacterial Communities and C, N, and C/N
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkhowa, D.J.; Bhattacharyya, P.N.; Sarma, A.K.; Mahanta, K. Diversity and distribution of earthworms in different soil habitats of Assam, north-east India, an Indo-Burma biodiversity hotspot. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 389–396. [Google Scholar] [CrossRef]
- Phillips, H.R.; Guerra, C.A.; Bartz, M.L.; Briones, M.J.; Brown, G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.B.; Hoogen, J.V.D.; Krebs, J.; et al. Global distribution of earthworm diversity. Science 2019, 366, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Schädler, M.; Demetrio, W.; Brown, G.G.; Eisenhauer, N. Climate change effects on earthworms—A review. Soil Org. 2019, 91, 114. [Google Scholar] [CrossRef]
- Chandran, M.S.; Sujatha, S.; Mohan, M.; Julka, J.M.; Ramasamy, E.V. Earthworm diversity at Nilgiri biosphere reserve, Western Ghats, India. Biodivers. Conserv. 2012, 21, 3343–3353. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cao, J.B.; He, X.X.; Liu, T.; Shao, Y.H.; Zhang, C.L.; Zhou, Q.Q.; Li, F.; Mao, P.; Tao, L.B.; et al. Plant leaf litter plays a more important role than roots in maintaining earthworm communities in subtropical plantations. Soil Biol. Biochem. 2020, 144, 107777. [Google Scholar] [CrossRef]
- McLean, M.A.; Migge-Kleian, S.; Parkinson, D. Earthworm invasions of ecosystems devoid of earthworms: Effects on soil microbes. Biol. Invasions 2006, 8, 1257–1273. [Google Scholar] [CrossRef]
- Zhang, W.X.; Shen, Z.F.; Shao, Y.H.; Shi, L.L.; Liu, S.J.; Shi, N.N.; Fu, S.L. Soil biota and sustainable agriculture: A review. Acta Ecol. Sin. 2020, 40, 3183–3206. (In Chinese) [Google Scholar] [CrossRef]
- Kim, G.; Jo, H.; Kim, H.S.; Kwon, M.; Son, Y. Earthworm effects on soil biogeochemistry in temperate forests focusing on stable isotope tracing: A review. Appl. Biol. Chem. 2022, 65, 88. [Google Scholar] [CrossRef]
- Klaminder, J.; Krab, E.J.; Larsbo, M.; Jonsson, H.; Fransson, J.; Koestel, J. Holes in the tundra: Invasive earthworms alter soil structure and moisture in tundra soils. Sci. Total Environ. 2023, 859, 160125. [Google Scholar] [CrossRef]
- Dong, Z.J.; Zhang, D.H.; Yang, Y.Y.; Lin, H.; Zhang, S.L. Effects of earthworm activities on soil nutrients in forest lands of the Qinling Mountains. J. Southwest For. Univ. 2020, 40, 100–107. (In Chinese) [Google Scholar] [CrossRef]
- Julia, S.; Guh, S.; Reinhard, L.; Stefan, S.; Erwin, M. The effect of macro-invertebrates and plant litter of different quality on the release of N from litter to plant on alpine pastureland. Biol. Fertil. Soils Coop. J. Int. Soc. Soil Sci. 2008, 44, 783–790. [Google Scholar] [CrossRef]
- Suarez, E.R.; Pelletier, D.M.; Fahey, T.J.; Groffman, P.M.; Bohlen, P.J.; Fisk, M.C. Effects of exotic earthworms on soil phosphorus cycling in two broadleaf temperate forests. Ecosystems 2004, 7, 28–44. [Google Scholar] [CrossRef]
- Adejuyigbe, C.; Tian, G.; Adeoye, G. Microcosmic study of soil microarthropod and earthworm interaction in litter decomposition and nutrient turnover. Nutr. Cycl. Agroecosystems 2006, 75, 47–55. [Google Scholar] [CrossRef]
- Don, A.; Steinberg, B.; Schöning, I.; Pritsch, K.; Joschko, M.; Gleixner, G.; Schulze, E.D. Organic carbon sequestration in earthworm burrows. Soil Biol. Biochem. 2008, 40, 1803–1812. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Jacquiod, S.; Puga-Freitas, R.; Spor, A.; Mounier, A.; Monard, C.; Mougel, C.; Philippot, L.; Blouin, M. A core microbiota of the plant-earthworm interaction conserved across soils. Soil Biol. Biochem. 2020, 144, 107754. [Google Scholar] [CrossRef]
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.-P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, 3–15. [Google Scholar] [CrossRef]
- Greiner, H.G.; Kashian, D.R.; Tiegs, S.D. Impacts of invasive Asian (Amynthas hilgendorfi) and European (Lumbricus rubellus) earthworms in a North American temperate deciduous forest. Biol. Invasions 2012, 14, 2017–2027. [Google Scholar] [CrossRef]
- Hallam, J.; Hodson, M.E. Impact of different earthworm ecotypes on water stable aggregates and soil water holding capacity. Biol. Fertil. Soils 2020, 56, 607–617. [Google Scholar] [CrossRef]
- Hallam, J.; Holden, J.; Robinson, D.A.; Hodson, M.E. Effects of winter wheat and endogeic earthworms on soil physical and hydraulic properties. Geoderma 2021, 400, 115126. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Scientific Publications: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dendooven, L.; Peres, G.; Tondoh, J.E.; Cluzeau, D.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Lavelle, P.; Charpentier, F.; Rossi, J.P.; Derouard, L.; Pashanasi, B.; Bernier, N. Effects of earthworms on soil organic matter and nutrient dynamics at a landscape scale over decades. In Earthworm Ecology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Devi, J.; Pegu, R.; Mondal, H.; Roy, R.; Bhattacharya, S.S. Earthworm stocking density regulates microbial community structure and fatty acid profiles during vermicomposting of lignocellulosic waste: Unraveling the microbe-metal and mineralization-humification interactions. Bioresour. Technol. 2023, 367, 128305. [Google Scholar] [CrossRef] [PubMed]
- Angst, Š.; Mueller, C.W.; Cajthaml, T.; Angst, G.; Lhotáková, Z.; Bartuška, M.; Špaldoňová, A.; Frouz, J. Stabilization of soil organic matter by earthworms is connected with physical protection rather than with chemical changes of organic matter. Geoderma 2017, 289, 29–35. [Google Scholar] [CrossRef]
- Chao, H.Z.; Sun, M.M.; Zhu, G.F.; Ye, M.; Zhang, S.T.; Liu, M.Q.; Hu, F. Ecological functioning of the earthworm intestinal bacteria and their role in toxicology research. Asian J. Ecotoxicol. 2020, 15, 35–48. (In Chinese) [Google Scholar] [CrossRef]
- Bouché, M.B.; Al-Addan, F. Earthworms, water infiltration and soil stability: Some new assessments. Soil Biol. Biochem. 1997, 29, 441–452. (In Chinese) [Google Scholar] [CrossRef]
- Bottinelli, N.; Hedde, M.; Jouquet, P.; Capowiez, Y. An explicit definition of earthworm ecological categories–Marcel Bouché’s triangle revisited. Geoderma 2020, 372, 114361. [Google Scholar] [CrossRef]
- Guhra, T.; Stolze, K.; Schweizer, S.; Totsche, K.U. Earthworm mucus contributes to the formation of organo-mineral associations in soil. Soil Biol. Biochem. 2020, 145, 107785. [Google Scholar] [CrossRef]
- Norgrove, L.; Csuzdi, C.; Hauser, S. Effects of cropping and tree density on earthworm community composition and densities in central Cameroon. Appl. Soil Ecol. 2011, 49, 268–271. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. The influence of environmental factors on earthworms. In Biology and Ecology of Earthworms; Springer: New York, NY, USA, 2022; pp. 191–232. [Google Scholar] [CrossRef]
- Ma, L.; Song, D.; Liu, M.; Li, Y.; Li, Y. Effects of earthworm activities on soil nutrients and microbial diversity under different tillage measures. Soil Tillage Res. 2022, 222, 105441. [Google Scholar] [CrossRef]
- Hedde, M.; Bureau, F.; Delporte, P.; Cécillon, L.; Decaëns, T. The effects of earthworm species on soil behaviour depend on land use. Soil Biol. Biochem. 2013, 65, 264–273. [Google Scholar] [CrossRef]
- Shipitalo, M.J.; Nuutinen, V.; Butt, K.P. Interaction of earthworm burrows and cracks in a clayey, subsurface-drained, soil. Appl. Soil Ecol. 2004, 26, 209–217. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.; Brown, G.G.; De Deyn, G.B.; Van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef] [PubMed]
- Eriksen-Hamel, N.S.; Whalen, J.K. Impacts of earthworms on soil nutrients and plant growth in soybean and maize agroecosystems. Agric. Ecosyst. Environ. 2007, 120, 442–448. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, D.; Veresoglou, S.D.; Chen, F.; Chen, Y.; Hao, Z.; Zhang, X.; Chen, B. Soil organic carbon and soil structure are driving microbial abundance and community composition across the arid and semi-arid grasslands in northern China. Soil Biol. Biochem. 2014, 77, 51–57. [Google Scholar] [CrossRef]
- Neupane, J.; Guo, W.; Cao, G.; Zhang, F.; Slaughter, L.; Deb, S. Spatial patterns of soil microbial communities and implications for precision soil management at the field scale. Precis. Agric. 2022, 23, 1008–1026. [Google Scholar] [CrossRef]
- Scullion, J.; Malik, A. Earthworm activity affecting organic matter, aggregation and microbial activity in soils restored after opencast mining for coal. Soil Biol. Biochem. 2000, 32, 119–126. [Google Scholar] [CrossRef]
- Medina-Sauza, R.M.; Álvarez-Jiménez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdán, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms building up soil microbiota, a review. Front. Environ. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Hendrix, P.F.; Baker, G.H.; Callaham, M.A.; Damoff, G.A.; Fragoso, C.; González, G.; James, S.W.; Lachnicht, S.L.; Winsome, T.; Zou, X. Invasion of exotic earthworms into ecosystems inhabited by native earthworms. Biol. Invasions Belowground Earthworms Invasive Species 2006, 8, 1287–1300. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Shang, Y.; Gao, Y.; Li, Y.; Wei, Q.; Dong, Y.; Mei, X.; Zhou, S.; Sun, G.; et al. High-altitude drives the convergent evolution of alpha diversity and indicator microbiota in the gut microbiomes of ungulates. Front. Microbiol. 2022, 13, 953234. [Google Scholar] [CrossRef]
- Belnap, J.; Weber, B.; Büdel, B. Biological Soil Crusts as an Organizing Principle in Drylands. In Biological Soil Crusts: An Organizing Principle in Drylands; Ecological Studies; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 226, pp. 56–73. [Google Scholar] [CrossRef]
- Hoeffner, K.; Monard, C.; Santonja, M.; Cluzeau, D. Feeding behaviour of epi-anecic earthworm species and their impacts on soil microbial communities. Soil Biol. Biochem. 2018, 125, 1–9. [Google Scholar] [CrossRef]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef] [PubMed]
- Angel, R.; Soares, M.I.M.; Ungar, E.D.; Gillor, O. Biogeography of soil archaea and bacteria along a steep precipitation gradient. ISME J. 2010, 4, 553–563. [Google Scholar] [CrossRef] [PubMed]
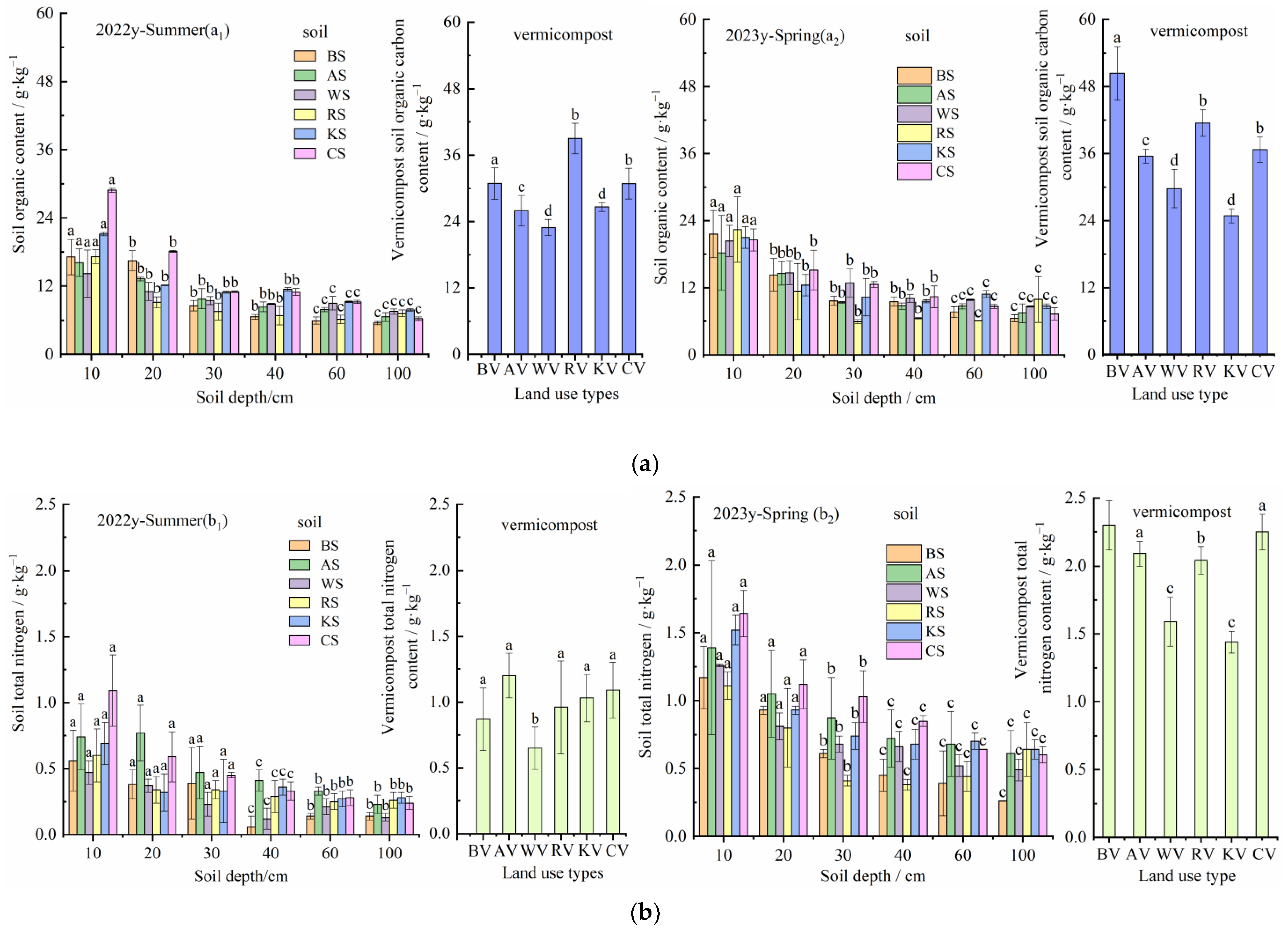
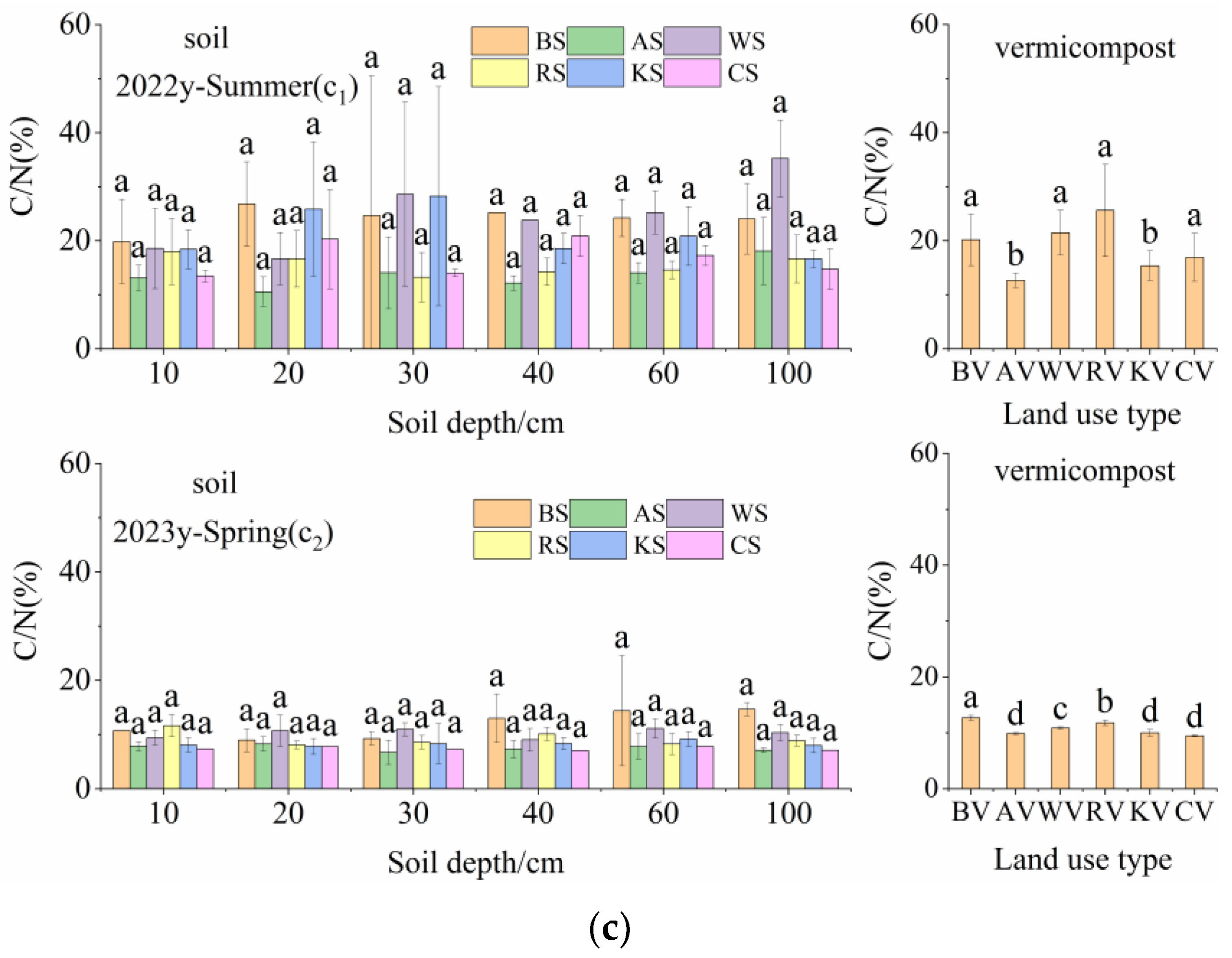
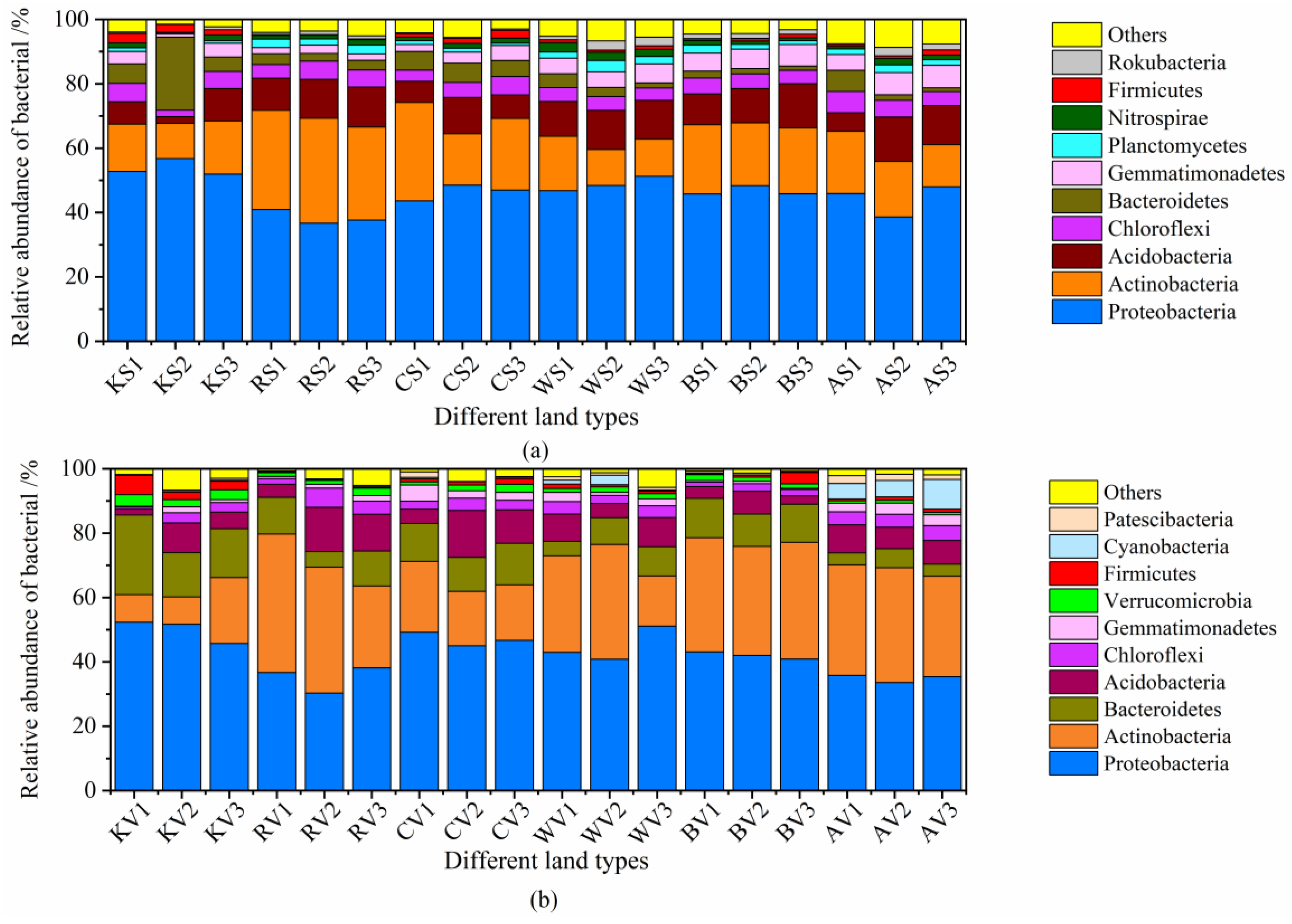

| Land-Use Soil Type | Location | Soil Water Content/% | Particle-Size Distribution/% | pH | Bulk Density/g·cm−3 | ||
|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||
| Buttonwood forest (BS) | 33.45 N, 107.63 E | 13.3 ± 0.01 | 28.95 ± 1.89 | 64.11 ± 1.14 | 6.94 ± 3.03 | 8.15 ± 0.01 | 1.39 ± 0.04 |
| Walnut forest (WS) | 34.48 N, 107.44 E | 18.7 ± 0.01 | 32.21 ± 1.13 | 63.19 ± 1.13 | 4.60 ± 2.02 | 8.14 ± 0.04 | 1.42 ± 0.06 |
| Apple orchard (AS) | 34.43 N, 107.88 E | 15.0 ± 0.01 | 33.27 ± 0.49 | 62.16 ± 0.49 | 4.57 ± 1.76 | 8.07 ± 0.19 | 1.30 ± 0.04 |
| Kiwi orchard (KS) | 34.26 N, 107.72 E | 21.3 ± 0.01 | 28.92 ± 1.93 | 65.12 ± 1.92 | 5.96 ± 2.76 | 8.00 ± 0.02 | 1.40 ± 0.08 |
| Ryegrass land (RS) | 34.35 N, 107.20 E | 11.0 ± 0.02 | 29.03 ± 2.53 | 59.04 ± 2.53 | 11.93 ± 4.56 | 8.19 ± 0.03 | 1.45 ± 0.05 |
| Corn field (CS) | 34.28 N, 107.64 E | 21.0 ± 0.01 | 29.30 ± 1.90 | 57.81 ± 1.90 | 13.88 ± 1.58 | 7.81 ± 0.04 | 1.36 ± 0.08 |
| Earthworm Species | Earthworm Populations/ ind·m−2 | Vermicompost Weight/ g·m−2 | |||
|---|---|---|---|---|---|
| Summer 2022 | Spring 2023 | Summer 2022 | Spring 2023 | ||
| Buttonwood forest (BS) | Amynthas carnosus planus, Metaphire baojiensis | 4 ± 0.6 c | 20 ± 3.6 b | 308.3 ± 25.1 b | 240.8 ± 25.4 a |
| Walnut forest (WS) | Amynthas carnosus carnosus, Metaphire baojiensis | 26 ± 2.3 a | 15 ± 5.3 b | 766.0 ± 35.4 a | 198.8 ± 8.5 b |
| Apple orchard (AS) | Amynthas carnosus planus | 2 ± 0 c | 40 ± 0.7 a | 296.7 ± 4.2 b | 286.0 ± 25.5 a |
| Kiwi orchard (KS) | Esienia foetida, Amynthas carnosus planus, Metaphire vulgaris | 13 ± 3.2 b | 32 ± 4.7 a | 732.7 ± 40.9 a | 235.4 ± 23.1 a |
| Ryegrass land (RS) | Esienia foetida, Amynthas carnosus carnosus | 18 ± 1.7 a | 19 ± 6.7 b | 434.0 ± 14.9 b | 189.2 ± 7.6 b |
| Corn field (CS) | Amynthas carnosus planus, Amynthas carnosus carnosu, Metaphire baojiensis | 17 ± 3.1 a | 38 ± 9.5 a | 482.0 ± 2.8 b | 232.2 ± 5.9 b |
| Land-Use Type Soil Species | Bacteria | ||
|---|---|---|---|
| Chao1 | Shannon | Simpson | |
| BS | 3819.87 ± 192 | 9.24 ± 0.35 | 0.9557 ± 0.0162 |
| AS | 3774.57 ± 209 | 9.70 ± 0.68 | 0.9737 ± 0.0240 |
| WS | 4227.74 ± 342 | 9.44 ± 0.62 | 0.9570 ± 0.0239 |
| RS | 3747.06 ± 375 | 10.44 ± 0.07 | 0.9954 ± 0.0019 |
| KS | 3880.09 ± 324 | 9.72 ± 0.32 | 0.9843 ± 0.0089 |
| CS | 4053.62 ± 520 | 10.35 ± 0.12 | 0.9927 ± 0.0047 |
| BV | 2660.24 ± 280 | 9.83 ± 0.42 | 0.9966 ± 0.0015 |
| AV | 3648.09 ± 277 | 10.58 ± 0.13 | 0.9979 ± 0.0002 |
| WV | 3350.85 ± 582 | 10.54 ± 0.33 | 0.9985 ± 0.004 |
| RV | 2796.03 ± 566 | 10.01 ± 0.58 | 0.9974 ± 0.0017 |
| KV | 3695.76 ± 693 | 10.14 ± 0.47 | 0.9973 ± 0.0007 |
| CV | 2831.19 ± 305 | 10.00 ± 0.58 | 0.9957 ± 0.0037 |
| C/ g·kg−1 | N/ g·kg−1 | C/N /% | ED | SWC /% | Chao1 | Proteobacteria | Actinobacteria | Acidobacteria | Chloroflexi | Bacteroidetes | Gemmatimonadetes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | C/ g·kg−1 | 1 | |||||||||||
| N/ g·kg−1 | 0.62 * | 1 | |||||||||||
| C/N/% | 0.15 | −0.81 * | 1 | ||||||||||
| ED | 0.60 * | −0.33 | 0.45 | 1 | |||||||||
| SWC/% | 0.82 * | 0.26 | 0.26 | 0.33 | 1 | ||||||||
| chao1 | −0.34 | −0.2 | 0.67 * | 0.73 * | 0.63 * | 1 | |||||||
| Proteobacteria | 0.48 | −0.18 | 0.57 * | 0.05 | 0.79 * | 0.44 | 1 | ||||||
| Actinobacteria | −0.19 | 0.19 | −0.57 * | 0.03 | −0.61 * | −0.48 | −0.85 * | 1 | |||||
| Acidobacteria | −0.77 * | −0.40 | 0.20 | 0.03 | −0.70 * | 0.04 | −0.56 * | 0.15 | 1 | ||||
| Chloroflexi | −0.07 | 0.48 | −0.69 * | −0.53 * | −0.61 * | −0.57 * | −0.75 * | 0.71 * | 0.28 | 1 | |||
| Bacteroidetes | 0.64 * | 0.24 | −0.05 | 0.12 | 0.75 * | 0.07 | 0.66 * | −0.32 | −0.95 ** | −0.53 * | 1 | ||
| Gemmatimonadetes | −0.26 | −0.17 | 0.30 | −0.53 * | −0.17 | 0.04 | 0.19 | −0.55 * | 0.51 * | 0.075 | −0.50 * | 1 | |
| Vermicompost | C/g·kg−1 | 1 | |||||||||||
| N/g·kg−1 | 0.18 | 1 | |||||||||||
| C/N/% | 0.61 * | −0.65 * | 1 | ||||||||||
| ED | 0.01 | −0.62 * | 0.55 * | 1 | |||||||||
| SWC/% | 0.61 * | 0.04 | −0.52 | - | 1 | ||||||||
| chao1 | −0.71 * | 0.22 | −0.64 * | −0.07 | 0.46 | 1 | |||||||
| Proteobacteria | −0.44 | −0.24 | −0.23 | 0.35 | 0.88 * | 0.16 | 1 | ||||||
| Actinobacteria | 0.38 | −0.12 | 0.42 | −0.31 | −0.94 ** | −0.41 | −0.89 * | 1 | |||||
| Acidobacteria | 0.09 | 0.05 | −0.07 | 0.10 | 0.53 * | −0.001 | 0.76 * | −0.76 * | 1 | ||||
| Chloroflexi | 0.44 | 0.31 | 0.2 | 0.37 | −0.11 | −0.2 | −0.38 | 0.13 | −0.37 | 1 | |||
| Bacteroidetes | 0.3 | 0.3 | 0.13 | 0.074 | −0.46 | 0.043 | −0.79 * | 0.52 | −0.75 | 0.80 * | 1 | ||
| Gemmatimonadetes | −0.38 | 0.42 | −0.56 * | −0.06 | 0.32 | 0.22 | −0.087 | −0.059 | −0.5 | 0.51 | 0.45 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Shao, M.; Wang, Y.; Li, T.; Jing, X.; Jia, K.; Zhang, Y. The Characteristics and Influential Factors of Earthworm and Vermicompost under Different Land Use in a Temperate Area, China. Forests 2024, 15, 1389. https://doi.org/10.3390/f15081389
Ma L, Shao M, Wang Y, Li T, Jing X, Jia K, Zhang Y. The Characteristics and Influential Factors of Earthworm and Vermicompost under Different Land Use in a Temperate Area, China. Forests. 2024; 15(8):1389. https://doi.org/10.3390/f15081389
Chicago/Turabian StyleMa, Li, Ming’an Shao, Yunqiang Wang, Tongchuan Li, Xuanxuan Jing, Kunyu Jia, and Yangyang Zhang. 2024. "The Characteristics and Influential Factors of Earthworm and Vermicompost under Different Land Use in a Temperate Area, China" Forests 15, no. 8: 1389. https://doi.org/10.3390/f15081389







