Exogenous Salicylic Acid Alleviates Physiological Stress in Salix matsudana Seedlings and Increases 2,4-Dinitrophenol Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Site
2.2. Test Materials
2.3. Experimental and Treatments
2.4. Measurement of Superoxide Dismutase (SOD), Peroxidase (POD), Catalase (CAT), and MDA Levels
2.5. Determination of Relative Conductivity (REC)
2.6. Dye the Leaves with 3,3-Diaminobenzidine Method (DAB) to Show the Accumulation of H2O2 in the Leaves
2.7. Staining the Leaves with Plant Superoxide Anion Staining Solution Method (NBT) to Show the Accumulation of O2−∙ in the Leaves
2.8. Determination of Photosynthetic Parameters
2.9. Chlorophyll Fluorescence Determination
2.10. Measurement of the Chlorophyll, H2O2, O2−∙ Levels
2.11. Root Vigor Determination
2.12. Determination of the Root Morphology, Biomass, Tolerance Index (TI) and Toxicity Factor
2.13. Percentage Removal of 2,4-DNP Was Determined
2.14. Plant Growth and Morphological Index Records
2.15. Data Analysis
3. Results
3.1. Photosynthetic Gas Exchange Parameters
3.2. Chlorophyll Fluorescence Parameters
3.3. Chlorophyll Content
3.4. Cell Membrane Damage Level
3.5. Antioxidant Enzyme Activity and Active Oxygen Level
3.6. Biomass
3.7. Growth Morphological Characteristics
3.8. 2,4-DNP Removal Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An overview of the environmental pollution and health effects associated with waste landfilling and open dum. Environ. Sci. Pollut. R. 2022, 29, 58514–58536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Liu, Z.L.; Tian, P.W. Research progress on plant effects of nitroaromatic compounds. J. Ecol. Environ. 2013, 22, 1079–1084. [Google Scholar] [CrossRef]
- Wang, J.Z.; Huang, W.D. 2,4-Dinitrophenol poisoning. Chin. J. Crit. Care Med. 2009, 2, 58–60. [Google Scholar] [CrossRef]
- Thang, P.Q.; Jitae, K.; Giang, B.L.; Viet, N.M.; Huong, P.T. Potential application of chicken manure biochar towards toxic phenol and 2,4-dinitrophenol in wastewaters. J. Environ. Manag. 2019, 251, 109556. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, H.; Lakzian, A.; Karimi, A.; Halajnia, A. Efficient removal of 2,4-dinitrophenol from synthetic wastewater and contaminated soil samples using free and immobilized laccases. J. Environ. Manag. 2020, 256, 109740. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.J.; Kalantary, R.R.; Dehghanifard, E.; Mahvi, A.H.; Faramarzi, M.A.; Kalhori, E.M. Investigation of immobilized laccase enzyme on nanoporous silica application for removal of 2,4-dinitrophenol from aqueous solution. J. Maz. Univ. Med. Sci. 2016, 26, 301–313. Available online: http://jmums.mazums.ac.ir/article-1-9268-en.html (accessed on 21 August 2016).
- Cao, X.; Oda, Y.; Shiraishi, F. Photocatalytic and adsorptive treatment of 2,4-dinitrophenol using a TiO2 film covering activated carbon surface. Chem. Eng. J. 2010, 156, 98–105. [Google Scholar] [CrossRef]
- Pan, J.Y.; Tu, W.M.C.; Guan, M.Y.; Yang, Y.J.; Xu, P.; Chen, M.X.; Cao, Z.Z. Research progress on mechanisms of salicylic acid in alleviating cadmium toxicity in crops. Chin. J. Ecol. 2020, 39, 4216–4223. [Google Scholar] [CrossRef]
- Wang, Y.K. Study on Salicylic Acid Alleviating Toxicity of Dioxyzine to Three Crops. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Guan, C.; Wang, C.; Wu, H.; Li, Q.; Zhang, Y.; Wang, G.; Ji, J.; Jin, C. Salicylic acid application alleviates the adverse effects of triclosan stress in tobacco plants through the improvement of plant photosynthesis and enhancing antioxidant system. Environ. Sci. Pollut. Res. 2020, 27, 1359–1372. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Varshney, P.; Ahmad, A. Salicylic acid minimizes nickel and/or salinity-induced toxicity in indian mustard (Brassica juncea) through an improved antioxidant system. Environ. Sci. Pollut. Res. 2012, 19, 8–18. [Google Scholar] [CrossRef]
- Christina, C.S.; Dani, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Saeed, R.; Abooalfazl, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- Qiu, Y.; An, K.; Sun, J.; Chen, X.; Gong, X.; Ma, L.; Wu, S.; Jiang, S.; Zhang, Z.; Wang, Y. Investigating the effect of methyl jasmonate and melatonin on resistance of Malus crabapple ‘hong Jiu’ to ozone stress. Environ. Sci. Pollut. Res. 2019, 26, 27761–27768. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Yang, Y.L.; Jia, L.Y.; Chen, H.J.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotox. Environ. Safe. 2013, 89, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.H. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shen, Q.D.; Ma, S.; Liu, L.; Cheng, J.L. Physiological and PIP transcriptional responses to progressive soil water deficit in three mulberry cultivars. Front. Plant Sci. 2020, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; You, J.; Xu, X.; Yang, Y.; Wang, J.; Zhang, D. Physiological and Biochemical Responses of Melilotus albas to Saline and Alkaline Stresses. Horticulturae 2024, 10, 297. [Google Scholar] [CrossRef]
- Nijs, I.; Ferris, R.; Blum, H.; Hendrey, G.; Impens, I. Stomatal regulation in a changing climate: A field study using free air temperature increase (FATI) and free air CO2 enrichment (FACE). Plant Cell Environ. 1997, 20, 1041–1050. [Google Scholar] [CrossRef]
- Lin, L.; Liu, L.; He, Y.Q.; Zhang, L.Y. Comparative Study on Different Photosynthetic Light-response and CO2 Response Models for Physalis pubescens L. North. Hortic. 2016, 8, 21–23. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Q.L.; Guo, Y.Y.; Zhang, Y.J.; Hou, L.Y. Effect of NaCl stress on photosynthetic chlorophyll fluorescence properties of Daphne giraldii leaves in Qilian Mountains. Northwest J. Botany. 2016, 36, 1182–1189. [Google Scholar] [CrossRef]
- Makeen, K.; Babu, G.S.; Lavanya, G.R.; Abraham, G. Studies of chlorophyll content by different methods in black gram (Vigna mungo L.). Int. J. Agric. Res. 2007, 2, 651–654. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Stefan, R.; Jagadis, G.K.; Werner, M.K. Oxidation of hydroxylamines to no by plant cells. Plant Signal. Behav. 2014, 4, 853–855. [Google Scholar] [CrossRef]
- Dillaway, D.N.; Stringer, J.W.; Rieske, L.K. Light availability influences root carbohydrates, and potentially vigor, in white oak advance regeneration. Forest Ecol. Manag. 2007, 250, 227–233. [Google Scholar] [CrossRef]
- Zhang, G.W.; Lu, H.L.; Zhang, L.; Chen, B.L.; Zhou, Z.G. Evaluation of salt tolerance and screening of salt tolerance indexes at germination and seedling stage of cotton. Chin. J. Appl. Ecol. 2011, 22, 2045–2053. [Google Scholar] [CrossRef]
- Li, H.C.; Zhao, P.Y.; Zhang, J.; Lv, Y.X.; Jing, Y.P.; Tuo, D.B.; Li, J. Response of new sunflower varieties to NaCl stress and their salt tolerance thresholds. Soil Bull. 2018, 49, 1452–1457. [Google Scholar] [CrossRef]
- Wu, X.L.; Liang, H.Y.; Yang, F.; Liu, W.G.; Yu, Y.H.; Yang, W.Y. Comprehensive evaluation and screening identification indexes of shade tolerance at seedling in soybean. Sci. Agric. Sin. 2015, 48, 2497–2507. [Google Scholar] [CrossRef]
- Wang, Z.Y. Physiological Characteristics of Flooding Stress in Seedlings of Nine Species of Woody Plants. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2014. [Google Scholar]
- Zhou, J.H.; Cheng, K.; Zheng, J.Y.; Liu, Z.Q.; Shen, W.B.; Fan, H.B.; Jin, Z.N. Physiological and biochemical characteristics of cinnamomum camphora in response to Cu-and Cd-contaminated soil. Water Air Soil Pollut. 2019, 230, 15. [Google Scholar] [CrossRef]
- Yan, Q.Q.; Zhang, J.S.; Dai, J.M.; Dou, Q.Q. Effect of betaine on photosynthesis and biomass accumulation in sea island cotton seedlings under saline stress. J. Crop Sci. 2019, 45, 1128–1135. [Google Scholar] [CrossRef]
- Pei, B. Effect of Soil Water Stress on Photosynthetic Physiological and Biochemical Properties of Sea Buckthorn. Master’s Thesis, Shandong Agricultural University, Taian, China, 2013. [Google Scholar]
- Zhang, Y.; Shuang, X.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumismelo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef]
- Fang, X.C. Characteristics of Light Response of Lonicera Japonica and Sour Date under Soil Water Stress. Master’s Thesis, Shandong Agricultural University, Taian, China, 2017. [Google Scholar]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Liu, L.N.; Zhang, W.Q.; Gan, X.H.; Tang, C.B.; Qiu, P.J. Effect of salt stress on photosynthetic characteristics and chlorophyll fluorescence parameters of Heritiera littoralis seedlings. J. For. Environ. 2019, 39, 601–607. [Google Scholar] [CrossRef]
- Hu, W.H.; Zhang, S.S.; Xiao, Y.A.; Yan, X.H. Physiological response of two species of Rhododendron to full light environment after prolonged shading and their photoprotection mechanism. J. Plant Ecol. 2015, 39, 1093–1100. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.; Bai, C. Supplementary calcium restores peanut (Arachis hypogaea) growth and photosynthetic capacity under low nocturnal temperature. Front. Plant Sci. 2020, 10, 1637–1646. [Google Scholar] [CrossRef]
- Yu, M.L.; Zuo, G.Q.; Li, Y.; Zheng, D.F.; Feng, N.J. Modulation of photosynthetic properties and protective enzyme activities of soybean seedlings under salinity stress by calcium cyclamate. Chin. J. Oil Crop 2019, 41, 741–749. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J. Plant Growth Regul. 2020, 39, 641–655. [Google Scholar] [CrossRef]
- Drazic, G.; Mihailovic, N. Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci. 2005, 168, 511–517. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Vincent, C.I.; Grosser, J.W.; Dutt, M. The response of salt-stressed Valencia sweet orange (Citrus sinensis) to salicylic acid and methyl jasmonate treatments. Plant Physiol. Rep. 2021, 26, 137–151. [Google Scholar] [CrossRef]
- Seyma, H.Y.; Mahmut, K.; Ridvan, T.; Semih, Y. Antioxidant enzyme responseof sorghum plant upon exposure to aluminum, chromium and lead heavy metals. Turk. J. Biochem. 2017, 42, 503–512. [Google Scholar] [CrossRef]
- Li, Y.M.; Guo, X.W.; Dai, H.P. Physiological response of Rubus crataegifolius seedlings to saline stress and their salt tolerance threshold. Northwest J. Bot. 2014, 34, 1213–1219. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wu, C.Y.; Yang, Z.; Yang, F.; Wu, Y.C. Effects of salinity stress on the growth and physiological and biochemical characteristics of jujube seedlings. Agric. Res. Arid Reg. 2018, 36, 153–160. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.L.; Yang, H. Toxicology of isoproturon to the food crop wheat as affected by salicylic acid. Environ. Sci. Pollut. Res. 2012, 19, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Yang, H.C.; Shao, H.B.; Zheng, A.Z.; Brestic, M. The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by heavy metals. Clean-Soil Air Water 2014, 42, 88–97. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan-Chemosphere, Q.M. Plant–bacteria PAR tnerships for the remedi-ation of hydrocarbon Chemosphere contaminatedsoils. Chemosphere 2013, 90, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.R.; Sklodowska, M. The oxidative stress and antioxidant systems in cucumber cells duringacclimation to salinity. Biol. Plant. 2014, 58, 47–54. [Google Scholar] [CrossRef]
- Xu, P.X.; Fei, L.; Chen, X.B.; Wang, Z.L. Tolerance and accumulation characteristics of cadmium in four cool-season turf plants. J. Grass Ind. 2014, 23, 176–188. [Google Scholar] [CrossRef]
- Jing, T.; Xie, H.C.; Sun, J.W.; Liu, H.D.; Li, H. Photosynthetic physiological response and purification effect of sunflower to aniline wastewater. Acta Ecol. Sinica 2017, 37, 6091–6098. [Google Scholar] [CrossRef][Green Version]
- Gu, Y.B.; Chen, F.Y.; Bai, J.S.; Lou, Y.J.; Tang, Z.H. Effect of salinity stress on functional traits of seedlings of Three Rivers cassis. J. Appl. Environ. Biol. 2020, 26, 10–16. [Google Scholar] [CrossRef]
- Weyens, N.; Vander, L.D.; Taghavi, S.; Vangronsveldl, J. Phytoremediation: Plant-endophyte PAR tnerships take the challenge. Curr. Opin. Biotech. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Li, C.H.; Shang, X.S.; Ou, Y.J.; Li, B.B.; Zhai, Y.Z.; Liu, X.J.; Zou, J.H. Effect of Ca on antioxidant enzyme activity of asexual lines of dry willow seedlings under Cd stress. Technol. Vis. 2018, 12, 85–88. [Google Scholar]
- Mnif, I.; Mnif, S.; Sahnoun, R.; Maktouf, S.; Ayedi, Y.; Ellouze-Chaabouni, S.; Ghribi, D. Biodegradation of diesel oil by a novel microbial consortium: Comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Environ. Sci. Pollut. Res. 2015, 22, 14852–14861. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.P. Effects of exogenous MeJA on the growth and antioxidant system of Chrysanthemum chinensis under cadmium stress. Acta Bot. Sin. 2019, 39, 1627–1635. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Zhao, R. Soil carbon dioxide flux from shelterbelts in farmland in temperate arid region, northwest China. Eur. J. Soil Biol. 2012, 48, 24–31. [Google Scholar] [CrossRef]
- Da, C.R.; Martinez, A.M.; Ferreira, S.T. 2,4-Dinitrophenol blocks neurodegeneration and preserves sciatic nerve function after trauma. J. Neurotrauma 2010, 27, 829–841. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
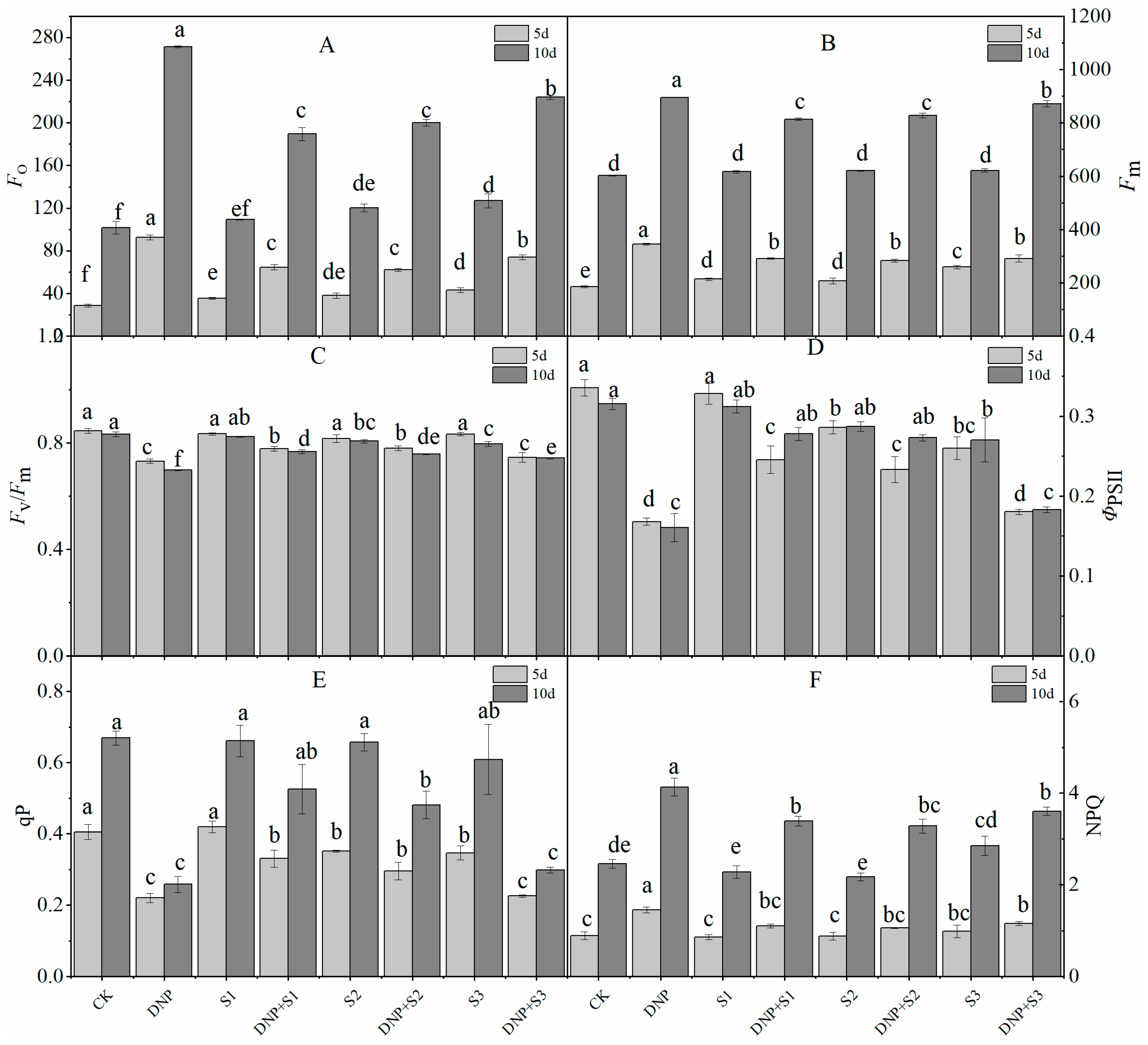
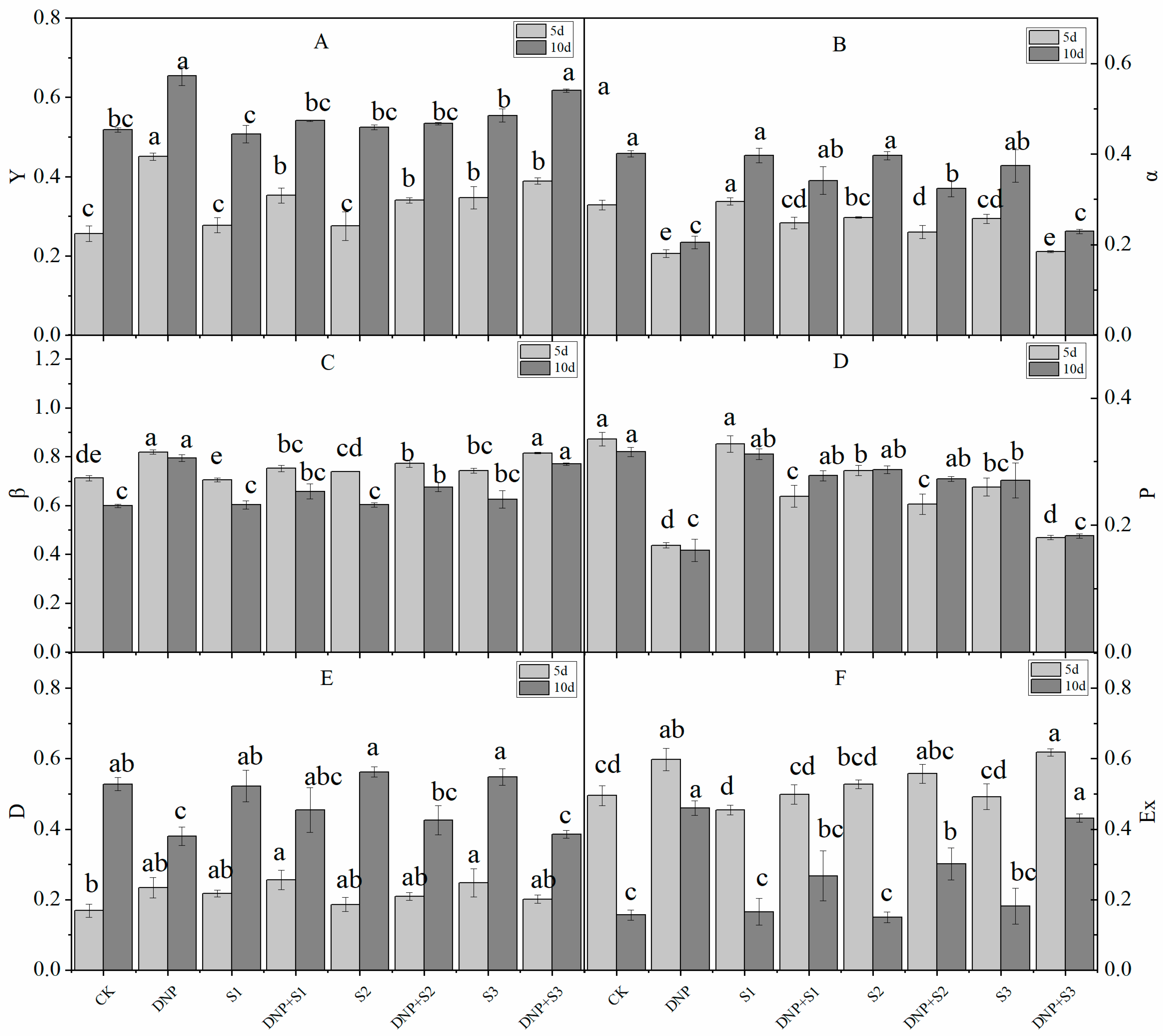
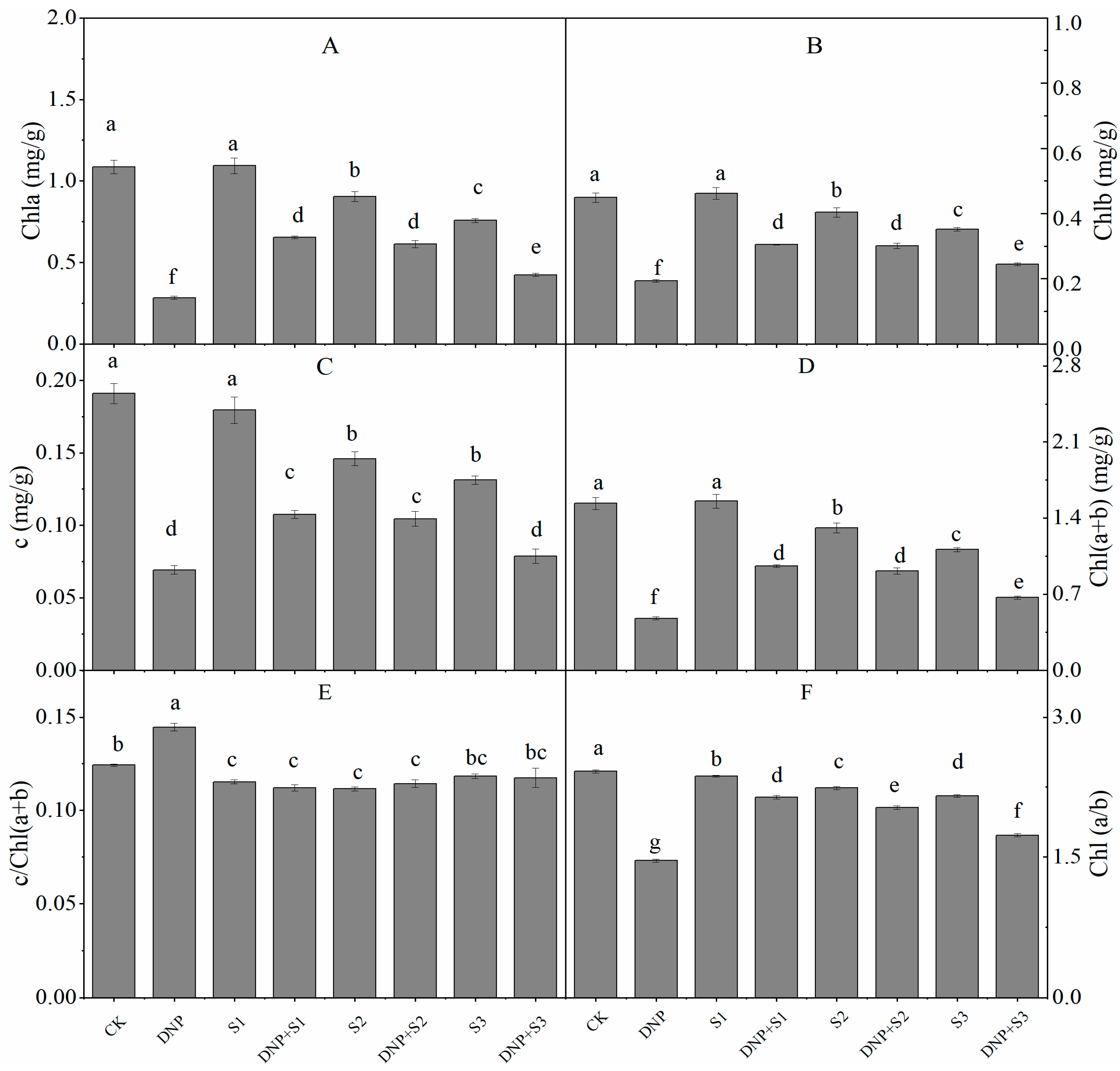

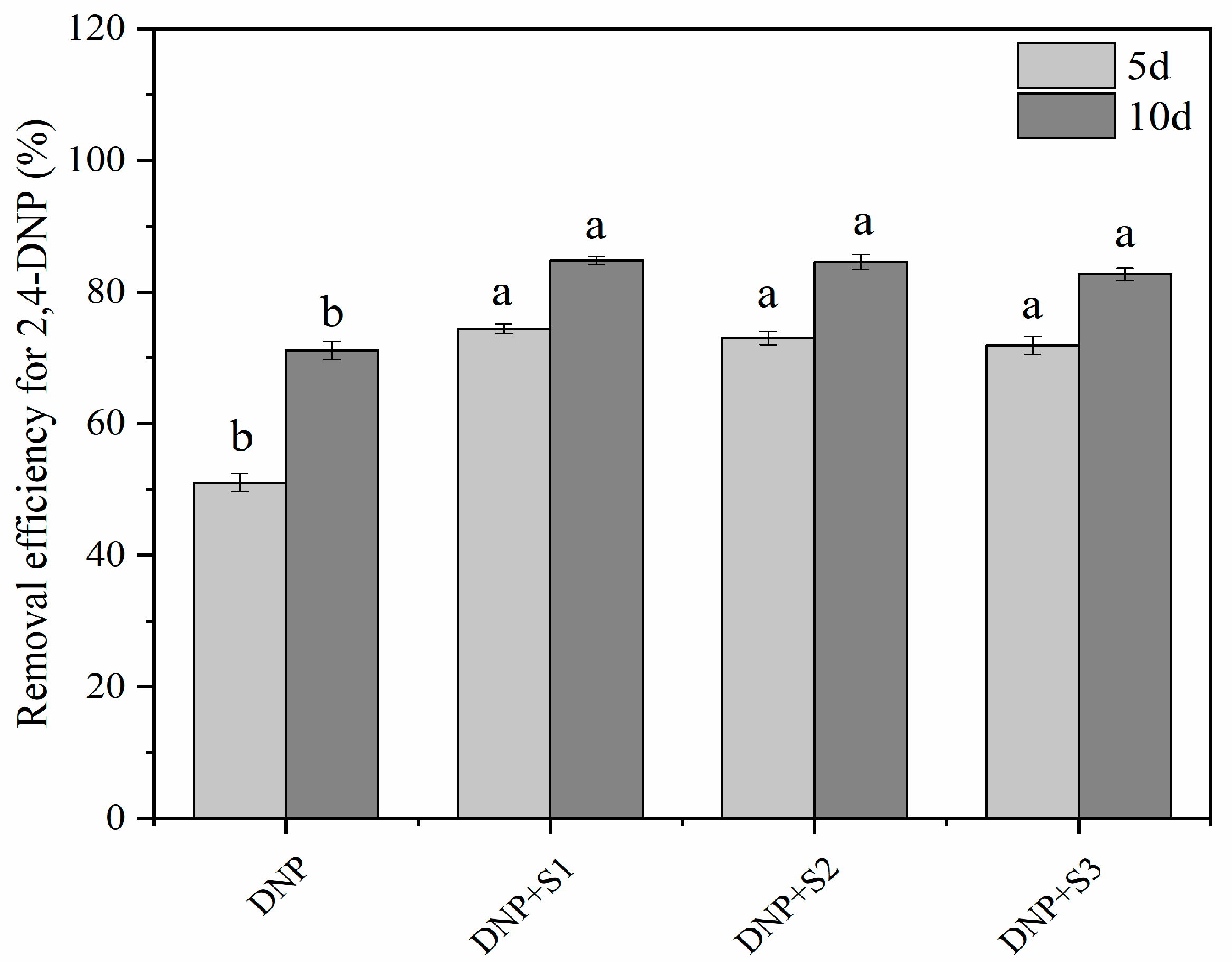
| Time (d) | Treatment | Pn (mmol∙m−2∙s−1) | Tr (mmol∙m−2∙s−1) | Ci (μmol∙mol−1) | Gs (mmol∙m−2∙s−1) | Ls (%) | WUE | LUE |
|---|---|---|---|---|---|---|---|---|
| 5 | CK | 13.1 ± 0.15 a | 4.5 ± 0.07 a | 237.5 ± 2.78 d | 219.1 ± 0.17 a | 0.41 ± 0.007 a | 2.9 ± 0.06 a | 1.09 ± 0.012 a |
| DNP | 6.6 ± 0.11 e | 3.0 ± 0.05 c | 329.1 ± 1.97 a | 114.8 ± 0.32 d | 0.18 ± 0.005 d | 2.3 ± 0.04 d | 0.55 ± 0.009 e | |
| S1 | 12.9 ± 0.07 a | 4.6 ± 0.06 a | 244.7 ± 3.13 d | 212.2 ± 2.99 a | 0.39 ± 0.008 a | 2.8 ± 0.05 a | 1.08 ± 0.006 a | |
| DNP + S1 | 12.4 ± 0.07 a | 4.7 ± 0.06 a | 247.1 ± 6.02 d | 207.30 ± 3.04 a | 0.38 ± 0.015 a | 2.7 ± 0.02 b | 1.04 ± 0.005 b | |
| S2 | 10.0 ± 0.04 c | 4.1 ± 0.09 b | 261.4 ± 2.95 c | 193.8 ± 6.05 b | 0.35 ± 0.007 b | 2.4 ± 0.05 c | 0.83 ± 0.003 c | |
| DNP + S2 | 9.9 ± 0.07 c | 4.1 ± 0.06 b | 263.6 ± 3.93 c | 190.4 ± 5.67 b | 0.34 ± 0.010 b | 2.4 ± 0.02 c | 0.82 ± 0.006 c | |
| S3 | 7.2 ± 0.11 d | 2.9 ± 0.06 c | 298.0 ± 2.78 b | 168.8 ± 5.78 c | 0.25 ± 0.007 c | 2.5 ± 0.04 c | 0.61 ± 0.009 d | |
| DNP + S3 | 7.2 ± 0.09 d | 3.0 ± 0.06 c | 303.4 ± 5.82 b | 160.0 ± 5.75 c | 0.24 ± 0.015 c | 2.5 ± 0.08 c | 0.60 ± 0.007 d | |
| 10 | CK | 10.9 ± 0.11 a | 2.8 ± 0.01 a | 233.5 ± 7.02 e | 147.1 ± 5.40 b | 0.42 ± 0.018 a | 4.0 ± 0.05 a | 0.91 ± 0.009 a |
| DNP | 4.6 ± 0.05 d | 1.4 ± 0.02 d | 338.5 ± 5.66 a | 53.4 ± 3.97 e | 0.15 ± 0.014 e | 3.4 ± 0.07 d | 0.39 ± 0.004 d | |
| S1 | 10.9 ± 0.10 a | 2.8 ± 0.02 a | 238.2 ± 6.01 e | 165.4 ± 7.05 a | 0.40 ± 0.015 a | 3.9 ± 0.03 ab | 0.91 ± 0.008 a | |
| DNP + S1 | 10.7 ± 0.06 a | 2.8 ± 0.05 a | 257.2 ± 5.60 d | 140.0 ± 4.78 b | 0.36 ± 0.014 b | 3.9 ± 0.05 ab | 0.89 ± 0.005 a | |
| S2 | 7.7 ± 0.08 b | 2.1 ± 0.02 b | 279.8 ± 4.13 c | 120.8 ± 3.21 c | 0.30 ± 0.010 c | 3.6 ± 0.03 c | 0.64 ± 0.008 b | |
| DNP + S2 | 7.5 ± 0.13 b | 2.1 ± 0.05 b | 288.5 ± 3.31 c | 115.8 ± 8.13 c | 0.28 ± 0.008 c | 3.6 ± 0.06 c | 0.63 ± 0.007 b | |
| S3 | 7.0 ± 0.03 c | 1.9 ± 0.03 c | 315.7 ± 5.53 b | 91.1 ± 3.21 d | 0.21 ± 0.014 d | 3.7 ± 0.07 bc | 0.58 ± 0.003 c | |
| DNP + S3 | 7.0 ± 0.07 c | 1.8 ± 0.03 c | 318.1 ± 5.49 b | 88.6 ± 4.35 d | 0.20 ± 0.013 d | 3.9 ± 0.08 ab | 0.89 ± 0.006 c |
| Time (d) | Treatment | One-Dimensional Linear Equation | 2,4-DNP Concentration When Pn Is Halved (mg·L−1) |
|---|---|---|---|
| 5 | DNP | Y = −0.6293X + 15 | 13.41 |
| DNP + S1 | Y = −0.3485X + 15 | 24.21 | |
| DNP + S2 | Y = −0.3881X + 15 | 21.74 | |
| DNP + S3 | Y = −0.5934X + 15 | 14.22 | |
| 10 | DNP | Y = −0.9484X + 15 | 10.06 |
| DNP + S1 | Y = −0.4862X + 15 | 19.62 | |
| DNP + S2 | Y = −0.6855X + 15 | 13.91 | |
| DNP + S3 | Y = −0.7366X + 15 | 12.95 |
| Treatment | MDA (mmol∙g−1) | REC | LD |
|---|---|---|---|
| CK | 13.8 ± 0.66 d | 7.4 ± 0.47 d | - |
| DNP | 38.2 ± 1.40 a | 48.6 ± 0.63 a | 44.5 ± 0.95 a |
| S1 | 14.0 ± 1.05 d | 8.1 ± 0.46 d | 0.7 ± 0.13 d |
| DNP + S1 | 32.6 ± 0.31 b | 40.1 ± 0.57 b | 35.3 ± 0.94 b |
| S2 | 16.4 ± 1.25 d | 8.5 ± 0.19 d | 1.2 ± 0.32 d |
| DNP + S2 | 35.7 ± 0.70 a | 41.0 ± 0.98 b | 36.3 ± 1.35 b |
| S3 | 29.6 ± 1.30 c | 21.7 ± 0.47 c | 15.4 ± 0.32 c |
| DNP + S3 | 38.7 ± 0.39 a | 47.3 ± 0.64 a | 43.1 ± 0.90 a |
| Time (d) | Treatment | SOD (U·g−1 FW) | POD (U·g−1·min−1) | CAT (U·g−1·min−1) | H2O2 (mmol·g−1) | O2−∙ (mmol·min−1·g−1) |
|---|---|---|---|---|---|---|
| 5 | CK | 336.6 ± 28.67 c | 1133.3 ± 120.19 c | 23.3 ± 3.33 d | 1.4 ± 0.02 b | 2.8 ± 0.20 b |
| DNP | 846.6 ± 14.08 a | 3166.7 ± 120.19 a | 73.3 ± 3.33 b | 3.0 ± 0.10 a | 6.8 ± 0.40 a | |
| S1 | 359.0 ± 52.57 c | 1200.0 ± 57.73 c | 26.7 ± 3.33 d | 1.5 ± 0.15 b | 2.9 ± 0.13 b | |
| DNP + S1 | 920.8 ± 10.09 a | 3400.0 ± 152.75 a | 76.7 ± 3.33 b | 2.9 ± 0.05 a | 6.8 ± 0.15 a | |
| S2 | 399.1 ± 5.31 c | 1400.0 ± 57.74 c | 33.3 ± 3.33 d | 1.5 ± 0.08 b | 2.9 ± 0.28 b | |
| DNP + S2 | 929.1 ± 6.36 a | 3333.3 ± 145.30 a | 86.7 ± 3.33 a | 2.9 ± 0.06 a | 6.7 ± 0.18 a | |
| S3 | 634.5 ± 62.09 b | 2433.3 ± 88.19 b | 46.7 ± 3.33 c | 1.5 ± 0.24 b | 2.7 ± 0.07 b | |
| DNP + S3 | 927.6 ± 34.02 a | 3100.0 ± 115.47 a | 66.7 ± 3.33 b | 2.8 ± 0.05 a | 6.9 ± 0.27 a | |
| 10 | CK | 319.7 ± 26.47 c | 1066.7 ± 66.67 e | 26.7 ± 3.33 d | 1.6 ± 0.12 b | 2.8 ± 0.52 b |
| DNP | 827.7 ± 32.94 a | 2766.7 ± 88.19 b | 66.7 ± 3.33 b | 3.0 ± 0.12 a | 6.8 ± 0.15 a | |
| S1 | 352.5 ± 27.58 c | 1133.3 ± 88.19 de | 33.3 ± 3.33 cd | 1.4 ± 0.08 b | 3.1 ± 0.22 b | |
| DNP + S1 | 864.1 ± 5.31 a | 3133.3 ± 88.19 a | 80.0 ± 5.77 a | 2.9 ± 0.09 a | 6.9 ± 0.13 a | |
| S2 | 375.7 ± 21.60 c | 1333.3 ± 33.33 d | 36.7 ± 3.33 cd | 1.5 ± 0.09 b | 2.8 ± 0.24 b | |
| DNP + S2 | 876.0 ± 10.01 a | 3200 ± 57.74 a | 90.0 ± 5.77 a | 3.0 ± 0.05 a | 6.9 ± 0.24 a | |
| S3 | 585.0 ± 43.21 b | 2033.3 ± 88.19 c | 43.3 ± 3.33 c | 1.6 ± 0.11 b | 3.1 ± 0.36 b | |
| DNP + S3 | 804.2 ± 15.90 a | 2833.3 ± 88.19 b | 63.3 ± 3.33 b | 2.7 ± 0.06 a | 6.8 ± 0.39 a |
| Treatment | Total Biomass (g) | Leaf Area (cm2) | Plant Height (cm) |
|---|---|---|---|
| CK | 52.1 ± 0.79 a | 43.2 ± 2.63 a | 71.5 ± 1.67 a |
| DNP | 21.7 ± 0.46 f | 18.6 ± 0.50 e | 55.4 ± 1.75 d |
| S1 | 44.4 ± 0.55 b | 33.9 ± 0.92 b | 66.0 ± 2.00 b |
| DNP + S1 | 40.5 ± 1.21 c | 30.5 ± 0.65 bc | 65.6 ± 1.73 b |
| S2 | 38.5 ± 0.91 c | 33.3 ± 0.24 b | 62.6 ± 1.38 bc |
| DNP + S2 | 28.4 ± 0.61 d | 27.9 ± 0.96 cd | 61.7 ± 1.25 bc |
| S3 | 25.8 ± 0.47 e | 26.2 ± 0.68 d | 61.1 ± 2.00 bc |
| DNP + S3 | 25.2 ± 0.60 e | 25.8 ± 1.11 d | 58.0 ± 1.66 cd |
| Treatment | Tolerability Index | Toxicity Coefficient |
|---|---|---|
| CK | - | - |
| DNP | 0.43 ± 0.02 d | 0.57 ± 0.02 a |
| DNP + S1 | 0.78 ± 0.02 a | 0.22 ± 0.02 d |
| DNP + S2 | 0.55 ± 0.01 b | 0.45 ± 0.01 c |
| DNP + S3 | 0.48 ± 0.01 c | 0.52 ± 0.01 b |
| Time (d) | One-Dimensional Linear Equation | 2,4-DNP Concentration When the TI of Total Biomass Is Halved (mg·L−1) |
|---|---|---|
| DNP | Y = −0.0382X + 1 | 13.09 |
| DNP + S1 | Y = −0.0148X + 1 | 33.78 |
| DNP + S2 | Y = −0.0302X + 1 | 16.56 |
| DNP + S3 | Y = −0.0343X + 1 | 14.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, L.; Fu, Y.; Fu, G.; Fu, D.; Li, H.; Su, S.; Xie, H.; Tian, H.; Wang, R.; et al. Exogenous Salicylic Acid Alleviates Physiological Stress in Salix matsudana Seedlings and Increases 2,4-Dinitrophenol Removal. Forests 2024, 15, 1392. https://doi.org/10.3390/f15081392
Wu C, Zhang L, Fu Y, Fu G, Fu D, Li H, Su S, Xie H, Tian H, Wang R, et al. Exogenous Salicylic Acid Alleviates Physiological Stress in Salix matsudana Seedlings and Increases 2,4-Dinitrophenol Removal. Forests. 2024; 15(8):1392. https://doi.org/10.3390/f15081392
Chicago/Turabian StyleWu, Chen, Liudong Zhang, Yikang Fu, Guilong Fu, Degang Fu, Hui Li, Shuai Su, Huicheng Xie, Hui Tian, Ruijiang Wang, and et al. 2024. "Exogenous Salicylic Acid Alleviates Physiological Stress in Salix matsudana Seedlings and Increases 2,4-Dinitrophenol Removal" Forests 15, no. 8: 1392. https://doi.org/10.3390/f15081392
APA StyleWu, C., Zhang, L., Fu, Y., Fu, G., Fu, D., Li, H., Su, S., Xie, H., Tian, H., Wang, R., & Li, K. (2024). Exogenous Salicylic Acid Alleviates Physiological Stress in Salix matsudana Seedlings and Increases 2,4-Dinitrophenol Removal. Forests, 15(8), 1392. https://doi.org/10.3390/f15081392







