Simulating the Long-Term Response of Forest Succession to Climate Change in the Boreal Forest of Northern Ontario, Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Gap Model ZELIG-CFS
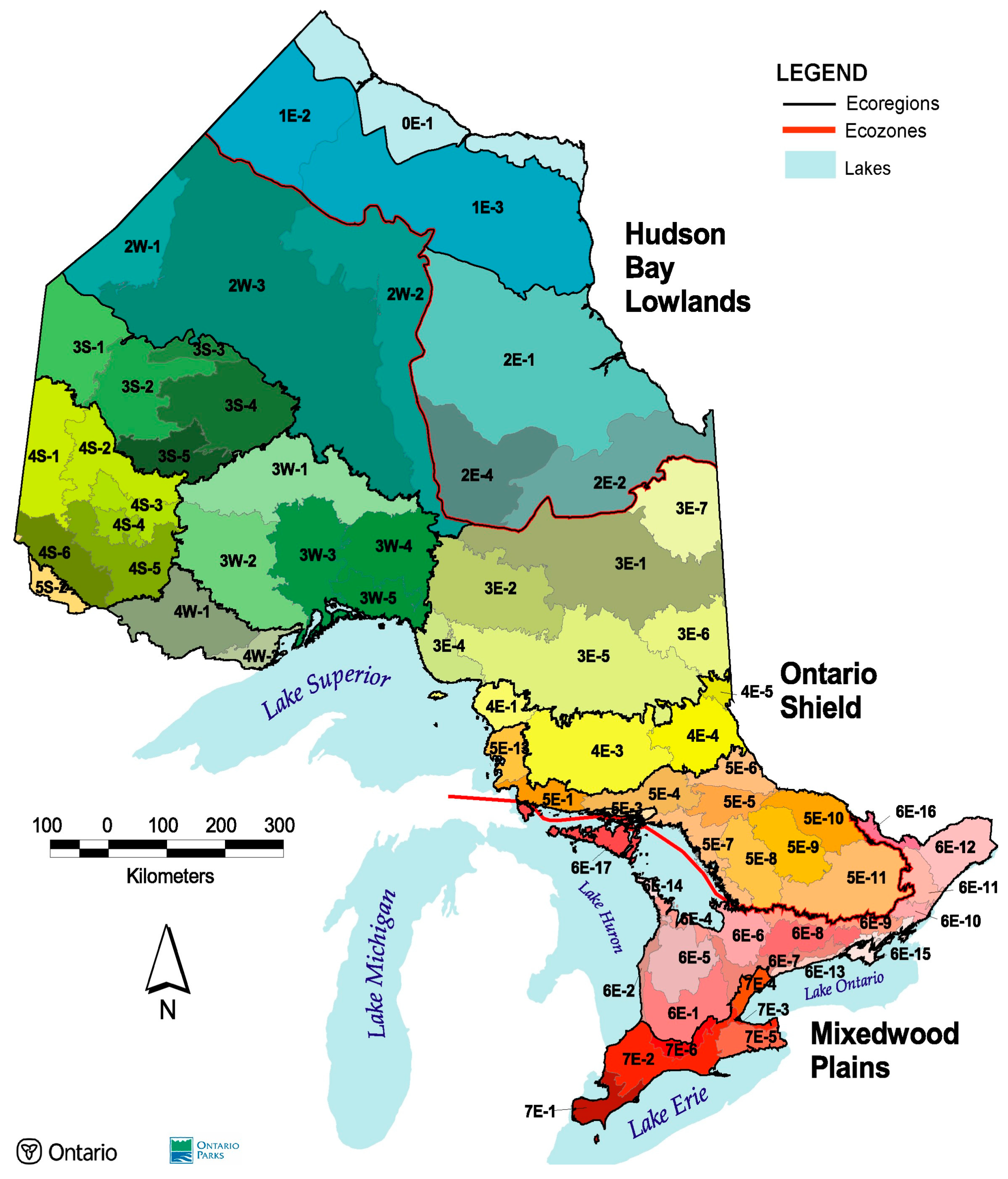
2.2. Study Area and Datasets
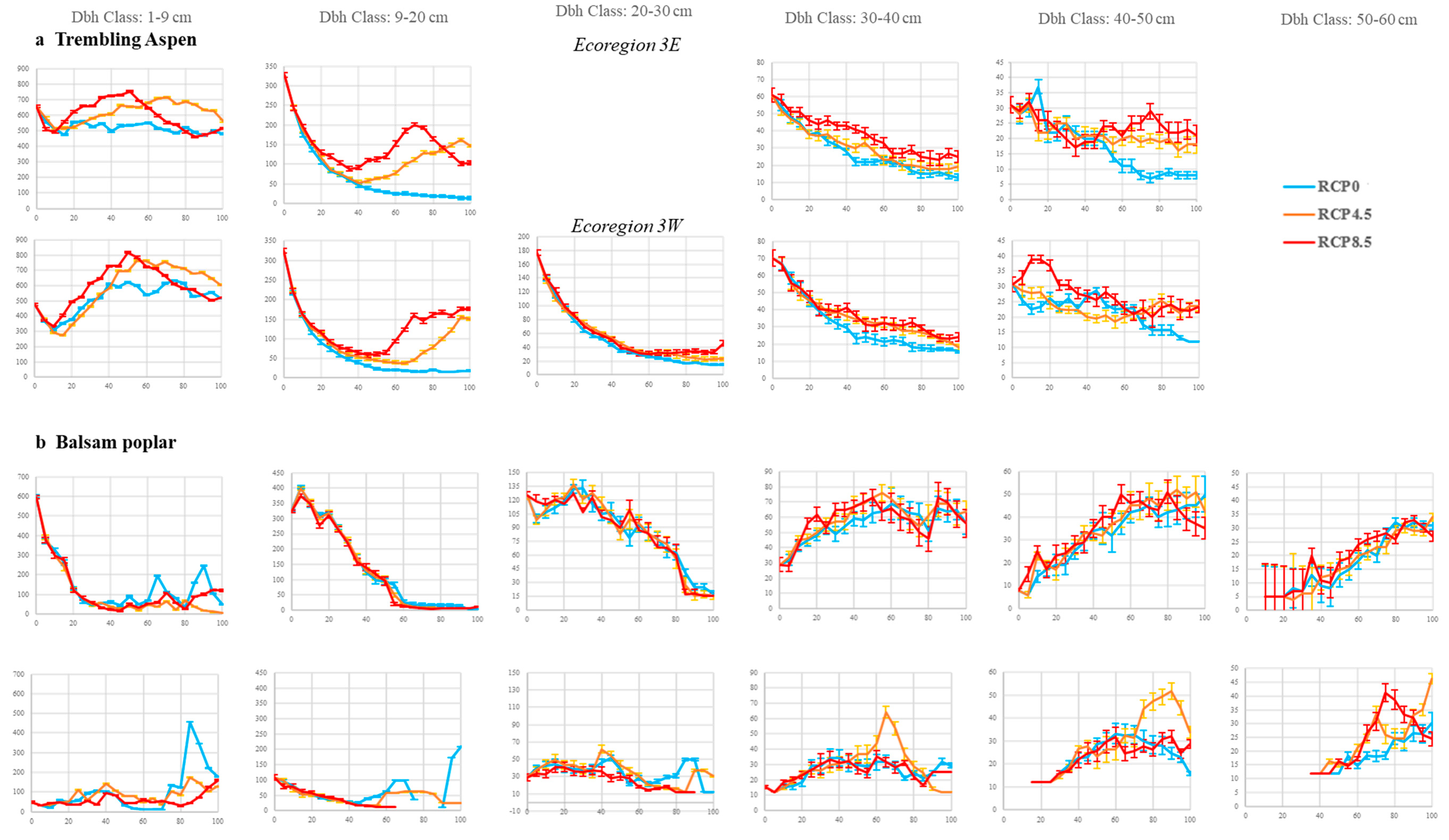
3. Results
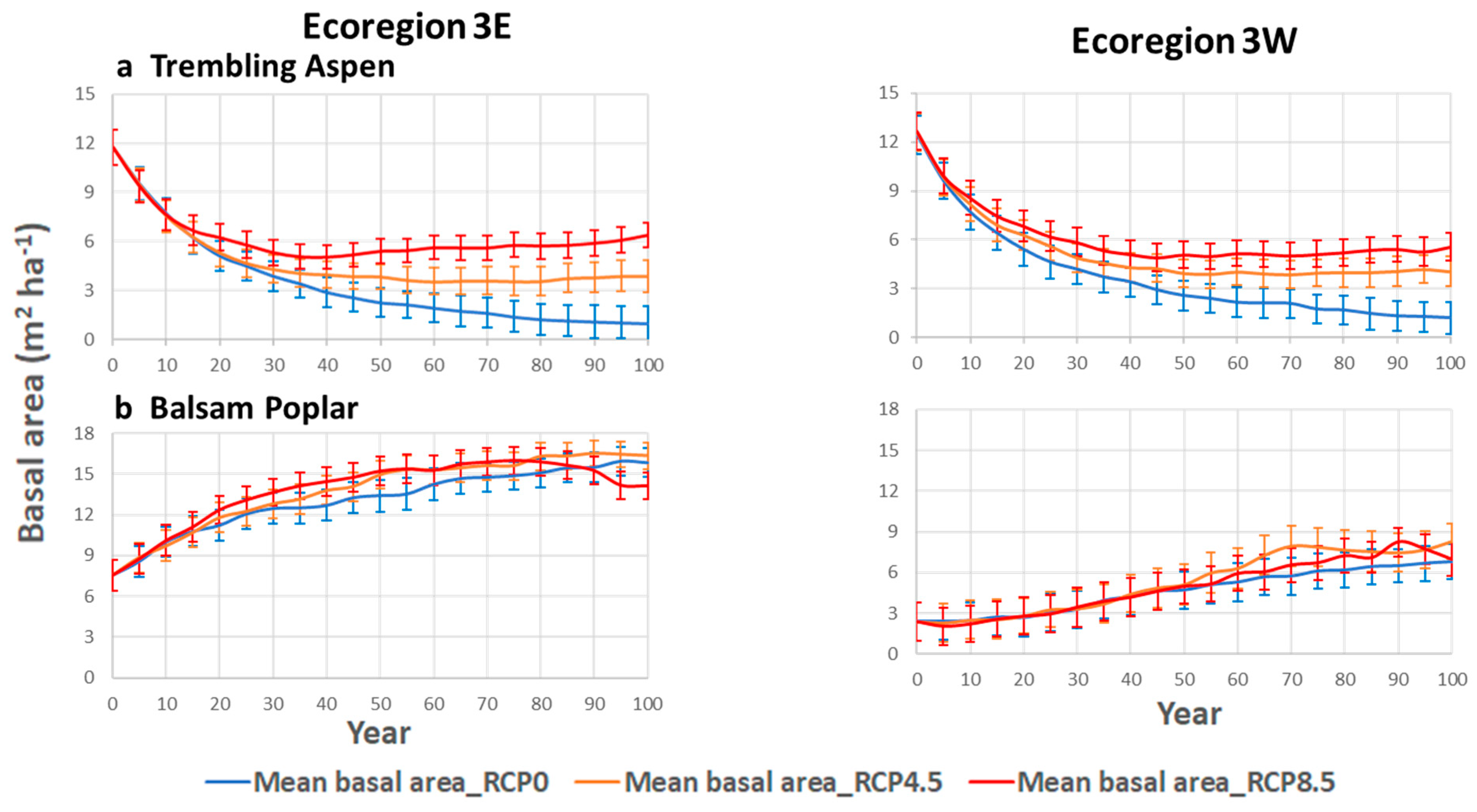
3.1. Mean Basal Area
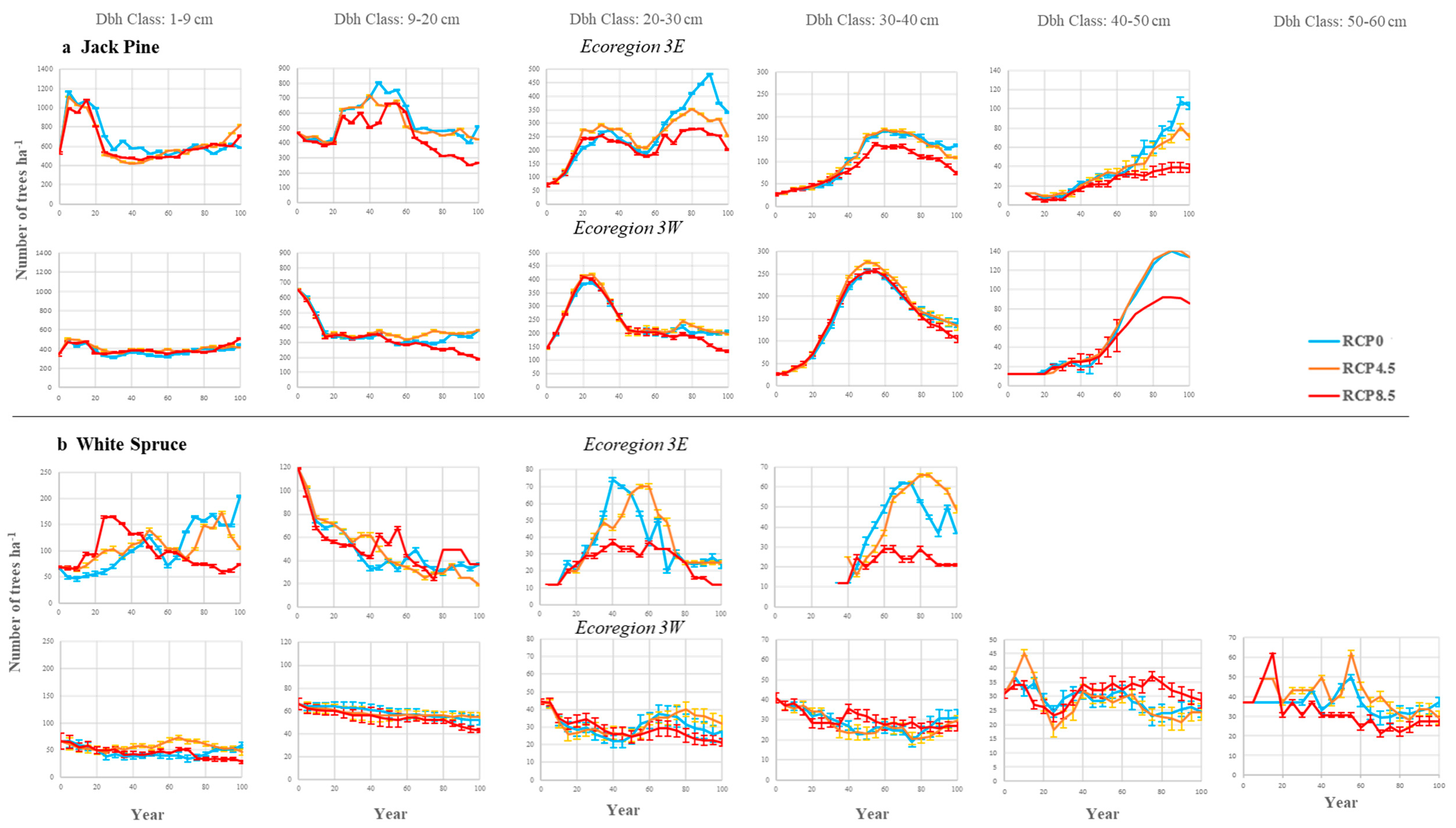
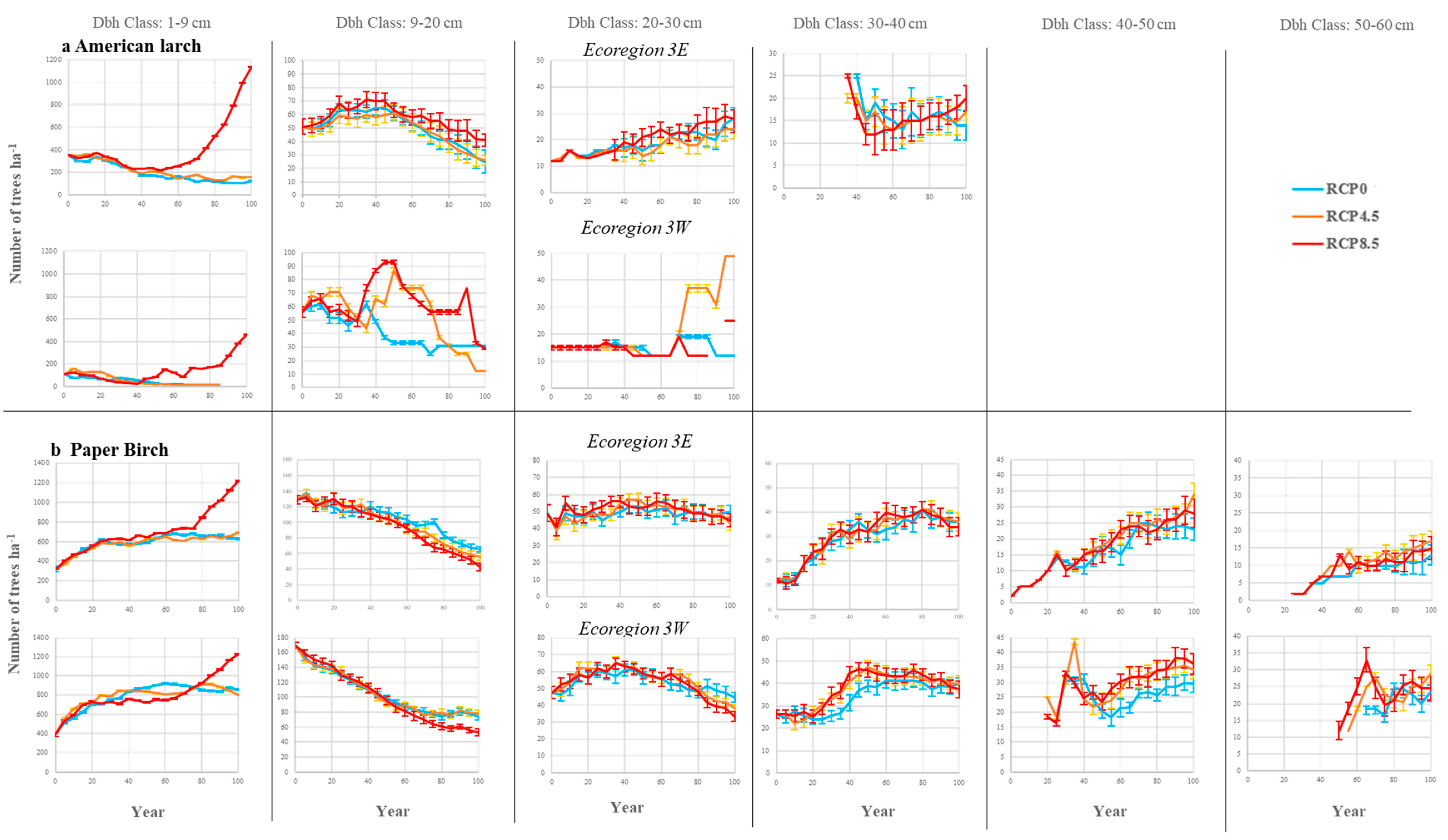
3.2. Stand Density
4. Discussion
4.1. Applicability of Gap Models
4.2. Impacts on Conifer Species
4.3. Impacts on Deciduous Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Refsland, T.K.; Cushman, J.H. Continent-wide synthesis of the long-term population dynamics of quaking aspen in the face of accelerating human impacts. Oecologia 2021, 197, 25–42. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. The impacts of climate change on forest development: A sustainable approach to management models applied to Mediterranean-type climate regions. Plants 2022, 11, 69. [Google Scholar] [CrossRef]
- Hofgaard, A.; Tardif, J.; Bergeron, Y. Dendroclimatic response of Picea mariana and Pinus banksiana along a latitudinal gradient in the eastern Canadian boreal forest. Can. J. For. Res. 1999, 29, 1333–1346. [Google Scholar] [CrossRef]
- Huang, J.-G.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Change Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Huang, J.-G.; Bergeron, Y.; Berninger, F.; Zhai, L.; Tardif, J.C.; Denneler, B. Impact of future climate on radial growth of four major boreal tree species in the eastern Canadian boreal forest. PLoS ONE 2013, 8, e56758. [Google Scholar] [CrossRef]
- Drobyshev, I.; Gewehr, S.; Berninger, F.; Bergeron, Y. Species specific growth responses of black spruce and trembling aspen may enhance resilience of boreal forest to climate change. J. Ecol. 2013, 101, 231–242. [Google Scholar] [CrossRef]
- Gauli, A.; Neupane, P.R.; Mundhenk, P.; Köhl, M. Effect of climate change on the growth of tree species: Dendroclimatological analysis. Forests 2022, 13, 493. [Google Scholar] [CrossRef]
- Devi, N.M.; Kukarskih, V.V.; Galimova, A.A.; Mazeppa, V.S.; Grigoriev, A.A. Climate change evidence in tree growth and stand productivity at the upper treeline ecotone in the Polar Ural Mountains. For. Ecosyst. 2020, 7, 7. [Google Scholar] [CrossRef]
- Searle, E.B.; Chen, H.Y.H. Persistent and pervasive compositional shifts of western boreal forest plots in Canada. Glob. Chang. Biol. 2017, 23, 857–866. [Google Scholar] [CrossRef]
- Wang, J.; Taylor, A.R.; D’Orangeville, L. Warming-induced tree growth may help offset increasing disturbance across the Canadian boreal forest. Proc. Natl. Acad. Sci. USA 2023, 120, e2212780120. [Google Scholar] [CrossRef]
- Lasch-Born, P.; Suckow, F.; Reyer, C.O.P.; Gutsch, M.; Kollas, C.; Badeck, F.-W.; Bugmann, H.K.M.; Grote, R.; Fűrstenau, C.; Schaber, J. Description and evaluation of the process-based forest model 4C v2.2 at four European forest sites. Geosci. Model Dev. 2020, 13, 5311–5343. [Google Scholar] [CrossRef]
- Boukhris, I.; Lahssini, S.; Collalti, A.; Moukrim, S.; Santini, M.; Chiti, T.; Valentioni, R. Calibrating a Process-Based Model to Enhance Robustness in Carbon Sequestration Simulations: The Case of Cedrus atlantica (Endl.) Manetti ex. Carrière. Forests 2023, 14, 401. [Google Scholar] [CrossRef]
- Hu, M.; Minunno, F.; Peltoniemi, M.; Akujärvi, A.; Mälelä, A. Testing the application of process-based forest growth model PREBAS to uneven-aged forests in Finland. For. Ecol. Manag. 2023, 529, 120702. [Google Scholar] [CrossRef]
- FAO. A Review of Existing Approaches and Methods to Assess Climate Change Vulnerability of Forests and Forest-Dependent People; Forestry Working group No. 5; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; 84p. [Google Scholar]
- Johnson, D.W. Progressive N limitation in forests: Review and implications for long-term responses to elevated CO2. Ecology 2006, 87, 64–75. [Google Scholar] [CrossRef]
- Gray, P.A. Impacts of climate change on diversity in forested ecosystems: Some examples. For. Chron. 2005, 81, 655–661. [Google Scholar] [CrossRef]
- Morin, X.; Damestoy, T.; Toigo, M.; Castagneyrol, B.; Jactel, H.; de Coligny, F.; Meredieu, C. Using forest gap models and experimental data to explore long-term effects of tree diversity on the productivity of mixed planted forests. Ann. For. Sci. 2020, 77, 50. [Google Scholar] [CrossRef]
- Larocque, G.R.; Shugart, H.H.; Xi, W.; Holm, J.A. Forest succession models. In Ecological Forest Management Handbook; Larocque, G.R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 179–221. [Google Scholar]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; de Mazancourt, C.; Scherer-Lorenzen, M.; Bugmann, H. Temporal stability in forest productivity increases with tree diversity due to asynchrony in species dynamics. Ecol. Lett. 2014, 17, 1526–1535. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; Garcia-Valdés, R.; Bugmann, H. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci. Rep. 2018, 8, 5627. [Google Scholar] [CrossRef]
- Bohn, F.J.; Huth, A. The importance of forest structure to biodiversity–productivity relationships. R. Soc. Open Sci. 2017, 4, 160521. [Google Scholar] [CrossRef]
- Bugmann, H. A review of forest gap models. Clim. Chang. 2001, 51, 259–301. [Google Scholar] [CrossRef]
- Huo, C.; Cheng, G.; Lu, X.; Fan, J. Simulating the effects of climate change on forest dynamics on Gongga Mountain, Southwest China. J. For. Res. 2010, 15, 176–185. [Google Scholar] [CrossRef]
- Dale, V.H.; Tharp, M.L.; Lannon, K.O.; Hodges, D.G. Modeling transient response of forests to climate change. Sci. Total Environ. 2010, 408, 1888–1901. [Google Scholar] [CrossRef]
- Shugart, H.H.; Wang, B.; Fischer, R.; Ma, J.; Fang, J.; Yan, X.; Huth, A.; Armstrong, A.H. Gap models and their individual-based relatives in the assessment of the consequences of global change. Environ. Res. Lett. 2018, 13, 033001. [Google Scholar] [CrossRef]
- Foster, A.C.; Shuman, J.K.; Shugart, H.H.; Dwire, K.A.; Fornwalt, P.J.; Sibold, J.; Negron, J. Validation and application of a forest gap model to the southern Rocky Mountains. Ecol. Model. 2017, 351, 109–128. [Google Scholar] [CrossRef]
- Foster, A.C.; Shuman, J.K.; Shugart, H.H.; Negron, J. Modeling the interactive effects of spruce beetle infestation and climate on subalpine vegetation. Ecosphere 2018, 9, e02437. [Google Scholar] [CrossRef]
- Schneider, R.; Franceschini, T.; Fortin, M.; Martin-Ducup, O.; Gauthray-Guyénet, V.; Larocque, G.R.; Marshall, P. Growth and yield models for predicting tree and stand productivity. In Ecological Forest Management Handbook; Larocque, G.R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 141–178. [Google Scholar]
- Ashraf, M.I.; Meng, F.-R.; Bopurque, C.P.-A.; MacLean, D.A. A novel modelling approach for predicting forest growth and yield under climate change. PLoS ONE 2015, 10, e0132066. [Google Scholar] [CrossRef]
- Morin, X.; Bugmann, H.; de Coligny, F.; Martin-StPaul, N.; Cailleret, M.; Limousin, J.-M.; Ourcival, J.-M.; Prevosto, B.; Simioni, G.; Toigo, M.; et al. Beyond forest succession: A gap model to study ecosystem functioning and tree community composition under climate change. Funct. Ecol. 2021, 35, 955–975. [Google Scholar] [CrossRef]
- Larocque, G.R.; Bell, F.W. Evaluating the performance of a forest succession model to predict the long-term dynamics of tree species in mixed boreal forests using historical data in northern Ontario, Canada. Forests 2021, 12, 1181. [Google Scholar] [CrossRef]
- Urban, D.L. A Versatile Model to Simulate Forest Pattern: A User’s Guide to ZELIG; Version 1.0; University of Virginia: Charlottesville, VA, USA, 1990. [Google Scholar]
- Urban, D.L. Using model analysis to design monitoring programs for landscape management and impact assessment. Ecol. Appl. 2000, 10, 1820–1832. [Google Scholar] [CrossRef]
- Urban, D.L.; Bonan, G.B.; Smith, T.M.; Shugart, H.H. Spatial applications of gap models. For. Ecol. Manag. 1991, 42, 95–110. [Google Scholar] [CrossRef]
- Botkin, D.B.; Janak, J.F.; Wallis, J.R. Some ecological consequences of a computer model of forest growth. J. Ecol. 1972, 60, 849–872. [Google Scholar] [CrossRef]
- Larocque, G.R.; Archambault, L.; Delisle, C. Development of the gap model ZELIG-CFS to predict the dynamics of North American mixed forest types with complex structures. Ecol. Model. 2011, 222, 2570–2583. [Google Scholar] [CrossRef]
- Elzein, T.; Larocque, G.R.; Sirois, L.; Arseneault, D. Comparing the predictions of gap model with vegetation and disturbance data in south-eastern Canadian mixed forests. For. Ecol. Manag. 2020, 455, 117649. [Google Scholar] [CrossRef]
- Johnsen, K.H.; Major, J.E. Black spruce family growth performance under ambient and elevated atmospheric CO2. New For. 1998, 15, 271–281. [Google Scholar] [CrossRef]
- Isebrands, J.G.; McDonald, E.P.; Kruger, E.; Hendrey, G.; Percy, K.; Pregitzer, K.; Sober, J.; Karnosky, D.F. Growth responses of Populus tremuloides clones to interacting elevated carbon dioxide and tropospheric ozone. Environ. Pollut. 2001, 115, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Isebrands, J.G.; McDonald, E.P.; Kruger, E.; Hendrey, G.R.; Percy, K.E.; Pregitzer, K.S.; Sober, H.; Karnosky, D.F. Growth responses of aspen clones to elevated carbon dioxide and ozone. In Air Pollution, Global Change and Forests in the New Millenium; Karmosky, D.F., Percy, K.E., Cappelka, A.H., Simpson, C., Pikkarainen, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; pp. 411–435. [Google Scholar]
- Kubiske, M.E.; Quinn, V.S.; Marquardt, P.E.; Karnosky, D.F. Effects of elevated atmospheric CO2 and/or O3 on intra- and interspecific competitive ability of aspen. Plant Biol. 2007, 9, 342–355. [Google Scholar] [CrossRef]
- Zhang, S.; Dang, Q.-L. Interactive effects of soil temperature and [CO2] on Morphological and Biomass Traits in Seedlings of Four Boreal Tree Species. For. Sci. 2007, 53, 453–460. [Google Scholar] [CrossRef]
- Cao, B.; Dang, Q.-L.; Yű, X.; Zhang, S. Effects of [CO2] and nitrogen on morphological and biomass traits of white birch (Betula papyrifera) seedlings. For. Ecol. Manag. 2008, 254, 217–224. [Google Scholar] [CrossRef]
- Marfo, J.; Dang, Q.-L. Interactive effects of carbon dioxide concentration and light on the morphological and biomass characteristics of black spruce and white spruce seedlings. Botany 2009, 87, 67–77. [Google Scholar] [CrossRef]
- Major, J.E.; Mosseler, A.; Johnsen, K.H.; Campbell, M.; Malcolm, J. Red and black spruce provenance growth and allocation under ambient and elevated CO2. Trees 2015, 29, 1313–1328. [Google Scholar] [CrossRef]
- Newaz, M.S.; Dang, Q.-L.; Man, R. Morphological Response of Jack Pine to the Interactive Effects of Carbon Dioxide, Soil Temperature and Photoperiod. Am. J. Plant Sci. 2016, 7, 879–893. [Google Scholar] [CrossRef]
- Crins, W.J.; Gray, P.A.; Uhlig, P.W.C.; Wester, M.C. Inventory, Monitoring and Assessment. In The Ecosystems of Ontario, Part 1: Ecozones and Ecoregions; SIB TER IMA TR-01; Ontario Ministry of Natural Resources: Peterborough, ON, Canada, 2009; 71p. [Google Scholar]
- Mackey, B.G.; McKenney, D.W.; Yang, Y.-Q.; McMahon, J.P.; Hutchinson, M.F. Site Regions Revisited: A Climatic Analysis of Hills’ Site Regions for the Province of Ontario Using a Parametric Method. Can. J. For. Res. 1996, 26, 333–354, Erratum in Can. J. For. Res. 1996, 26, 1112. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Fortin, M.; White, J.C.; Sattler, D.; Kurz, W.A.; Penner, M.; Edwards, J.; Hays-Byl, W.; Comeau, R.; Roy, V. Climate sensitive growth and yield models in Canadian forestry: Challenges and opportunities. For. Chron. 2024, 100, 88–106. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Bugmann, H.; Pitelka, L.F. How much physiology is needed in forest gap models for simulating long-term vegetation response to global change? Challenges, limitations, and potentials. Clim. Chang. 2001, 51, 541–557. [Google Scholar] [CrossRef]
- D’Orangeville, L.; Houle, D.; Duchesne, L.; Phillips, R.P.; Bergeron, Y.; Kneeshaw, D. Beneficial effects of climate warming on boreal tree growth may be transitory. Nat. Comm. 2018, 9, 3213. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; Post, W.M. Development of a Linked Forest Productivity-Soil Process Model; ORNL/TM-9519; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1985; p. 168.
- Viereck, L.A.; Johnston, W.F. Picea mariana (Mill.) B.S.P. In Silvics of North America; Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 227–237. [Google Scholar]
- Sims, R.A.; Kershaw, H.M.; Wickware, G.M. The Autecology of Major Tree Species in the North Central Region of Ontario; COFRDA Rep. 3302 NWOFTDU Tech. Rep. 48; Forestry Canada, Ontario Region, Great Lakes Forest Research Centre: Thunder Bay, ON, Canada, 1990; p. 126. [Google Scholar]
- Burns, R.M.; Honkala, B.H. Silvics of North America: 1. Conifers; Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990; Volume 1.
- Burns, R.M.; Honkala, B.H. Silvics of North America: 2. Hardwoods; Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990; Volume 2, p. 877.
- Marquis, D.A.; Bjorkbom, J.C.; Yelenosky, G. Effect of seedbed condition and light exposure on paper birch regeneration. J. For. 1964, 62, 876–881. [Google Scholar] [CrossRef]
- Messier, C.; Doucet, R.; Ruel, J.-C.; Claveau, Y.; Kelly, C.; Lechowicz, M.J. Functional ecology of advance regeneration in relation to light in boreal forests. Can. J. For. Res. 1999, 29, 812–823. [Google Scholar] [CrossRef]
- Drescher, M.; Perera, A.H.; Buse, L.J.; Arnup, R.; Bowling, C.; Etheridge, D.; Niznowski, G.; Ride, K.; Vasiliauskas, S. Boreal forest succession in Ontario: An analysis of the knowledge space. Appl. Res. Dev. 2008, 171, 59. [Google Scholar]
- Dietrich, R.; Bell, F.W.; Silva, L.C.R.; Cecile, A.; Horwath, W.R.; Anand, M. Climate sensitivity, water-use efficiency, and growth decline in boreal jack pine (Pinus banksiana) forests in Northern Ontario. J. Geophys. Res. Biogeosci. 2016, 121, 2761–2774. [Google Scholar] [CrossRef]
- Sang, Z.; Sebastian-Azcona, J.; Hamann, A.; Menzel, A.; Hacke, U. Adaptive limitations of white spruce populations to drought imply vulnerability to climate change in its western range. Evol. Appl. 2019, 12, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Nienstaedt, H.; Zasada, J.C. Picea glauca (Moench). In Silvics of North America; Burns, R.M., Honkala, B.H., technical coordinators, Eds.; Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, Conifers; pp. 204–226. [Google Scholar]
- Man, R.; Lieffers, V.J. Are mixtures of aspen and white spruce more productive than single species stands. For. Chron. 1999, 75, 505–513. [Google Scholar] [CrossRef]
- Johnston, W.F. Larix laricina (Du Roi) K. Koch. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 141–151. [Google Scholar]
- McCarthy, J. Gap dynamics of forest trees: A review with particular attention to boreal forests. Environ. Rev. 2001, 9, 1–59. [Google Scholar] [CrossRef]
- Keane, R.E.; Austin, M.; Field, C.; Huth, A.; Lexer, M.J.; Peters, D.; Solomon, A.; Wyckoff, P. Tree mortality in gap models: Application to climate change. Clim. Chang. 2001, 51, 509–540. [Google Scholar] [CrossRef]
- Howard, J.L. Populus tremuloides. In Fire Effects Information System, [Online]; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer): Washington, DC, USA, 1996. Available online: https://www.fs.usda.gov/database/feis/plants/tree/poptre/all.html (accessed on 7 December 2023).
- Zasada, J.C.; Phipps, H.M. Populus balsamifera L., Balsam Poplar. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990; Volume 2, Hardwoods; pp. 518–529. [Google Scholar]



| Species | Agemax 1 | Dmax | Hmax | DDmin | DDmax | Toler | Drought | Nutri |
|---|---|---|---|---|---|---|---|---|
| Black spruce | 250 | 40 | 2000 | 265 | 1929 | 2 | 3 | 3 |
| Balsam fir | 200 | 65 | 3000 | 250 | 2404 | 1 | 1 | 3 |
| Jack pine | 150 | 50 | 2500 | 213 | 2234 | 5 | 4 | 3 |
| White spruce | 200 | 64 | 3000 | 447 | 1929 | 2 | 3 | 3 |
| Trembling aspen | 125 | 75 | 3700 | 889 | 5556 | 5 | 3 | 2 |
| Paper birch | 250 | 150 | 4500 | 1420 | 3084 | 3 | 2 | 2 |
| Northern white cedar | 400 | 100 | 2900 | 1000 | 2188 | 2 | 4 | 3 |
| American larch | 335 | 75 | 2500 | 280 | 2660 | 5 | 4 | 3 |
| Balsam poplar | 150 | 75 | 2500 | 555 | 2491 | 5 | 1 | 3 |
| Species | Number of Seedlings per m2 | Stocking |
|---|---|---|
| Black spruce | 0.4552 (0.5135) 1 | 0.262 (0.191) |
| Balsam fir | 5.7385 (65.8297) | 0.278 (0.253) |
| Jack pine | 0.0096 (0.0195) | 0.119 (0.123) |
| Paper birch | 0.0534 (0.1246) | 0.314 (0.304) |
| White spruce | 0.0045 (0.0230) | 0.010 (0.026) |
| Balsam poplar | 0.0027 (0.0226) | 0.057 (0.116) |
| Trembling aspen | 0.7808 (2.3265) | 0.119 (0.151) |
| Northern white cedar | 0.1421 (0.2519) | 0.145 (0.039) |
| American larch | 0.0069 (0.0254) | 0.079 (0.164) |
| Species | Slope | Intercept |
|---|---|---|
| (Atmospheric CO2 Concentration) | ||
| Black spruce | 0.0492 | –17.71 |
| White spruce | 0.0556 | −20 |
| Jack pine | 0.0694 | −25 |
| Paper birch | 0.05 | −18 |
| Trembling aspen | 0.0806 | −29 |
| Balsam fir | 0.0492 | −17.71 |
| Northern white cedar | 0.0492 | −17.71 |
| American larch | 0.0556 | −20 |
| Balsam poplar | 0.0806 | −29 |
| Species | Ecoregion 3E | Ecoregion 3W |
|---|---|---|
| Black spruce | 17.89 (0.005, 46.61) 1 | 14.23 (0.007, 49.05) |
| Balsam fir | 2.53 (0.001, 22.19) | 2.01 (0.006, 20.25) |
| Jack pine | 7.59 (0.016, 44.18) | 17.49 (0.053, 47.23) |
| White spruce | 1.79 (0.006, 5.91) | 1.37 (0.004, 12.99) |
| Trembling aspen | 20.31 (0.012, 45.38) | 13.85 (0.028, 65.70) |
| Paper birch | 2.30 (0.002, 19.82) | 3.43 (0.009, 15.70) |
| Northern white cedar | 0.72 (0.008, 8.25) | 0.95 (0.58, 2.17) |
| American larch | 3.22 (0.133, 9.14) | 1.08 (0.072, 5.46) |
| Balsam poplar | 7.22 (0.005, 34.37) | 2.92 (0.006, 18.19) |
| Species | Stand Density | Dbh (cm) |
|---|---|---|
| (Number of Trees per ha 1) | ||
| American Can dataset (n = 148) | ||
| Black spruce | 1604 (12,7515) 1 | 7.8 (1.0,56.9) |
| Balsam fir | 390 (12,2707) | 6.2 (1.0,34.0) |
| Trembling aspen | 898 (12,5278) | 12.8 (1.3,59.9) |
| Paper birch | 762 (12,9456) | 6.5 (0.3,36.6) |
| Jack pine | 961 (12,7713) | 12.2 (1.3,53.8) |
| American larch | 805 (12,2842) | 5.3 (1.3,22.4) |
| Northern white cedar | 54 (12,124) | 5.9 (1.3,16.0) |
| White spruce | 136 (12,1125) | 6.6 (1.3,22.4) |
| Balsam poplar | 266 (12,1211) | 9.6 (1.3,25.9) |
| Kimberly Clark dataset (n = 114) | ||
| Black spruce | 1211 (12,4574) | 11.1 (1.3,53.6) |
| Jack pine | 864 (12,6440) | 14.5 (2.2,38.1) |
| Trembling aspen | 447 (12,3115) | 14.7 (2.2,49.3) |
| Paper birch | 274 (12,853) | 10.1 (2.2,32.5) |
| Balsam fir | 85 (12,346) | 10.1 (1.5,29.2) |
| Balsam poplar | 113 (12,457) | 15.9 (6.1,31.2) |
| White spruce | 66 (12,420) | 17.9 (1.3,53.6) |
| American larch | 25 (12,37) | 11.6 (9.1,14.5) |
| Spruce Falls Power and Paper Co. dataset (n = 113) | ||
| Black spruce | 3244 (5,16343) | 6.6 (2.5,40.6) |
| Balsam fir | 602 (2,12344) | 5.4 (2.5,35.6) |
| Trembling aspen | 739 (2,5437) | 7.7 (2.5,48.3) |
| Paper birch | 199 (2989) | 9.4 (2.5,40.6) |
| Balsam poplar | 917 (2,4745) | 8.8 (2.5,48.3) |
| Jack pine | 452 (2,2839) | 11.7 (2.5,35.6) |
| American larch | 121 (2781) | 7.0 (2.5,20.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larocque, G.R.; Bell, F.W.; Searle, E.B.; Mayor, S.J.; Schiks, T.; Kalantari, P. Simulating the Long-Term Response of Forest Succession to Climate Change in the Boreal Forest of Northern Ontario, Canada. Forests 2024, 15, 1417. https://doi.org/10.3390/f15081417
Larocque GR, Bell FW, Searle EB, Mayor SJ, Schiks T, Kalantari P. Simulating the Long-Term Response of Forest Succession to Climate Change in the Boreal Forest of Northern Ontario, Canada. Forests. 2024; 15(8):1417. https://doi.org/10.3390/f15081417
Chicago/Turabian StyleLarocque, Guy R., F. Wayne Bell, Eric B. Searle, Stephen J. Mayor, Thomas Schiks, and Parvin Kalantari. 2024. "Simulating the Long-Term Response of Forest Succession to Climate Change in the Boreal Forest of Northern Ontario, Canada" Forests 15, no. 8: 1417. https://doi.org/10.3390/f15081417








