Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatment
2.2. Photosynthetic Pigment Content Determination
2.3. Chlorophyll Fluorescence Parameter Examination
2.4. Photosynthetic Parameter Measurement
2.5. The Area of Latex Burst Determination
2.6. Tensile Property Test
2.7. Chemical Composition Analysis of Rubber Tree Bark
2.8. Statistical Analysis
3. Results
3.1. Changes in Photosynthetic Pigment Content under Chilling Stress
3.2. Effect of Chilling Stress on Fluorescence Parameters in Rubber Trees
3.3. Impact of Continuous Chilling Stress on Photosynthetic Parameters in Rubber Trees
3.4. Latex Burst and Nectar Secretion Represented the Visual Damage of Chilling Stress
3.5. Different Tensile Properties in Barks of Different Rubber Tree Varieties
3.6. Chemical Compositions Exhibited Diversities in Rubber Tree Barks of Different Varieties
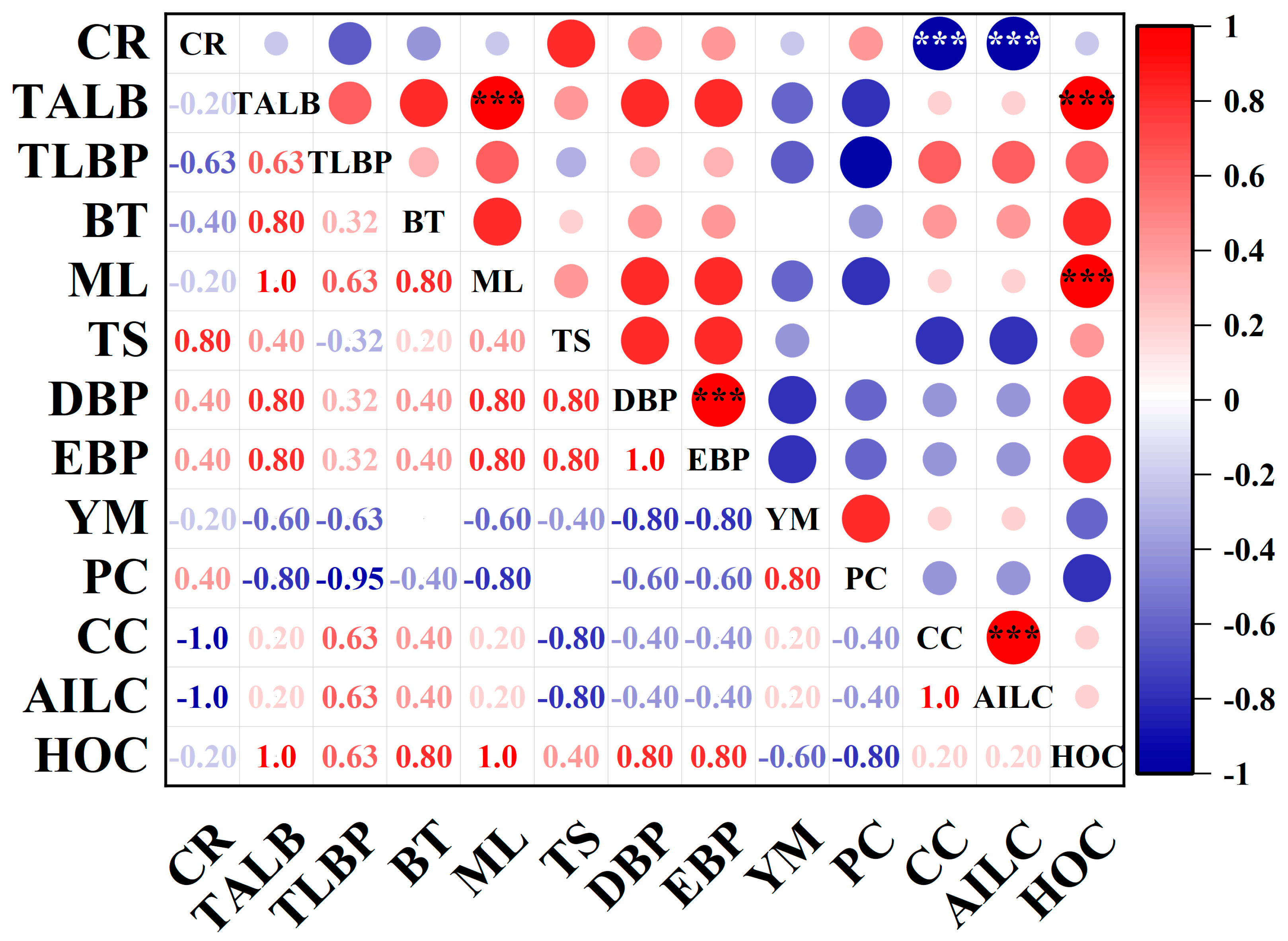
3.7. A Comprehensive Evaluation of Different Rubber Tree Varieties for Chilling Resistance
4. Discussion
4.1. Changes in Photosynthetic Pigment Content and Photosynthetic Capacity of Rubber Tree Seedlings in Response to Chilling Stress
4.2. Chilling Stress-Induced Bark Latex Burst and Nectar Secretion in Rubber Tree Seedlings
4.3. Bark Physical Properties, Chemical Components Were Correlated with Chilling Resistance and Latex Burst in Rubber Tree Seedlings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, M.C.; Boatwright, L.; da Silva, C.C.; Scaloppi, E.J.; de Souza, G.P.; Barbazuk, W.B.; Pereira, D.S.A. Deep expression analysis reveals distinct cold-response strategies in rubber tree (Hevea brasiliensis). BMC Genom. 2019, 20, 455. [Google Scholar]
- Priyadarshan, P.M. Biology of Hevea Rubber, 2nd ed.; Springer International Publishing AG: Cham, Switzerland, 2017; p. 139, p. 134, p. 215. [Google Scholar]
- Long, X.; Lu, J.; Kav, N.V.K.; Qin, Y.; Fang, Y. Identification and evaluation of suitable reference genes for gene expression analysis in rubber tree leaf. Mol. Biol. Rep. 2020, 47, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts—Beyond energy capture and carbon fixation: Tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Wang, J.; Thaler, P.; Luo, Y.; Xu, J. Contrasted effects of temperature during defoliation vs. refoliation periods on the infection of rubber powdery mildew (Oidium heveae) in Xishuangbanna, China. Int. J. Biometeorol. 2020, 64, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, J.; Fu, Y.; Duan, B.; Hongchang, A.; Zhang, L.; Tarno, H. Infestation of platypodine beetles (Coleoptera: Curculionidae) on rubber trees in China. Coleopt. Bull. 2020, 74, 626–631. [Google Scholar] [CrossRef]
- Hiremath, S.R.; Prathapan, K.D. First report of the invasive south American pinhole borer, Euplatypus parallelus (Fabricius) (Coleoptera: Curculionidae: Platypodinae), on rubber in India. Coleopt. Bull. 2019, 73, 714–717. [Google Scholar] [CrossRef]
- Rodríguez, C.S.; Cognato, A.I.; Righi, C.A. Bark and ambrosia beetle (Curculionidae: Scolytinae) diversity found in agricultural and fragmented forests in Piracicaba-SP, Brazil. Environ. Entomol. 2017, 46, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Wu, J. Laticifer differentiation in Hevea brasiliensis: Induction by exogenous jasmonic acid and linolenic acid. Ann. Bot. 2000, 85, 37–43. [Google Scholar] [CrossRef]
- He, K.; Huang, Z. Rubber Culture in the Northern Part of Tropical Area, 1st ed.; GuangDong Science & Technology Press: GuangZhou, China, 1987; p. 114, p. 122, pp. 379–382. [Google Scholar]
- Petrova, A.; Gorshkova, T.; Kozlova, L. Gradients of cell wall nano-mechanical properties along and across elongating primary roots of maize. J. Exp. Bot. 2021, 72, 1764–1781. [Google Scholar] [CrossRef]
- Chanliaud, E.; Burrows, K.M.; Jeronimidis, G.; Gidley, M.J. Mechanical properties of primary plant cell wall analogues. Planta 2002, 215, 989–996. [Google Scholar] [CrossRef]
- Hu, J.Q.; Qi, Q.; Zhao, Y.; Tian, X.; Lu, H.; Gai, Y.; Jiang, X. Unraveling the impact of Pto4CL1 regulation on the cell wall components and wood properties of perennial transgenic Populus tomentosa. Plant Physiol. Biochem. 2019, 139, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef]
- Roig-Oliver, M.; Fullana-Pericàs, M.; Bota, J.; Flexas, J. Adjustments in photosynthesis and leaf water relations are related to changes in cell wall composition in Hordeum vulgare and Triticum aestivum subjected to water deficit stress. Plant Sci. 2021, 311, 111015. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F. Experimental Guidance for Plant Physiology; Higher Education Press: Beijing, China, 2006; pp. 74–76. [Google Scholar]
- Cheng, L.; An, F.; Xie, G.; Jiang, H.; Wang, J.; Peng, W.; Zhou, L. Cold resistance of Hevea brasiliensis based on phloem fiber morphology and bark tensile properties. J. Northwest For. Univ. 2023, 38, 47–54. [Google Scholar]
- GB/T 10742-2008; Raw Fiber Material—Determination of Pectin Content. Standards Press of China: Beijing, China, 2008.
- Wang, L.; Cheng, C. Determination the content of cellulose by nitric acid-ethanol method. Chem. Res. 2011, 22, 52–55, 71. [Google Scholar]
- GB/T 2677.8-1994; Fibrous Raw Material—Determination of Acid-Insoluble Lignin. Standards Press of China: Beijing, China, 1994.
- GBT 2677.10-1995; Fibrous Raw Material—Determination of Holocellulose. Standards Press of China: Beijing, China, 1995.
- Han, Y.; Liu, J.; Li, J.; Jiang, Z.; Ma, B.; Chu, C.; Geng, Z. Novel risk assessment model of food quality and safety considering physical-chemical and pollutant indexes based on coefficient of variance integrating entropy weight. Sci. Total Environ. 2023, 877, 162730. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Deng, X.; Jiang, S. Global warming, grain production and its efficiency: Case study of major grain production region. Ecol. Indic. 2019, 105, 563–570. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Tian, X.; Fu, Y.; Jiang, X.; Li, Y.; Wang, G. Influence of light intensity on chloroplast development and pigment accumulation in wild-type and etiolated mutant plants of Anthurium andraeanum ‘Sonate’. Plant Signal. Behav. 2018, 13, 1482174. [Google Scholar]
- Foyer, C.H.; Vanacker, H.; Gómez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Review. Plant Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, J.; Sha, X.; Zhao, Y.; Hu, T.; Yang, P. Effect of rhizobium symbiosis on low-temperature tolerance and antioxidant response in alfalfa (Medicago sativa L.). Front. Plant Sci. 2019, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.; Mishra, K.B.; Kuusisto, I.; Mishra, A.; Novotná, K.; Šebela, D.; Tyystjärvi, E. Effects of low temperature on photoinhibition and singlet oxygen production in four natural accessions of Arabidopsis. Planta 2020, 252, 19. [Google Scholar] [CrossRef]
- Brunetti, C.; Tattini, M.; Guidi, L.; Velikova, V.; Ferrini, F.; Fini, A. An integrated overview of physiological and biochemical responses of Celtis australis to drought stress. Urban for. Urban Green. 2019, 46, 126480. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Yahia, E.M.; González-Aguilar, G.A. Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J. Sci. Food Agric. 2010, 90, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Wang, C.; Xu, J.; Cheng, Y. Carotenoid accumulation in postharvest “Cara Cara” navel orange (Citrus sinensis Osbeck) fruits stored at different temperatures was transcriptionally regulated in a tissue-dependent manner. Plant Cell Rep. 2012, 31, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Teng, R.; Han, M.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Facundo, H.V.D.V.; Gurak, P.D.; Mercadante, A.Z.; Lajolo, F.M.; Cordenunsi, B.R. Storage at low temperature differentially affects the colour and carotenoid composition of two cultivars of banana. Food Chem. 2015, 170, 102–109. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Xu, B.; Fu, J.; Du, Y.; Fang, Q.; Dong, B.; Zhao, H. Temperature regulation of carotenoid accumulation in the petals of sweet osmanthus via modulating expression of carotenoid biosynthesis and degradation genes. BMC Genom. 2022, 23, 418. [Google Scholar] [CrossRef] [PubMed]
- León-Chan, R.G.; López-Meyer, M.; Osuna-Enciso, T.; Sañudo-Barajas, J.A.; Heredia, J.B.; León-Félix, J. Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ. Exp. Bot. 2017, 139, 143–151. [Google Scholar] [CrossRef]
- Chung, S.W.; Rho, H.; Lim, C.K.; Jeon, M.K.; Kim, S.; Jang, Y.J.; An, H.J. Photosynthetic response and antioxidative activity of ‘Hass’ avocado cultivar treated with short-term low temperature. Sci. Rep. 2022, 12, 11593. [Google Scholar] [CrossRef] [PubMed]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental stress—What can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagasuga, K.; Okada, M. The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol. 2008, 49, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.B.; Mishra, A.; Kubásek, J.; Urban, O.; Heyer, A.G.; Govindjee. Low temperature induced modulation of photosynthetic induction in non-acclimated and cold-acclimated Arabidopsis thaliana: Chlorophyll a fluorescence and gas-exchange measurements. Photosynth. Res. 2019, 139, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Bhat, H.A.; Zargar, M.A.; Ahmad, P.; John, R. Combined gas exchange characteristics, chlorophyll fluorescence and response curves as selection traits for temperature tolerance in maize genotypes. Photosynth. Res. 2021, 150, 213–225. [Google Scholar] [CrossRef]
- Gao, H.; Wu, F. Physiological and transcriptomic analysis of tomato in response to sub-optimal temperature stress. Plant Signal. Behav. 2024, 19, 2332018. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Bode, R.; Dahal, K.; Hollis, L.; Rosso, D.; Krol, M.; Ivanov, A.G. Chloroplast redox imbalance governs phenotypic plasticity: The “grand design of photosynthesis” revisited. Front. Plant Sci. 2012, 3, 255. [Google Scholar] [CrossRef]
- Bag, P.; Chukhutsina, V.; Zhang, Z.; Paul, S.; Ivanov, A.G.; Shutova, T.; Croce, R.; Holzwarth, A.R.; Jansson, S. Direct energy transfer from photosystem II to photosystem I confers winter sustainability in Scots Pine. Nat. Commun. 2020, 11, 6388. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, J.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.B.M.; Chen, L.; Fu, J. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Biol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Wu, X.; He, J.; Zhu, Z.; Yang, S.; Zha, D. Protection of photosynthesis and antioxidative system by 24-epibrassinolide in Solanum melongena under cold stress. Biol. Plant. 2014, 58, 185–188. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Z.; Luo, J.; Wang, C. Quantifying chilling injury on the photosynthesis system of strawberries: Insights from photosynthetic fluorescence characteristics and hyperspectral inversion. Plants 2023, 12, 3138. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A.P.M. Photorespiration is crucial for dynamic response of photosynthetic metabolism and stomatal movement to altered CO2 availability. Mol. Plant. 2017, 10, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Atif, M.J.; Meng, H.; Ghani, M.I.; Ali, M.; Wang, X.; Ding, Y.; Li, X.; Cheng, Z. Biochemical and physiological responses of Cucumis sativus cultivars to different combinations of low-temperature and high humidity. J. Plant Growth Regul. 2023, 42, 390–406. [Google Scholar] [CrossRef]
- Lindfors, L.; Hölttä, T.; Lintunen, A.; Porcar-Castell, A.; Nikinmaa, E.; Juurola, E. Dynamics of leaf gas exchange, chlorophyll fluorescence and stem diameter changes during freezing and thawing of Scots pine seedlings. Tree Physiol. 2015, 35, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; An, F.; Xie, G.; Wang, J.; Peng, W.; Zhou, L. Comprehensive evaluation on cold resistance of Hevea brasiliensis varieties and comparison of bark latex bursting differences between varieties. Southwest China J. Agric. Sci. 2022, 35, 2326–2333. [Google Scholar]
- Wang, J. The rubber honey source in Hainan Island is highly developable. Apic. China 1988, 88, 31. [Google Scholar]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef]
- Bold, G.; Langer, M.; Börnert, L.; Speck, T. The protective role of bark and bark fibers of the giant sequoia (Sequoiadendron giganteum) during high-energy impacts. Int. J. Mol. Sci. 2020, 21, 3355. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, 207373. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Raja, K.; Kumar, V.V.; Ganeshan, P. Characterization study of Holoptelea integrifolia tree bark fibres reinforced epoxy composites. Nat. Prod. Res. 2024, 38, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Bjurhager, I.; Olsson, A.M.; Zhang, B.; Gerber, L.; Kumar, M.; Berglund, L.A.; Burgert, I.; Sundberg, B.; Salmén, L. Ultrastructure and mechanical properties of Populus wood with reduced lignin content caused by transgenic down-regulation of cinnamate 4-hydroxylase. Biomacromolecules 2010, 11, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Verger, S.; Chabout, S.; Gineau, E.; Mouille, G. Cell adhesion in plants is under the control of putative O-fucosyltransferases. Development 2016, 143, 2536–2540. [Google Scholar] [PubMed]
- Wang, X.; Keplinger, T.; Gierlinger, N.; Burgert, I. Plant material features responsible for bamboo’s excellent mechanical performance: A comparison of tensile properties of bamboo and spruce at the tissue, fibre and cell wall levels. Ann. Bot. 2014, 114, 1627–1635. [Google Scholar] [CrossRef]
- Shibly, M.A.H.; Islam, M.I.; Rahat, M.N.H.; Billah, M.M.; Rahman, M.M.; Bashar, M.S.; Abdul, B.; Alorfi, H.S. Extraction and characterization of a novel cellulosic fiber derived from the bark of Rosa hybrida plant. Int. J. Biol. Macromol. 2024, 257, 128446. [Google Scholar] [CrossRef]
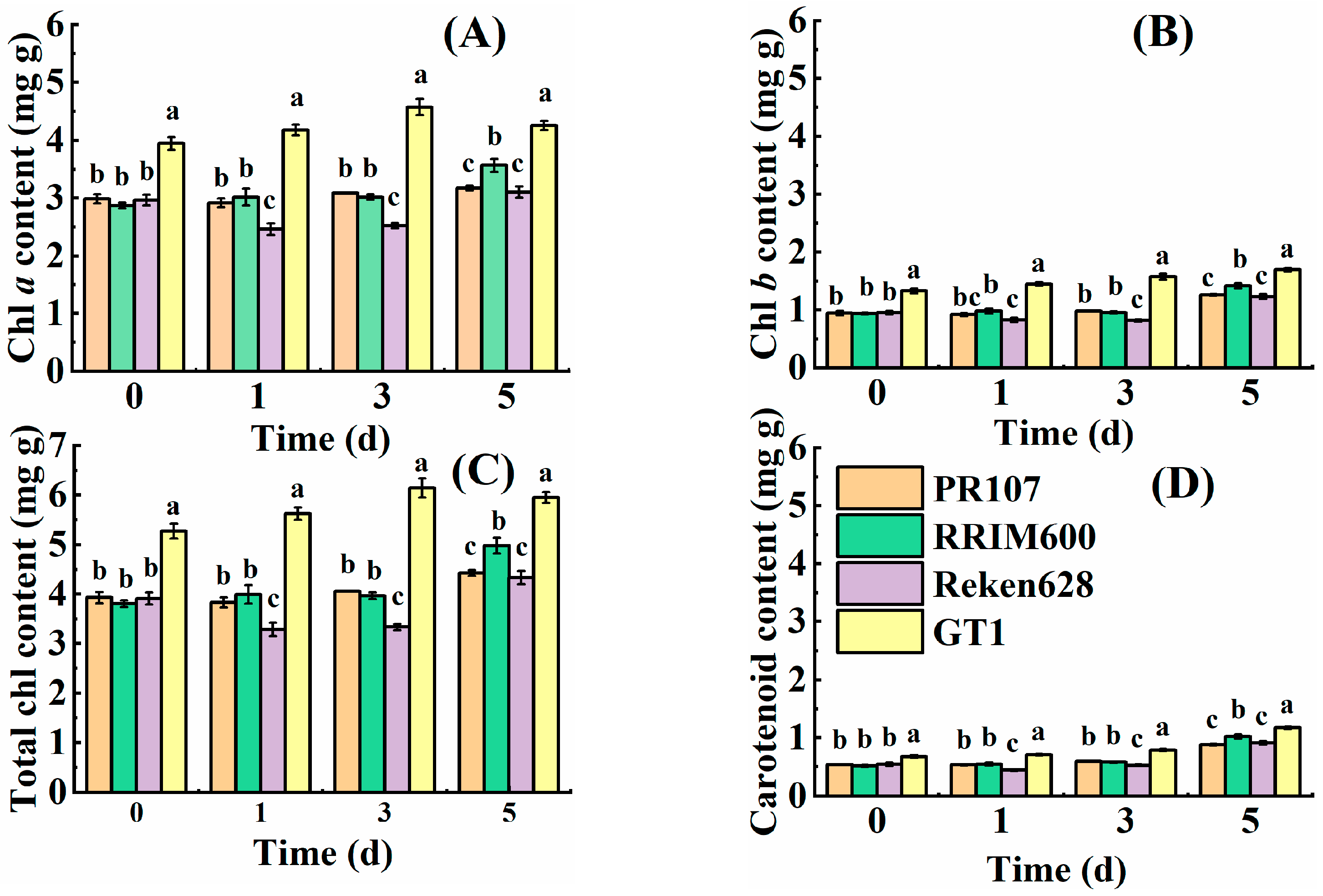
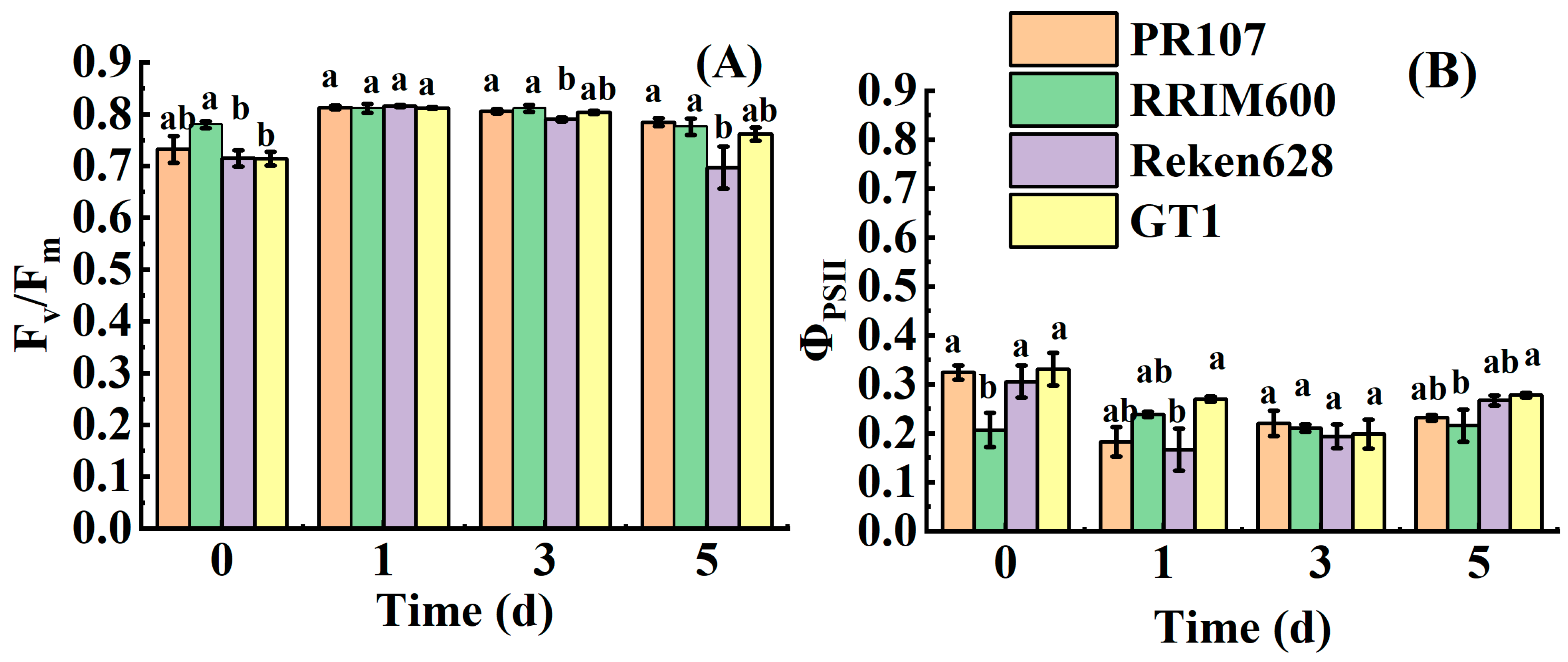
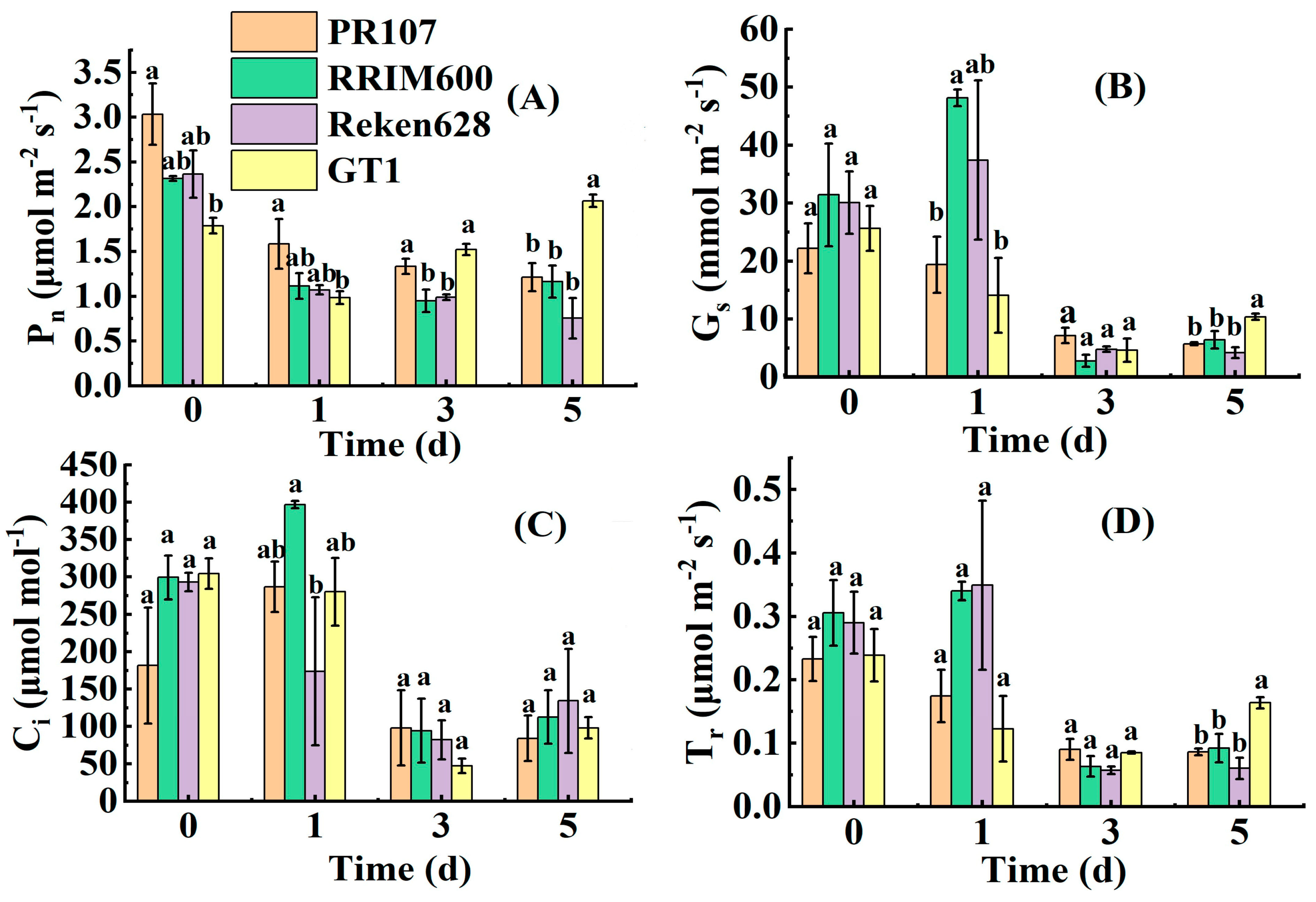

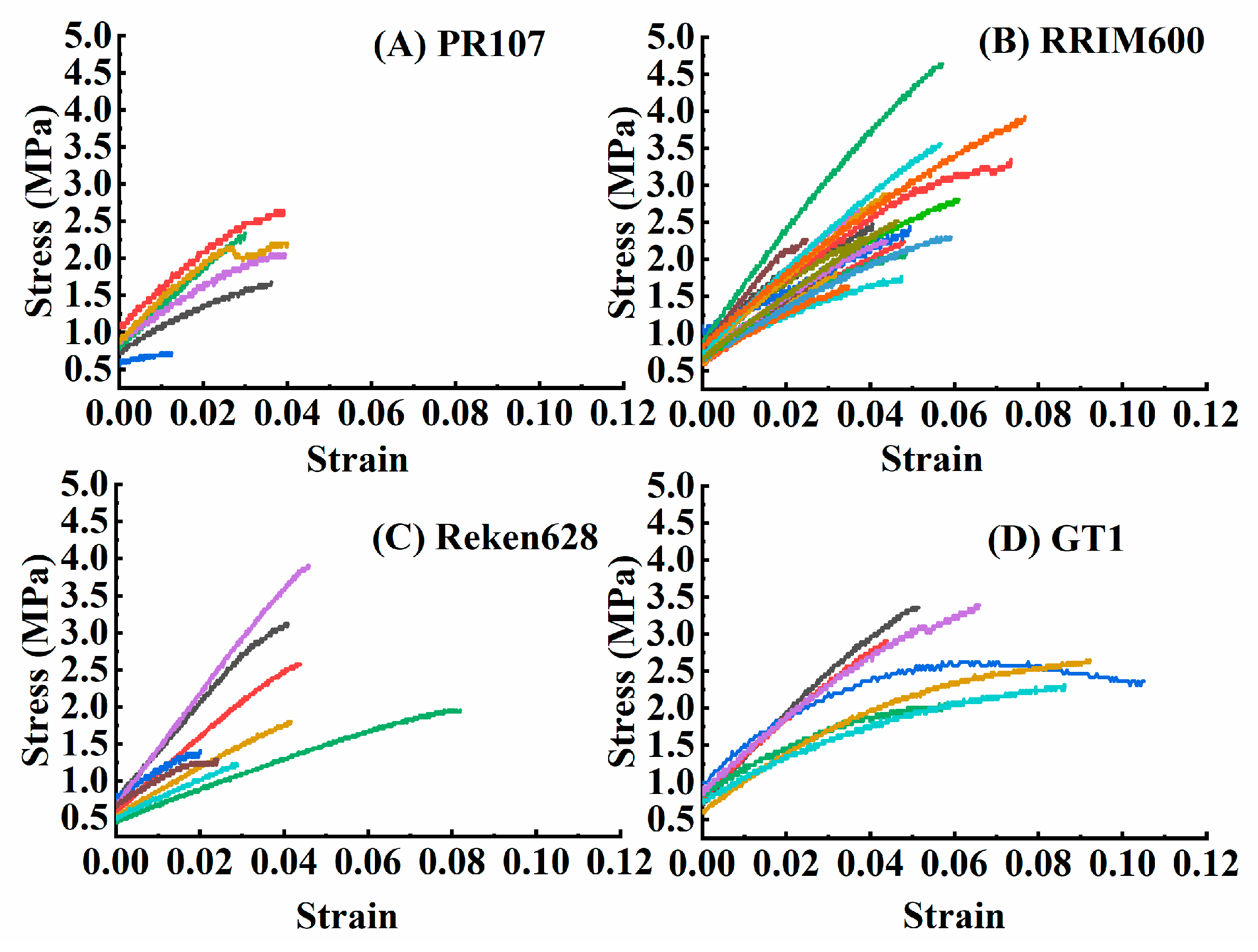
| Variety | Chilling Stress Time (Day) | Chl a (mg g−1) | Chl b (mg g−1) | T Chl (mg g−1) | Caro (mg g−1) |
|---|---|---|---|---|---|
| PR107 | 0 | 2.9870 ± 0.0805 ab | 0.9472 ± 0.0351 b | 3.9341 ± 0.1156 b | 0.5353 ± 0.0091 c |
| 1 | 2.9182 ± 0.0766 b | 0.9198 ± 0.0259 b | 3.8380 ± 0.1025 b | 0.5340 ± 0.0125 c | |
| 3 | 3.0843 ± 0.0054 ab | 0.9794 ± 0.0021 b | 4.0637 ± 0.0071 b | 0.5908 ± 0.0002 b | |
| 5 | 3.1734 ± 0.0412 a | 1.2600 ± 0.0166 a | 4.4334 ± 0.0578 a | 0.8804 ± 0.0119 a | |
| RRIM600 | 0 | 2.8712 ± 0.0503 b | 0.9370 ± 0.0160 b | 3.8083 ± 0.0663 b | 0.5124 ± 0.0154 b |
| 1 | 3.0186 ± 0.1447 b | 0.9794 ± 0.0424 b | 3.9980 ± 0.1872 b | 0.5464 ± 0.0225 b | |
| 3 | 3.0179 ± 0.0462 b | 0.9533 ± 0.0175 b | 3.9712 ± 0.0637 b | 0.5758 ± 0.0085 b | |
| 5 | 3.5649 ± 0.1123 a | 1.4169 ± 0.0449 a | 4.9818 ± 0.1572 a | 1.0204 ± 0.0353 a | |
| Reken628 | 0 | 2.9633 ± 0.0925 a | 0.9515 ± 0.0289 b | 3.9148 ± 0.1213 b | 0.5459 ± 0.0289 b |
| 1 | 2.4659 ± 0.1003 b | 0.8293 ± 0.0349 c | 3.2952 ± 0.1351 c | 0.4430 ± 0.0167 c | |
| 3 | 2.5231 ± 0.0477 b | 0.8193 ± 0.0157 c | 3.3423 ± 0.0634 c | 0.5240 ± 0.0107 b | |
| 5 | 3.1033 ± 0.0969 a | 1.2338 ± 0.0384 a | 4.3371 ± 0.1353 a | 0.9104 ± 0.0288 a | |
| GT1 | 0 | 3.9467 ± 0.1118 b | 1.3295 ± 0.0410 c | 5.2762 ± 0.1525 c | 0.6756 ± 0.0264 c |
| 1 | 4.1778 ± 0.0923 b | 1.4486 ± 0.0326 bc | 5.6265 ± 0.1249 bc | 0.7087 ± 0.0140 c | |
| 3 | 4.5727 ± 0.1405 a | 1.5734 ± 0.0494 ab | 6.1461 ± 0.1898 ab | 0.7902 ± 0.0188 b | |
| 5 | 4.2558 ± 0.0786 ab | 1.6952 ± 0.0312 a | 5.9510 ± 0.1098 a | 1.1751 ± 0.0219 a |
| Variety | NDP | NSP | Total Number | Area of DP (mm2) | Area of SP (mm2) | Total Area (mm2) | Location |
|---|---|---|---|---|---|---|---|
| PR107 | 6 | 0 | 6 | 14.2874 | 0 | 14.2874 | Petiole |
| RRIM600 | 4 | 0 | 4 | 32.9117 | 0 | 32.9117 | Petiole, stem |
| Reken628 | 8 | 1 | 9 | 35.9893 | 12.9428 | 48.9321 | Petiole, stem, leaf scar |
| GT1 | 6 | 0 | 6 | 32.9124 | 0 | 32.9124 | Petiole, stem |
| Variety | BT (mm) | ML (N) | TS (MPa) | EBP (%) | YM (MPa) |
|---|---|---|---|---|---|
| PR107 | 0.7383 ± 0.0577 b | 6.7783 ± 0.7080 b | 1.9400 ± 0.2762 b | 3.2861 ± 0.4361 b | 59.5229 ± 4.7126 a |
| RRIM600 | 0.8005 ± 0.0256 b | 9.8736 ± 0.5952 a | 2.5005 ± 0.1692 ab | 4.5051 ± 0.3424 b | 72.7703 ± 16.4655 a |
| Reken628 | 0.9875 ± 0.0706 a | 10.7900 ± 1.3989 a | 2.2237 ± 0.3224 ab | 4.7181 ± 0.7977 b | 54.1421 ± 8.2707 a |
| GT1 | 0.7643 ± 0.0452 b | 10.5743 ± 0.9489 a | 2.7643 ± 0.1917 a | 7.1811 ± 0.8670 a | 42.8443 ± 6.8711 a |
| Variety | PC (%) | CC (%) | AILC (%) | HOC (%) |
|---|---|---|---|---|
| PR107 | 13.4000 ± 0.5859 b | 1.8500 ± 0.0436 b | 21.5000 ± 0.4359 a | 58.9000 ± 0.5686 c |
| RRIM600 | 16.5000 ± 0.1732 a | 0.8700 ± 0.0529 c | 20.0000 ± 0.2646 b | 60.1000 ± 0.0577 bc |
| Reken628 | 7.3000 ± 0.0700 c | 3.1000 ± 0.0513 a | 22.1000 ± 0.1528 a | 69.6000 ± 0.5132 a |
| GT1 | 7.94000 ± 0.0208 c | 0.3600 ± 0.0115 d | 19.4000 ± 0.1155 b | 60.4000 ± 0.3215 b |
| Variety | Chilling-Resistance Coefficient | DM | Rank | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl a | Chl b | T Chl | Caro | Pn | Gs | Ci | Tr | Fv/Fm | ΦPSII | |||
| PR107 | 0.0615 | 0.0446 | 0.0092 | 0.0051 | 0.0388 | 0 | 0 | 0 | 0.0043 | 0.0101 | 0.1736 | 3 |
| RRIM600 | 0.0788 | 0.0741 | 0.0124 | 0.0101 | 0.0071 | 0.0656 | 0.0402 | 0.0413 | 0.0059 | 0 | 0.3255 | 2 |
| Reken628 | 0 | 0 | 0 | 0 | 0 | 0.0422 | 0.0052 | 0.0329 | 0 | 0.0071 | 0.0874 | 4 |
| GT1 | 0.3278 | 0.3624 | 0.0539 | 0.0403 | 0.0231 | 0.0006 | 0.0127 | 0.0050 | 0.0027 | 0.0239 | 0.8524 | 1 |
| Indicator | Wvj | Ej | Wej | Wj |
|---|---|---|---|---|
| Chl a | 0.3278 | 0.5887 | 0.1046 | 0.2109 |
| Chl b | 0.3624 | 0.5210 | 0.1218 | 0.2394 |
| T chl | 0.0539 | 0.5725 | 0.1087 | 0.0872 |
| Caro | 0.0403 | 0.5492 | 0.1146 | 0.0774 |
| Pn | 0.0388 | 0.6660 | 0.0849 | 0.0654 |
| Gs | 0.0656 | 0.5042 | 0.1261 | 0.1036 |
| Ci | 0.0402 | 0.5787 | 0.1071 | 0.0748 |
| Tr | 0.0413 | 0.6345 | 0.0929 | 0.0706 |
| Fv/Fm | 0.0059 | 0.7580 | 0.0615 | 0.0217 |
| ΦPSII | 0.0239 | 0.6949 | 0.0776 | 0.0490 |
| Variety | D | Rank |
|---|---|---|
| PR107 | 0.1957 | 3 |
| RRIM600 | 0.4217 | 2 |
| Reken628 | 0.1471 | 4 |
| GT1 | 0.7457 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Jiang, H.; Xie, G.; Wang, J.; Peng, W.; Zhou, L.; An, F. Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis. Forests 2024, 15, 1408. https://doi.org/10.3390/f15081408
Cheng L, Jiang H, Xie G, Wang J, Peng W, Zhou L, An F. Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis. Forests. 2024; 15(8):1408. https://doi.org/10.3390/f15081408
Chicago/Turabian StyleCheng, Linlin, Huichuan Jiang, Guishui Xie, Jikun Wang, Wentao Peng, Lijun Zhou, and Feng An. 2024. "Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis" Forests 15, no. 8: 1408. https://doi.org/10.3390/f15081408
APA StyleCheng, L., Jiang, H., Xie, G., Wang, J., Peng, W., Zhou, L., & An, F. (2024). Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis. Forests, 15(8), 1408. https://doi.org/10.3390/f15081408







