Association of Carbon Pool with Vegetation Composition along the Elevation Gradients in Subtropical Forests in Pakistan
Abstract
:1. Introduction
2. Materials and Methods
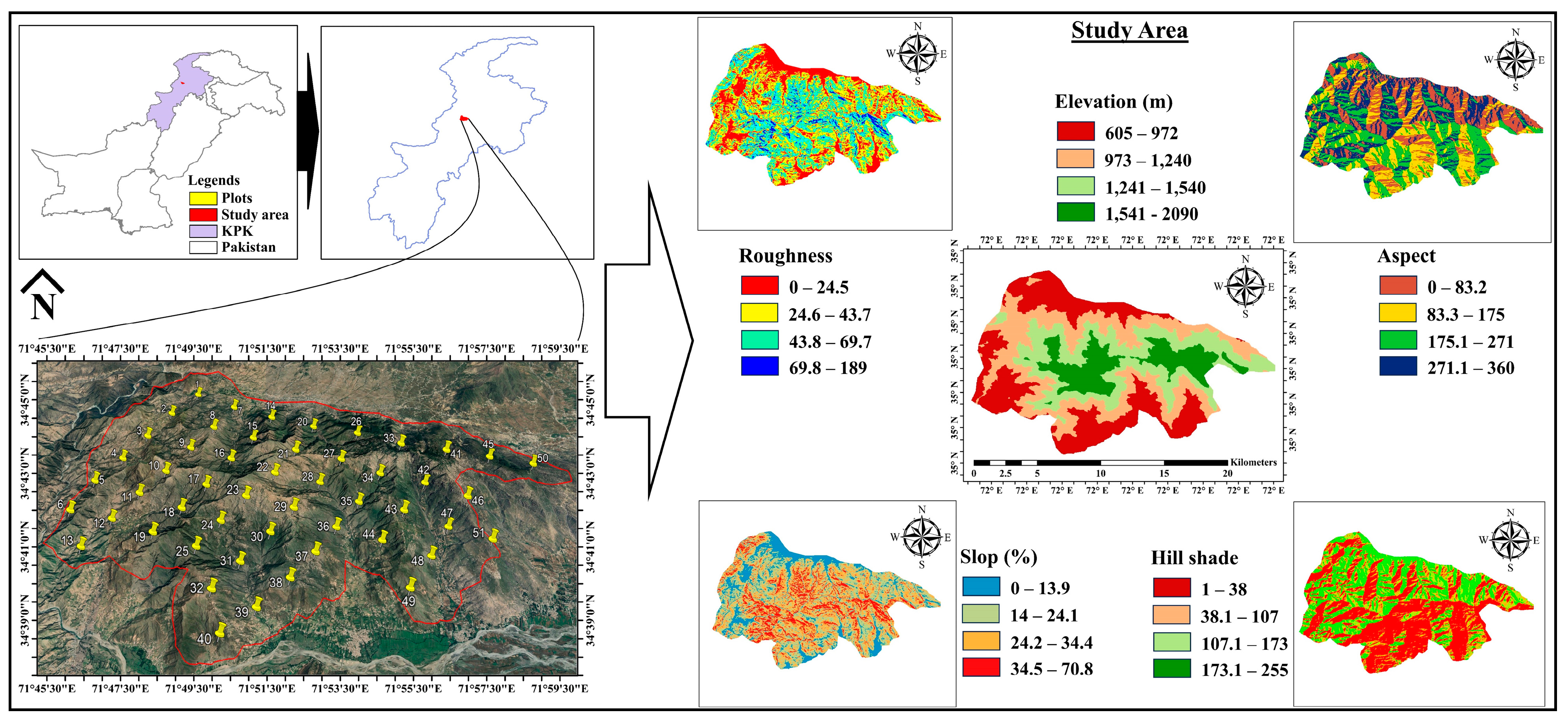
2.1. Study Area
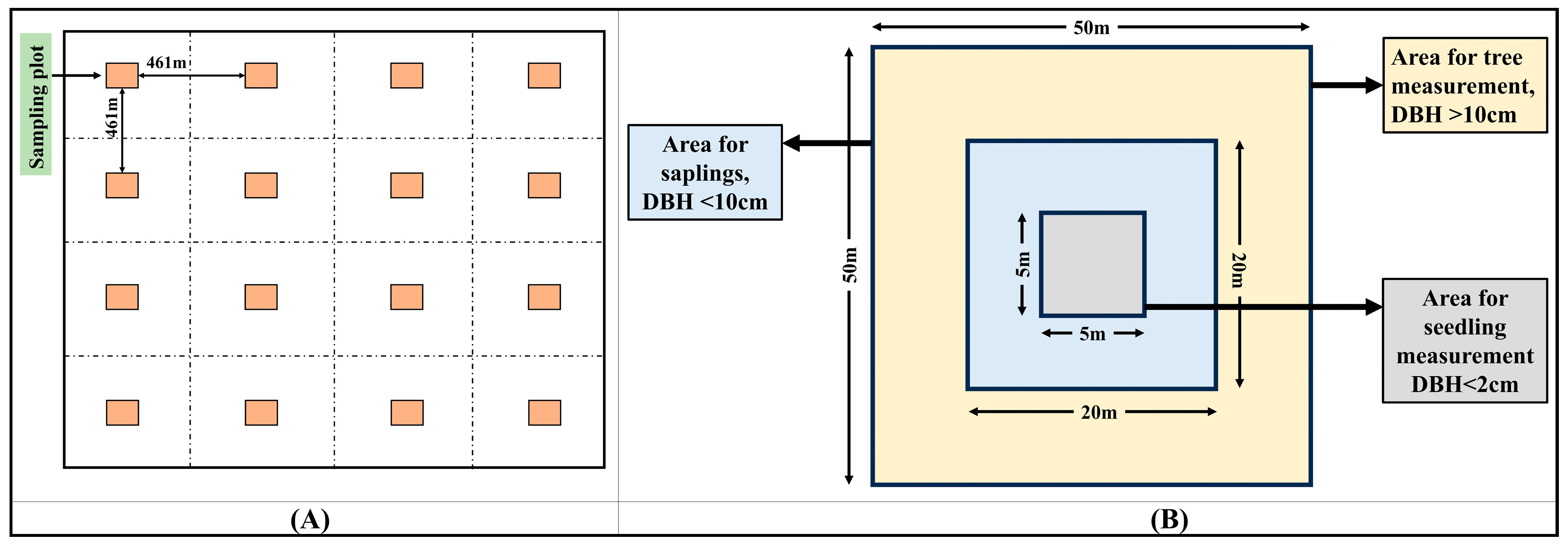
2.2. Sampling and Inventory
2.2.1. Litter Sampling
2.2.2. Deadwood Sampling
2.2.3. Soil Sampling
2.3. Biomass Calculation
2.3.1. Tree and Sapling Biomass
2.3.2. Litter Biomass
2.3.3. Deadwood Biomass
2.3.4. Soil Biomass
2.4. Species Dominance
2.5. Vegetation Diversity
2.6. Carbon Pool Calculation
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Stands at Different Elevations
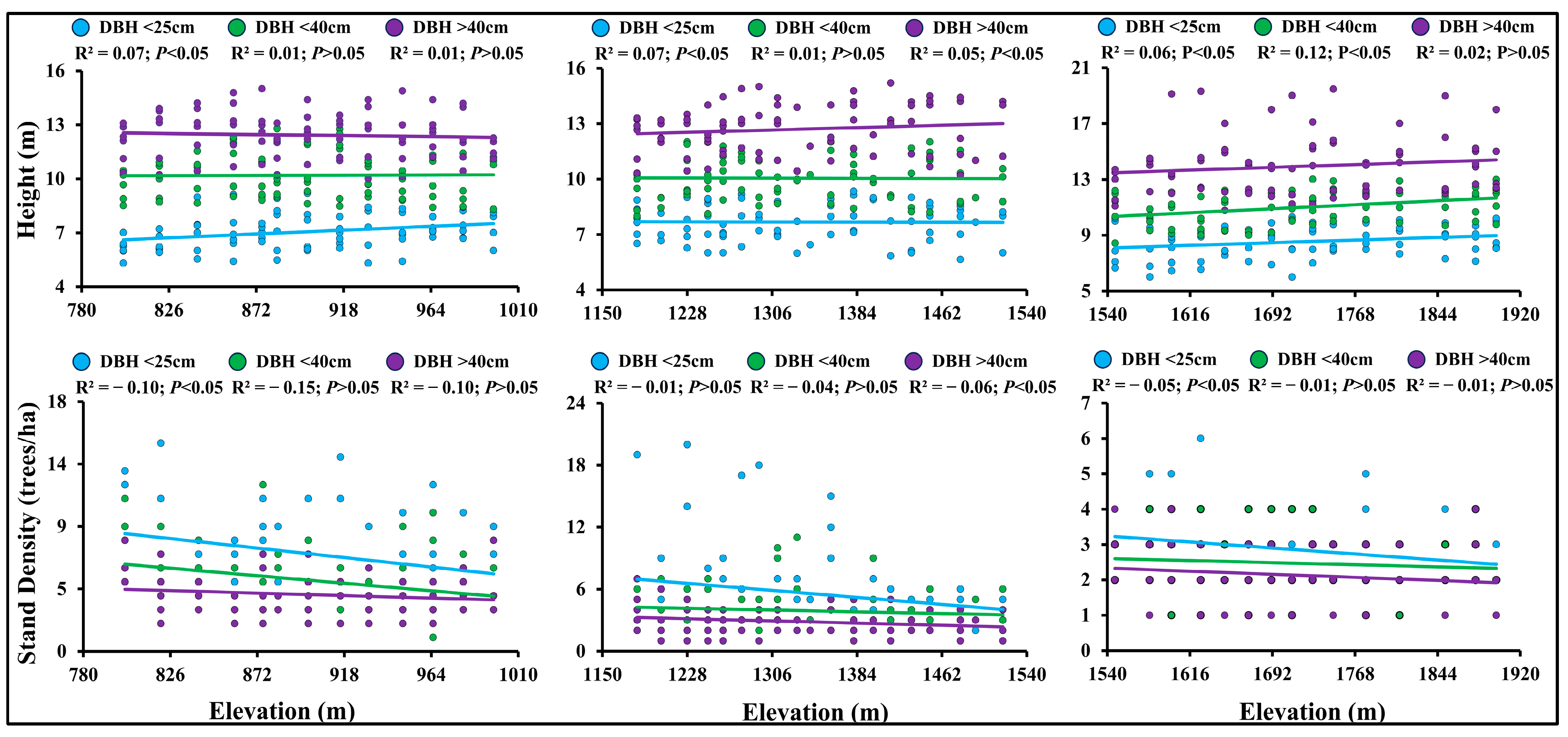
3.2. Association of Tree Height and Density of Different DBH Classes with Elevation
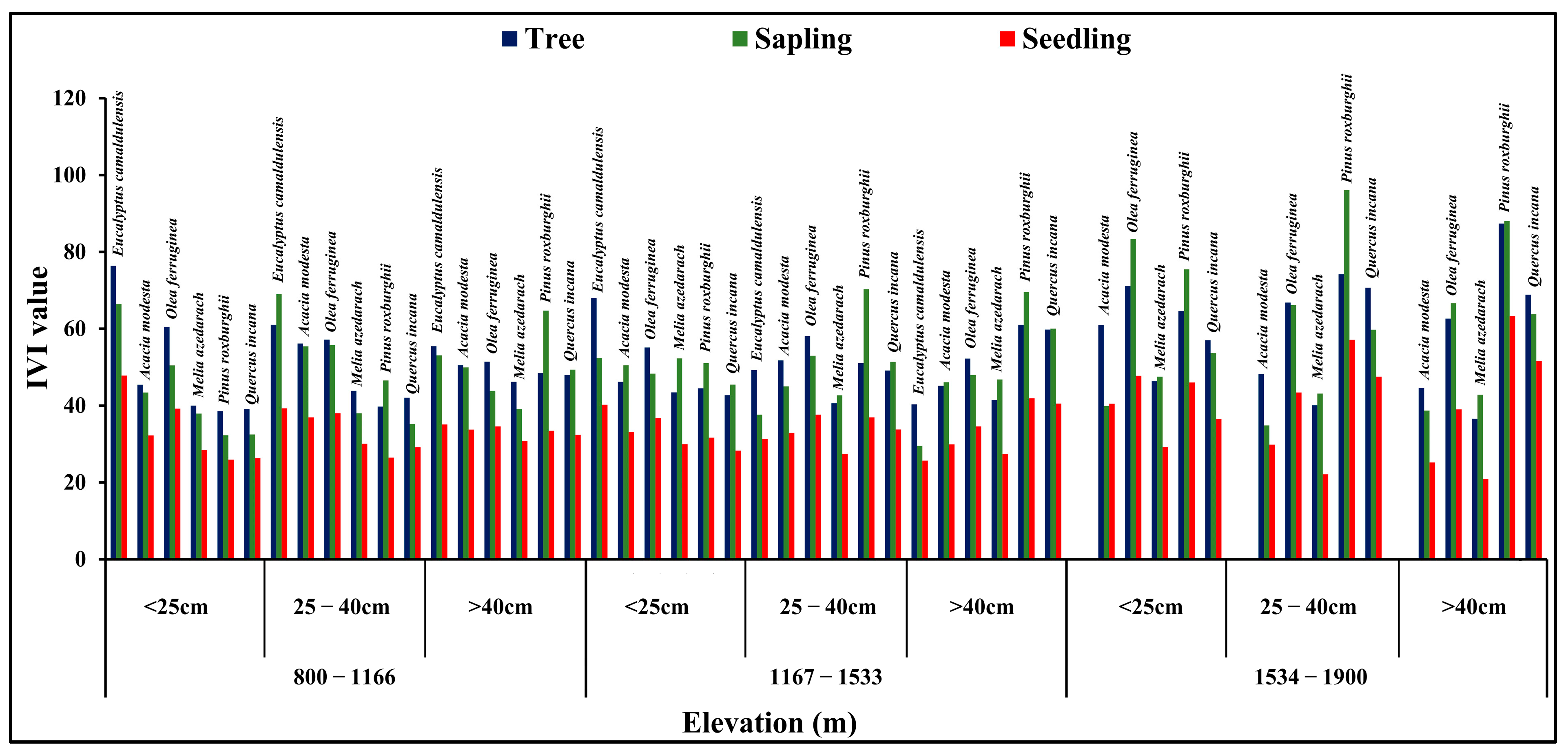
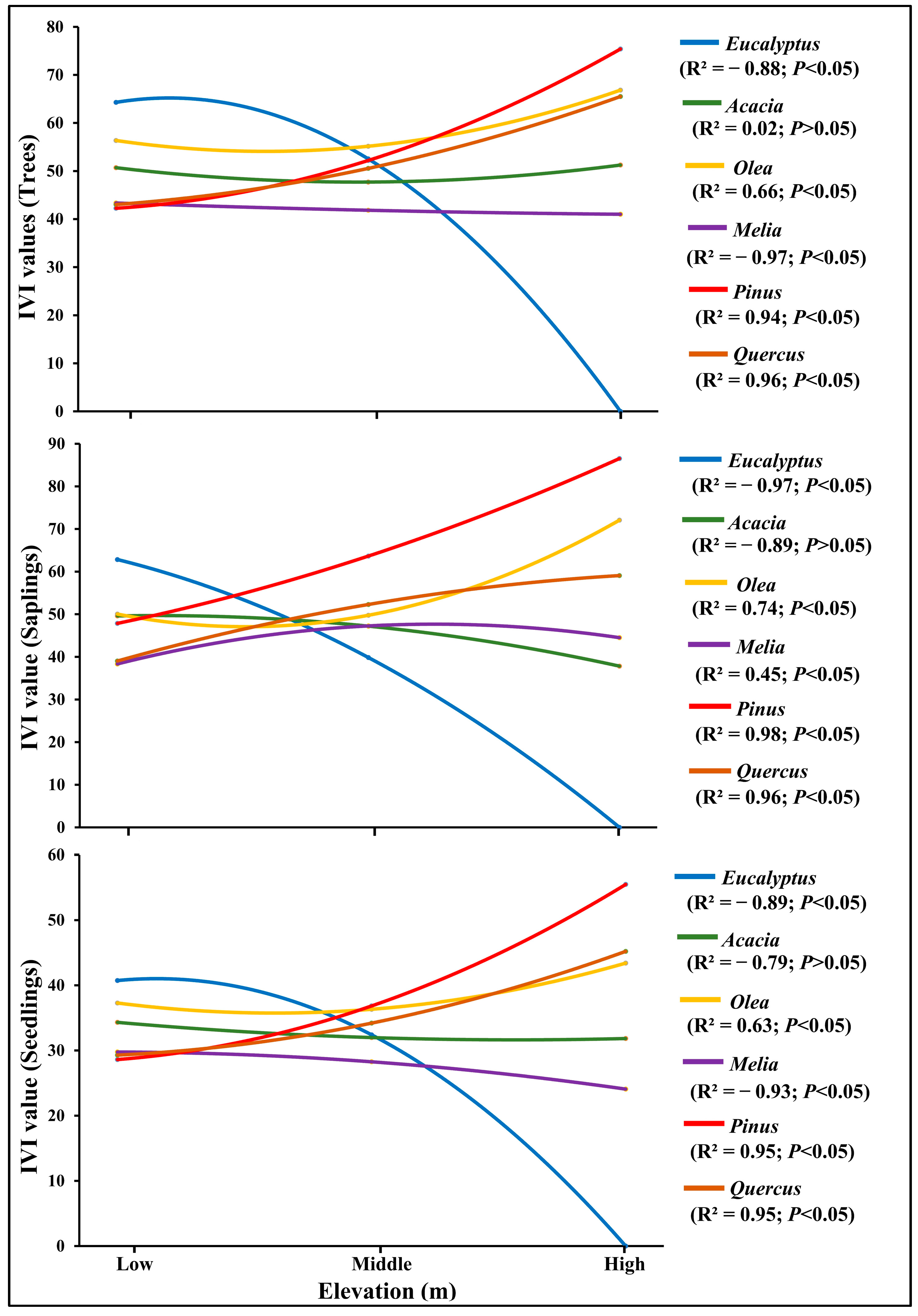
3.3. Association between IVI Value with Elevation Gradient and DBH Classes
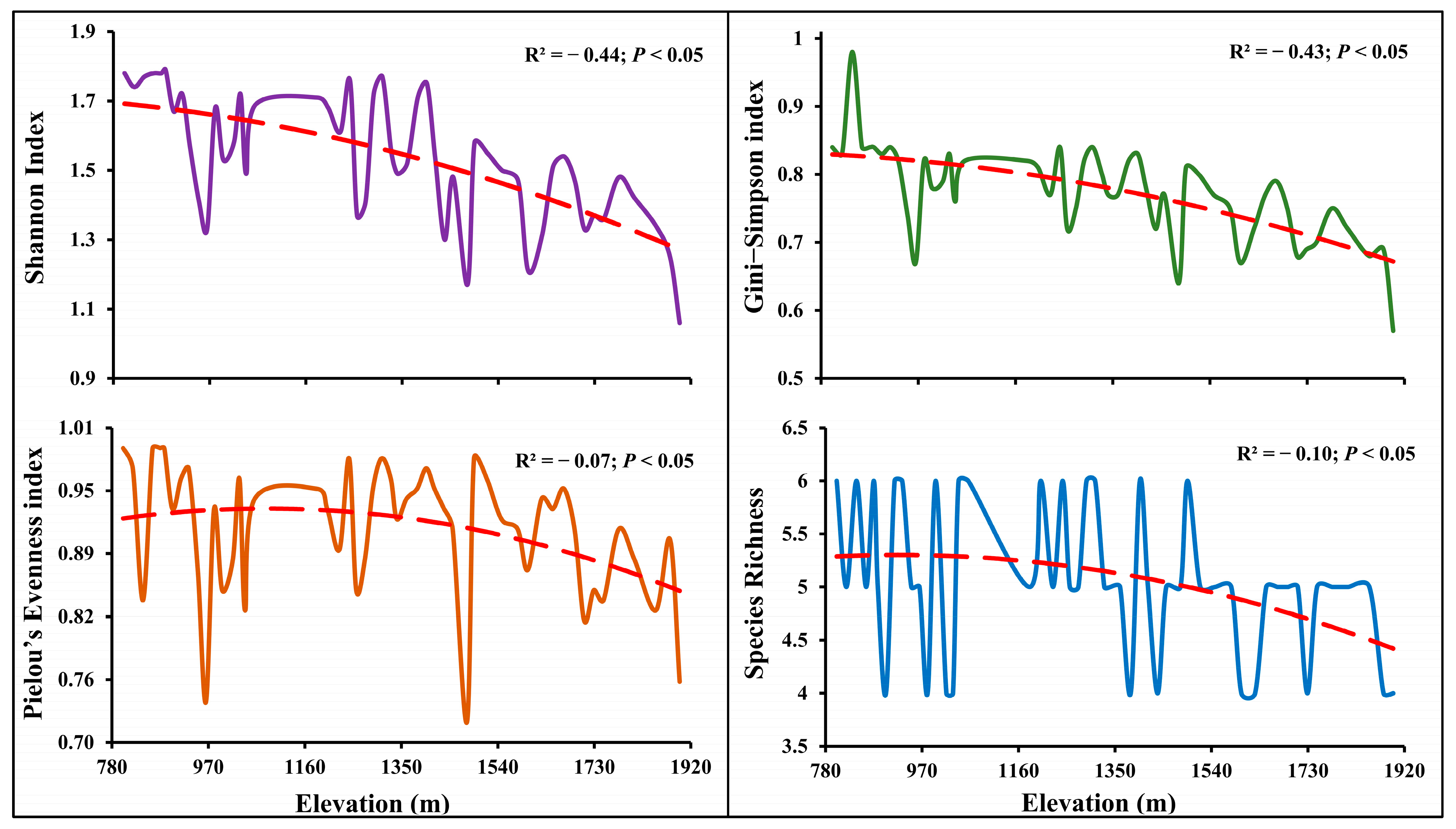
3.4. Association of Species Diversity with Elevation Gradients
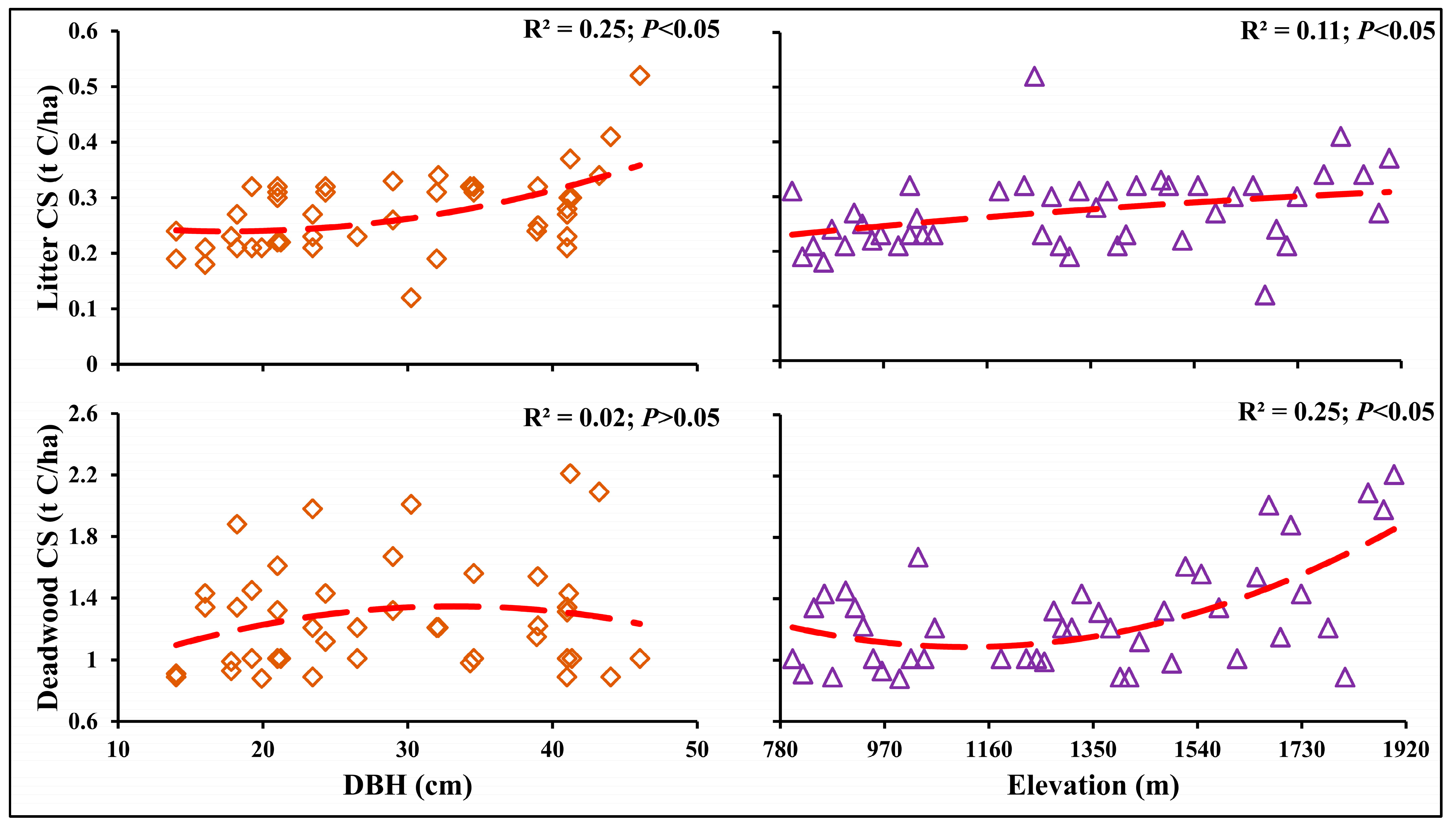
3.5. Association between Litter and Deadwood Carbon with Elevation Gradients
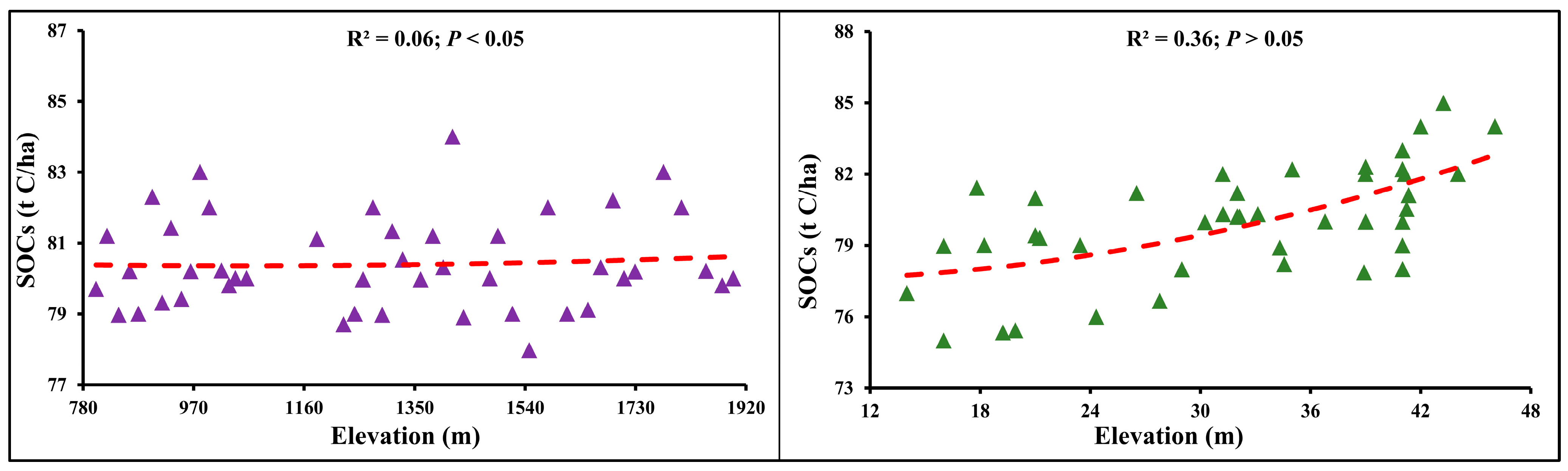
3.6. SOC Association with Elevation Gradients and DBH Classes
3.7. Carbon Pool Association with Elevation Gradients and DBH Classes
4. Discussion
4.1. Elevation and DBH Classes Impact Tree Growth Parameters, Litter, and Deadwood Accumulation
4.2. Species Dominance and Diversity along Elevation Gradients
4.3. Elevation and DBH as a Driver of SOC and Carbon Pool
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aryal, S.; Shrestha, S.; Maraseni, T.; Wagle, P.; Gaire, N. Carbon stock and its relationships with tree diversity and density in community forests in Nepal. Int. For. Rev. 2018, 20, 263–273. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar]
- Aryal, S.; Bhattarai, D.R.; Devkota, R.P. Comparison of Carbon Stocks Between Mixed and Pine-Dominated Forest Stands Within the Gwalinidaha Community Forest in Lalitpur District, Nepal. Small-Scale For. 2013, 12, 659–666. [Google Scholar] [CrossRef]
- Pandey, S.S.; Maraseni, T.N.; Cockfield, G. Carbon stock dynamics in different vegetation dominated community forests under REDD+: A case from Nepal. For. Ecol. Manag. 2014, 327, 40–47. [Google Scholar] [CrossRef]
- Neupane, P.; Gauli, A.; Maraseni, T.; Kübler, D.; Mundhenk, P.; Dang, M.; Köhl, M. A segregated assessment of total carbon stocks by the mode of origin and ecological functions of forests: Implication on restoration potential. Int. For. Rev. 2017, 19, 120–147. [Google Scholar] [CrossRef]
- Thuille, A.; Schulze, E. Carbon dynamics in successional and afforested spruce stands in Thuringia and the Alps. Glob. Chang. Biol. 2006, 12, 325–342. [Google Scholar] [CrossRef]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For. Ecol. Manag. 2010, 260, 2170–2179. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finér, L.; Jaroszewicz, B.; Vesterdal, L. Is Tree Species Diversity or Species Identity the More Important Driver of Soil Carbon Stocks, C/N Ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Rajput, B.S.; Bhardwaj, D.R.; Pala, N.A. Factors influencing biomass and carbon storage potential of different land use systems along an elevational gradient in temperate northwestern Himalaya. Agrofor. Syst. 2017, 91, 479–486. [Google Scholar] [CrossRef]
- Andivia, E.; Rolo, V.; Jonard, M.; Formánek, P.; Ponette, Q. Tree species identity mediates mechanisms of top soil carbon sequestration in a Norway spruce and European beech mixed forest. Ann. For. Sci. 2016, 73, 437–447. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Z.; Ryan, M.G.; You, Y.; Sun, O.J. Changes in soil organic carbon contents and fractionations of forests along a climatic gradient in China. For. Ecosyst. 2019, 6, 1. [Google Scholar] [CrossRef]
- Reyna-Bowen, L.; Lasota, J.; Vera-Montenegro, L.; Vera-Montenegro, B.; Błońska, E. Distribution and Factors Influencing Organic Carbon Stock in Mountain Soils in Babia Góra National Park, Poland. Appl. Sci. 2019, 9, 3070. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snall, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Hounkpatin, O.K.; de Hipt, F.O.; Bossa, A.Y.; Welp, G.; Amelung, W. Soil organic carbon stocks and their determining factors in the Dano catchment (Southwest Burkina Faso). CATENA 2018, 166, 298–309. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Andrade, A.B.; Araujo, M.A.; Lal, R. Soil organic carbon retention more affected by altitude than texture in a forested mountain range in Brazil. Soil Res. 2018, 56, 284–295. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Qin, Y.; Cao, J.; Li, H.; Zhao, Y. Soil organic carbon as functions of slope aspects and soil depths in a semiarid alpine region of Northwest China. CATENA 2017, 152, 94–102. [Google Scholar] [CrossRef]
- Hofhansl, F.; Chacón-Madrigal, E.; Fuchslueger, L.; Jenking, D.; Morera-Beita, A.; Plutzar, C.; Silla, F.; Andersen, K.M.; Buchs, D.M.; Dullinger, S.; et al. Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 2020, 10, 5066. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kobayashi, T.; Nashimoto, M. Altitudinal differences in bark stripping by sika deer in the subalpine coniferous forest of Mt. Fuji. For. Ecol. Manag. 2011, 261, 2089–2095. [Google Scholar] [CrossRef]
- Swetnam, T.L.; Brooks, P.D.; Barnard, H.R.; Harpold, A.A.; Gallo, E.L. Topographically driven differences in energy and water constrain climatic control on forest carbon sequestration. Ecosphere 2017, 8, e01797. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Meng, J. Effects of Plot Design on Estimating Tree Species Richness and Species Diversity. Forests 2022, 13, 2003. [Google Scholar] [CrossRef]
- Dieler, J.; Uhl, E.; Biber, P.; Müller, J.; Rötzer, T.; Pretzsch, H. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. Eur. J. For. Res. 2017, 136, 739–766. [Google Scholar] [CrossRef]
- Silaeva, T.; Andreychev, A.; Kiyaykina, O.; Balčiauskas, L. Taxonomic and ecological composition of forest stands inhabited by forest dormouse Dryomys nitedula (Rodentia: Gliridae) in the Middle Volga. Biologia 2021, 76, 14751482. [Google Scholar] [CrossRef]
- Wang, J.; Hu, A.; Meng, F.; Zhao, W.; Yang, Y.; Soininen, J.; Shen, J.; Zhou, J. Embracing mountain microbiome and ecosystem functions under global change. New Phytol. 2022, 234, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Tashi, S.; Singh, B.; Keitel, C.; Adams, M. Soil carbon and nitrogen stocks in forests along an altitudinal gradient in the eastern Himalayas and a meta-analysis of global data. Glob. Chang. Biol. 2016, 22, 2255–2268. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, B.S.S.; Haider, A.; Laeeq, M.T. Land Cover Atlas of Pakistan; Pakistan Forest Institute: Peshawar, Pakistan, 2012; p. 140. [Google Scholar]
- MOCC. Readiness Preparation Proposal (RPP) for Pakistan; Ministry of Climate Change, Government of Pakistan Islamabad: Islamabad, Pakistan, 2013. [Google Scholar]
- Hayat, U.; Abbas, A.; Shi, J. Public Attitudes towards Forest Pest Damage Cost and Future Control Extent: A Case Study from Two Cities of Pakistan. Forests 2024, 15, 544. [Google Scholar] [CrossRef]
- Hayat, U.; Akram, M.; Kour, S.; Arif, T.; Shi, J. Pest Risk Assessment of Aeolesthes sarta (Coleoptera: Cerambycidae) in Pakistan under Climate Change Scenario. Forests 2023, 14, 253. [Google Scholar] [CrossRef]
- Khan, I.; Hayat, U.; Mujahid, A.; Majid, A.; Chaudhary, A.; Badshah, M.; Huang, J. Evaluation of growing stock, biomass and soil carbons and their association with a diameter: A case study from a planted chir pine (pinus roxburghii) forest. Appl. Ecol. Environ. Res. 2021, 19, 1457–1472. [Google Scholar] [CrossRef]
- Ali, A.; Ashraf, M.I.; Gulzar, S.; Akmal, M. Estimation of forest carbon stocks in temperate and subtropical mountain systems of Pakistan: Implications for REDD+ and climate change mitigation. Environ. Monit. Assess. 2020, 192, 198. [Google Scholar] [CrossRef]
- Nizami, S.M. The inventory of the carbon stocks in sub tropical forests of Pakistan for reporting under Kyoto Protocol. J. For. Res. 2012, 23, 377–384. [Google Scholar] [CrossRef]
- Ahmad, A.; Nizami, S.M. Carbon stocks of different land uses in the Kumrat valley, Hindu Kush Region of Pakistan. J. For. Res. 2015, 26, 57–64. [Google Scholar] [CrossRef]
- Amir, M.; Liu, X.; Ahmad, A.; Saeed, S.; Mannan, A.; Muneer, M.A. Patterns of Biomass and Carbon Allocation across Chronosequence of Chir Pine (Pinus roxburghii) Forest in Pakistan: Inventory-Based Estimate. Adv. Meteorol. 2018, 2018, 3095891. [Google Scholar] [CrossRef]
- Hayat, U.; Khan, I.; Roman, M. A response of tropical tree Terminalia arjuna (Ar-jun)[Combretaceae-Myrtales] seeds and seedlings towards the presowing seed treatment in forest nursery of Sargodha, Paki-stan. Int. J. Biosci. 2020, 16, 495–505. [Google Scholar] [CrossRef]
- PMD. Pakistan Metrological Department. 2023. Available online: https://www.pmd.gov.pk/en/ (accessed on 29 September 2023).
- Bhishma, P.S.; Shiva, S.P.; Ajay, P.; Eak, B.R.; Sanjeeb, B.; Tibendra, R.B.; Shambhu, C.; Rijan, T. Forest Carbon Stock Measurement: Guidelines for Measuring Carbon Stocks in Community-Managed Forests; Funded by Norwegian Agency for Development Cooperation (NORAD); Asia Network for Sustainable Agriculture and Bioresources (ANSAB) Publishing: Kathmandu, Nepal, 2020; pp. 17–43. [Google Scholar]
- Yohannes, H.; Soromessa, T.; Argaw, M. Carbon stock analysis along slope and slope aspect gradient in Gedo Forest: Im-plications for climate change mitigation. J. Earth Sci. Clim. Change 2015, 6, 6–11. [Google Scholar] [CrossRef]
- Pearson, T.R.; Walker, S.; Brown, S. Sourcebook for Land-Use, Land-Use Change and Forestry Projects; Winrock International and the Bio-carbon fund of the World Bank: Arlington, TX, USA, 2005; pp. 19–35. Available online: https://winrock.org/wp-content/uploads/2016/03/Winrock-BioCarbon_Fund_Sourcebook-compressed.pdf (accessed on 10 January 2024).
- IPCC [Intergovernmental Panel on Climate Change]. Good Practice Guidance for Land Use, Land-Use Change and Forestry; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2003; p. 590. [Google Scholar]
- Ali, A.; Ashraf, M.I.; Gulzar, S.; Akmal, M. Development of an Allometric Model for Biomass Estimation of Pinus roxburghii, Growing in Subtropical Pine Forests of Khyber Pakhtunkhwa, Pakistan. Sarhad J. Agric. 2020, 36, 236–244. [Google Scholar] [CrossRef]
- Ali, A. Biomass and Carbon Tables for Major Tree Species of Khyber Pakhtunkhwa; Pakistan Forest Institute: Peshawar, Pakistan, 2020. [Google Scholar]
- Miah, D.; Islam, K.N.; Kabir, H.; Koike, M. Allometric models for estimating aboveground biomass of selected homestead tree species in the plain land Narsingdi district of Bangladesh. Trees For. People 2020, 2, 100035. [Google Scholar] [CrossRef]
- Khan, I.A.; Khan, W.R.; Ali, A.; Nazre, M. Assessment of Above-Ground Biomass in Pakistan Forest Ecosystem’s Carbon Pool: A Review. Forests 2021, 12, 586. [Google Scholar] [CrossRef]
- Hairiah, K.; Sitompul, S.M.; Van Noordwijk, M.; Palm, C. Methods for Sampling Carbon Stocks above and Below Ground; ICRAF: Bogor, Indonesia, 2001; pp. 1–23. Available online: https://apps.worldagroforestry.org/downloads/Publications/PDFS/LN01114.PDF (accessed on 10 January 2024).
- Morisada, K.; Ono, K.; Kanomata, H. Organic carbon stock in forest soils in Japan. Geoderma 2003, 119, 21–32. [Google Scholar] [CrossRef]
- Hartley, J.; Marchant, J. Methods of Determining the Moisture Content of Wood; Technical Paper No. 41; Research Division, State Forests of New South Wales: Sydney, Austria, 1995; p. 61. [Google Scholar]
- Agus, F.; Hairiah, K.; Mulyani, A. Measuring Carbon Stock in Peat Soils: Practical Guidelines; World Agroforestry Centre (ICRAF) Southeast Asia Regional Program, Indonesian Centre for Agricultural Land Resources Research and Development: Bogor, Indonesia, 2011; 60p, Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=e639e6e20348e8db564cf12b832b7f5286b84db3 (accessed on 10 January 2024).
- Rahman, M.H.; Holmes, A.W.; Deurer, M.; Saunders, S.J.; Mowat, A.; Clothier, B.E. Comparison of three methods to estimate organic carbon in allophanic soils in New Zealand. In Proceedings of the 24th Annual FLRC Workshop, Auckland, New Zealand, 8–10 February 2011; Massey University: Auckland, New Zealand, 2011; p. 4. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Chapagain, U.; Chapagain, B.P.; Nepal, S.; Manthey, M. Impact of Disturbances on Species Diversity and Regeneration of Nepalese Sal (Shorea robusta) Forests Managed under Different Management Regimes. Earth 2021, 2, 826–844. [Google Scholar] [CrossRef]
- Gracia, M.; Montané, F.; Piqué, J.; Retana, J. Overstory structure and topographic gradients determining diversity and abundance of understory shrub species in temperate forests in central Pyrenees (NE Spain). For. Ecol. Manag. 2007, 242, 391–397. [Google Scholar] [CrossRef]
- Yeom, D.-J.; Kim, J.H. Comparative evaluation of species diversity indices in the natural deciduous forest of Mt. Jeombong. For. Sci. Technol. 2011, 7, 68–74. [Google Scholar] [CrossRef]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communities; University of Illinois Press: Urbana, IL, USA, 1963; p. 117. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.H.; Fastie, C.L. Spatial and Temporal Variability in the Growth and Climate Response of Treeline Trees in Alaska. Clim. Change 2002, 52, 481–509. [Google Scholar] [CrossRef]
- Liang, M.; Liu, X.; Etienne, R.S.; Wang, Y.; Staehelin, C. Does facilitation and competition of two shrubs with different eleva-tion distribution patterns change with environmental stress? Plant Soil 2016, 408, 393–404. [Google Scholar]
- Cao, J.; Wang, X.; Tian, Y.; Wen, Z.; Zha, T. Pattern of carbon allocation across three different stages of stand development of a Chinese pine (Pinus tabulaeformis) forest. Ecol. Res. 2012, 27, 883–892. [Google Scholar] [CrossRef]
- Li, C.; Zha, T.; Liu, J.; Jia, X. Carbon and nitrogen distribution across a chronosequence of secondary lacebark pine in China. For. Chron. 2013, 89, 192–198. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Skov, F. Mesoscale distribution of understorey plants in temperate forest (Kalø, Denmark): The importance of environment and dispersal. Plant Ecol. 2002, 160, 169–185. [Google Scholar] [CrossRef]
- Kang, B.; Liu, S.; Zhang, G.; Chang, J.; Wen, Y.; Ma, J.; Hao, W. Carbon accumulation and distribution in Pinus massoniana and Cunninghamia lanceolata mixed forest ecosystem in Daqingshan, Guangxi, China. Acta Ecol. Sin. 2006, 26, 1320–1327. [Google Scholar] [CrossRef]
- Ming, A.; Jia, H.; Zhao, J.; Tao, Y.; Li, Y. Above- and Below-Ground Carbon Stocks in an Indigenous Tree (Mytilaria laosensis) Plantation Chronosequence in Subtropical China. PLoS ONE 2014, 9, e109730. [Google Scholar] [CrossRef] [PubMed]
- MOCC. In Ministry of Climate Change and Environmental Coordination, Government of Pakistan; 2023. Available online: https://www.mocc.gov.pk/ProjectDetail/M2QzOWJmMjUtZTU3MC00NmFkLWE4YmMtZDFhMmRlOGU2NGRh (accessed on 10 January 2024).
- Lindenmayer, D.; Hobbs, R. Fauna conservation in Australian plantation forests—A review. Biol. Conserv. 2004, 119, 151–168. [Google Scholar] [CrossRef]
- Vetaas, O.R.; Grytnes, J. Distribution of vascular plant species richness and endemic richness along the Himalayan elevation gradient in Nepal. Glob. Ecol. Biogeogr. 2002, 11, 291–301. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. Available online: https://www.jstor.org/stable/3682769 (accessed on 10 January 2024). [CrossRef]
- Rickart, E.A. Elevational diversity gradients, biogeography and the structure of montane mammal communities in the intermountain region of North America. Glob. Ecol. Biogeogr. 2001, 10, 77–100. [Google Scholar] [CrossRef]
- Sánchez-Cordero, V. Elevation gradients of diversity for rodents and bats in Oaxaca, Mexico. Glob. Ecol. Biogeogr. 2001, 10, 63–76. [Google Scholar] [CrossRef]
- Rana, P.; Koirala, M.; Bhuju, D.R.; Boonchird, C. Population structure of Rhododendron campanulatum D. Don and asso-ciated tree species along the elevational gradient of Manaslu conservation area, Nepal. J. Inst. Sci. Technol. 2016, 21, 95–102. [Google Scholar] [CrossRef]
- Tesfaye, G.; Teketay, D.; Fetene, M.; Beck, E. Regeneration of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Flora 2009, 205, 135–143. [Google Scholar] [CrossRef]
- Wardle, P. An explanation for alpine timberline. N. Z. J. Bot. 1971, 9, 371–402. [Google Scholar] [CrossRef]
- Qing, L. The effects of gap size and within gap position on the survival and growth of naturally regenerated abies georgei seedlings. Chin. J. Plant Ecol. 2004, 28, 204–209. [Google Scholar] [CrossRef]
- Colwell, R.K.; Hurtt, G.C. Nonbiological Gradients in Species Richness and a Spurious Rapoport Effect. Am. Nat. 1994, 144, 570–595. [Google Scholar] [CrossRef]
- Körner, C. Why are there global gradients in species richness? mountains might hold the answer. Trends Ecol. Evol. 2000, 15, 513–514. [Google Scholar] [CrossRef]
- Brown, J.H. Mammals on mountainsides: Elevational patterns of diversity. Glob. Ecol. Biogeogr. 2001, 10, 101–109. [Google Scholar] [CrossRef]
- D’antonio, C.M.; Tunison, J.T.; Loh, R.K. Variation in the impact of exotic grasses on native plant composition in relation to fire across an elevation gradient in Hawaii. Austral Ecol. 2000, 25, 507–522. [Google Scholar] [CrossRef]
- Jimbo, T.; Saulei, S.; Moses, J.; Lawong, B.; Kaina, G.; Kiapranis, R.; Hitofumi, A.; Novotny, V.; Attorre, F.; Testolin, R.; et al. Beyond the Trees: A Comparison of Nonwoody Species, and Their Ecology, in Papua New Guinea Elevational Gradient Forest. Case Stud. Environ. 2023, 7, 1831407. [Google Scholar] [CrossRef]
- Haq, S.M.; Calixto, E.S.; Rashid, I.; Srivastava, G.; Khuroo, A.A. Tree diversity, distribution and regeneration in major forest types along an extensive elevational gradient in Indian Himalaya: Implications for sustainable forest management. For. Ecol. Manag. 2022, 506, 119968. [Google Scholar] [CrossRef]
- Colwell, R.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
- Hayat, U.; Shi, J.; Wu, Z.; Rizwan, M.; Haider, M.S. Which SDM Model, CLIMEX vs. MaxEnt, Best Forecasts Aeolesthes sarta Distribution at a Global Scale under Climate Change Scenarios? Insects 2024, 15, 324. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Mora, J.; Guerra, J.; Armas-Herrera, C.; Arbelo, C.; Rodríguez-Rodríguez, A. Storage and depth distribution of organic carbon in volcanic soils as affected by environmental and pedological factors. CATENA 2014, 123, 163–175. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Song, M. Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. For. Ecol. Manag. 2012, 277, 90–97. [Google Scholar] [CrossRef]
- Choudhury, B.U.; Fiyaz, A.R.; Mohapatra, K.P.; Ngachan, S. Impact of Land Uses, Agrophysical Variables and Altitudinal Gradient on Soil Organic Carbon Concentration of North-Eastern Himalayan Region of India. Land Degrad. Dev. 2014, 27, 1163–1174. [Google Scholar] [CrossRef]
- Gairola, S.; Ghildiyal, S.K.; Sharma, C.M.; Suyal, S. Species richness and diversity along an altitudinal gradient in moist temperate forest of Garhwal Himalaya. Am. J. Sci. 2009, 5, 119–128. [Google Scholar]
- Moser, G.; Hertel, D.; Leuschner, C. Altitudinal Change in LAI and Stand Leaf Biomass in Tropical Montane Forests: A Transect Study in Ecuador and a Pan-Tropical Meta-Analysis. Ecosystems 2007, 10, 924–935. [Google Scholar] [CrossRef]
- Simegn, T.Y.; Soromessa, T. Carbon Stock Variations Along Altitudinal and Slope Gradient in the Forest Belt of Simen Mountains National Park, Ethiopia. Am. J. Environ. Prot. 2015, 4, 199. [Google Scholar] [CrossRef]
- Tsui, C.-C.; Chen, Z.-S.; Hsieh, C.-F. Relationships between soil properties and slope position in a lowland rain forest of southern Taiwan. Geoderma 2004, 123, 131–142. [Google Scholar] [CrossRef]
- Griffiths, R.; Madritch, M.D.; Swanson, A. The effects of topography on forest soil characteristics in the Oregon Cascade Mountains (USA): Implications for the effects of climate change on soil properties. For. Ecol. Manag. 2008, 257, 1–7. [Google Scholar] [CrossRef]

| Months | Temperature °C | Ppt mm | Humidity % | ||||
|---|---|---|---|---|---|---|---|
| Max. | Min. | Avg. | Max. | Min. | Avg. | ||
| January | 11.58 | 0.9 | 6.24 | 70.67 | 48.21 | 28.88 | 38.55 |
| February | 12.68 | 2.7 | 7.69 | 207.7 | 60.32 | 40.23 | 50.28 |
| March | 17.53 | 6.95 | 12.24 | 178.24 | 58.32 | 39.23 | 48.78 |
| April | 23.47 | 11.73 | 17.60 | 150.35 | 53.43 | 36.61 | 45.02 |
| May | 29.5 | 16.49 | 23.00 | 81.01 | 45.43 | 23.32 | 34.38 |
| June | 32.48 | 20.43 | 26.46 | 66.7 | 42.82 | 22.59 | 32.71 |
| July | 31.1 | 21.81 | 26.46 | 250.48 | 68.34 | 39.74 | 54.04 |
| August | 28.32 | 18.93 | 23.63 | 255.65 | 82.67 | 46.98 | 64.83 |
| September | 26.13 | 15.13 | 20.63 | 85.86 | 67.11 | 40.01 | 53.56 |
| October | 22.39 | 10.89 | 16.64 | 45.42 | 47.11 | 27.18 | 37.15 |
| November | 17.05 | 5.47 | 11.26 | 52.19 | 44.4 | 23.08 | 33.74 |
| December | 13.86 | 2.32 | 8.09 | 28.05 | 40.01 | 18.01 | 29.01 |
| Tree Species | Allometric Equation | Reference |
|---|---|---|
| Eucalyptus camaldulensis | [43] | |
| Acacia modesta | [32,44] | |
| Olea ferruginea | [32,44] | |
| Melia azedarach | [45] | |
| Pinus roxburghii | [43] | |
| Quercus incana | [32] |
| Fixed Variable | Growth Variables | Unstandardized Coefficients | Standardized Coefficients | |||||
|---|---|---|---|---|---|---|---|---|
| B | S.E | Beta | t | Sig. | Adj. R2 | F-Value | ||
| Elevation Gradient | Spp. C | −0.42 | 0.19 | −0.16 | −2.90 | 0.03 | −0.10 | 4.91 |
| SD | −1.43 | 0.11 | −0.41 | −13.64 | 0.00 | −0.41 | 186.01 | |
| DBH | 0.34 | 0.59 | 0.02 | 0.57 | 0.56 | 0.02 | 0.33 | |
| TH | 0.21 | 0.15 | 0.04 | 1.38 | 0.16 | 0.05 | 1.92 | |
| BA | −0.55 | 0.09 | −0.20 | −6.27 | 0.00 | −0.20 | 39.32 | |
| Vol | −1.39 | 0.41 | −0.11 | −3.39 | 0.00 | −0.11 | 11.48 | |
| TB | −56.15 | 4.16 | −0.41 | −13.49 | 0.00 | −0.40 | 181.99 | |
| Elevation Gradients | DBH Classes | No. of Trees | DBH (cm) | Height (m) | BA (m2/ha) | Vol (m3/ha) | AGB (t/ha) | BGB (t/ha) | TB (t/ha) | CS (t C/ha) |
|---|---|---|---|---|---|---|---|---|---|---|
| Low | I | 6.36 ± 0.54 | 19.16 ± 0.53 | 7.42 ± 0.19 | 1.76 ± 0.12 | 4.43 ± 0.34 | 209.88 ± 18.12 | 41.98 ± 3.62 | 251.86 ± 21.75 | 125.93 ± 10.87 |
| II | 4.46 ± 0.38 | 29.12 ± 0.83 | 10.23 ± 0.15 | 2.86 ± 0.22 | 9.78 ± 0.82 | 143.74 ± 12.52 | 28.75 ± 2.50 | 172.49 ± 15.02 | 86.24 ± 7.51 | |
| III | 3.53 ± 0.30 | 44.13 ± 0.64 | 12.54 ± 0.14 | 5.30 ± 0.40 | 22.15 ± 1.67 | 109.86 ± 9.32 | 21.97 ± 1.86 | 131.83 ± 11.18 | 65.92 ± 5.59 | |
| Middle | I | 4.19 ± 0.50 | 19.42 ± 0.81 | 7.66 ± 0.31 | 1.38 ± 0.16 | 3.82 ± 0.47 | 137.50 ± 16.64 | 27.50 ± 3.33 | 165.00 ± 19.97 | 82.50 ± 9.98 |
| II | 3.06 ± 0.40 | 30.81 ± 1.55 | 9.99 ± 0.36 | 2.48 ± 0.31 | 8.88 ± 1.15 | 97.70 ± 13.01 | 19.54 ± 2.60 | 117.24 ± 15.61 | 58.62 ± 7.81 | |
| III | 2.25 0.25 | 46.16 ± 1.64 | 12.67 ± 0.45 | 4.17 ± 0.44 | 18.73 ± 1.94 | 69.12 ± 7.60 | 13.82 ± 1.52 | 82.94 ± 9.12 | 41.47 ± 4.56 | |
| High | I | 2.26 ± 0.20 | 19.11 ± 0.37 | 7.29 ± 0.17 | 0.94 ± 0.10 | 2.78 ± 0.34 | 73.77 ± 6.58 | 14.75 ± 1.32 | 88.52 ± 7.89 | 44.26 ± 3.95 |
| II | 2.06 ± 0.21 | 31.03 ± 0.93 | 9.43 ± 0.13 | 2.22 ± 0.21 | 8.42 ± 0.83 | 64.92 ± 6.57 | 12.98 ± 1.31 | 77.90 ± 7.89 | 38.95 ± 3.94 | |
| III | 1.72 ± 0.19 | 42.98 ± 0.69 | 11.88 ± 0.23 | 3.56 ± 0.34 | 17.13 ± 1.53 | 52.55 ± 5.86 | 10.51 ± 1.17 | 63.06 ± 7.03 | 31.53 ± 3.51 |
| Elevation (m) | Size Class | Shannon-Weiner Index | Gini-Simpson Index | Evenness Index | Species Richness | Abundance |
|---|---|---|---|---|---|---|
| Low | Tree | 1.64 ± 0.04 | 0.81 ± 0.02 | 0.92 ± 0.02 | 5.88 ± 0.09 | 63.47 ± 4.55 |
| Sapling | 1.35 ± 0.1 | 0.74 ± 0.04 | 0.84 ± 0.03 | 5 ± 0.3 | 57.12 ± 4.9 | |
| Seedling | 1.27 ± 0.12 | 0.76 ± 0.03 | 0.8 ± 0.06 | 4.82 ± 0.26 | 51.88 ± 5.2 | |
| Middle | Tree | 1.55 ± 0.04 | 0.78 ± 0.01 | 0.92 ± 0.02 | 5.38 ± 0.15 | 54.88 ± 3.8 |
| Sapling | 1.2 ± 0.1 | 0.61 ± 0.06 | 0.69 ± 0.06 | 4.94 ± 0.2 | 50.59 ± 3.29 | |
| Seedling | 1.07 ± 0.14 | 0.59 ± 0.06 | 0.79 ± 0.05 | 4.59 ± 0.35 | 48.88 ± 3.42 | |
| High | Tree | 1.39 ± 0.03 | 0.72 ± 0.01 | 0.89 ± 0.01 | 4.75 ± 0.11 | 49.41 ± 2.27 |
| Sapling | 1.03 ± 0.12 | 0.59 ± 0.07 | 0.68 ± 0.03 | 4.18 ± 0.29 | 44.17 ± 2.96 | |
| Seedling | 0.98 ± 0.14 | 0.55 ± 0.06 | 0.66 ± 0.08 | 4.06 ± 0.22 | 44.47 ± 2.57 |
| Elevation (m) | DBH Class (cm) | LB (kg/ha) | LCS (t C/ha) | DWB (kg/ha) | DWCS (t C/ha) |
|---|---|---|---|---|---|
| Low | I | 0.73 ± 0.02 | 0.23 ± 0.01 | 3.60 ± 0.06 | 1.12 ± 0.04 |
| II | 0.76 ± 0.02 | 0.24 ± 0.01 | 3.84 ± 0.07 | 1.19 ± 0.04 | |
| III | 0.80 ± 0.02 | 0.25 ± 0.01 | 3.99 ± 0.07 | 1.24 ± 0.4 | |
| Middle | I | 0.80 ± 0.02 | 0.25 ± 0.01 | 3.97 ± 0.08 | 1.23 ± 0.05 |
| II | 0.90 ± 0.03 | 0.28 ± 0.01 | 4.15 ± 0.09 | 1.29 ± 0.05 | |
| III | 0.91 ± 0.02 | 0.28 ± 0.01 | 4.20 ± 0.08 | 1.30 ± 0.06 | |
| High | I | 0.93 ± 0.03 | 0.29 ± 0.02 | 3.81 ± 0.07 | 1.18 ± 0.06 |
| II | 0.99 ± 0.03 | 0.31 ± 0.02 | 4.85 ± 0.09 | 1.50 ± 0.07 | |
| III | 1.05 ± 0.04 | 0.33 ± 0.02 | 4.46 ± 0.08 | 1.38 ± 0.07 |
| Elevation (m) | DBH Classes | BD (g/cm3) | SOC (kg/ha) | SOCS (t C/ha) |
|---|---|---|---|---|
| Low | I | 1.38 ± 0.03 | 42.64 ± 0.17 c | 78.53 ± 0.31 b |
| II | 1.56 ± 0.04 | 43.39 ± 0.17 b | 80.54 ± 0.32 a | |
| III | 1.73 ± 0.05 | 44.30 ± 0.18 a | 81.25 ± 0.33 a | |
| Middle | I | 1.39 ± 0.03 | 44.55 ± 0.18 a | 80.01 ± 0.33 b |
| II | 1.65 ± 0.05 | 44.75 ± 0.18 a | 81.67 ± 0.34 a | |
| III | 1.71 ± 0.05 | 45.21 ± 0.19 a | 82.09 ± 0.34 a | |
| High | I | 1.63 ± 0.04 | 44.68 ± 0.19 b | 81.05 ± 0.33 b |
| II | 1.72 ± 0.05 | 44.84 ± 0.19 b | 81.17 ± 0.34 b | |
| III | 1.90 ± 0.06 | 45.58 ± 0.20 a | 82.15 ± 0.35 a |
| Parameters | Carbon Pools | Adj. R2 | p-Value |
|---|---|---|---|
| Elevation | AGCS | −0.72 ** | 0.00 |
| BGCS | −0.72 ** | 0.00 | |
| SCS | −0.28 | 0.06 | |
| LCS | 0.18 ** | 0.00 | |
| DCS | 0.23 ** | 0.00 | |
| SOCS | 0.06 | 0.16 | |
| TCS | −0.73 ** | 0.00 | |
| DBH | AGCS | −0.50 ** | 0.00 |
| BGCS | −0.50 ** | 0.00 | |
| SCS | −0.36 * | 0.02 | |
| LCS | 0.19 * | 0.04 | |
| DCS | 0.05 | 0.76 | |
| SOCS | 0.39 ** | 0.00 | |
| TCS | −0.45 ** | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Hayat, U.; Lushuang, G.; Khan, F.; Xinyi, H.; Shufan, W. Association of Carbon Pool with Vegetation Composition along the Elevation Gradients in Subtropical Forests in Pakistan. Forests 2024, 15, 1395. https://doi.org/10.3390/f15081395
Khan I, Hayat U, Lushuang G, Khan F, Xinyi H, Shufan W. Association of Carbon Pool with Vegetation Composition along the Elevation Gradients in Subtropical Forests in Pakistan. Forests. 2024; 15(8):1395. https://doi.org/10.3390/f15081395
Chicago/Turabian StyleKhan, Inam, Umer Hayat, Gao Lushuang, Faiza Khan, He Xinyi, and Wu Shufan. 2024. "Association of Carbon Pool with Vegetation Composition along the Elevation Gradients in Subtropical Forests in Pakistan" Forests 15, no. 8: 1395. https://doi.org/10.3390/f15081395






