The Interaction between Climate and Soil Properties Influences Tree Species Richness in Tropical and Subtropical Forests of Southern China
Abstract
1. Introduction
2. Materials and Methods
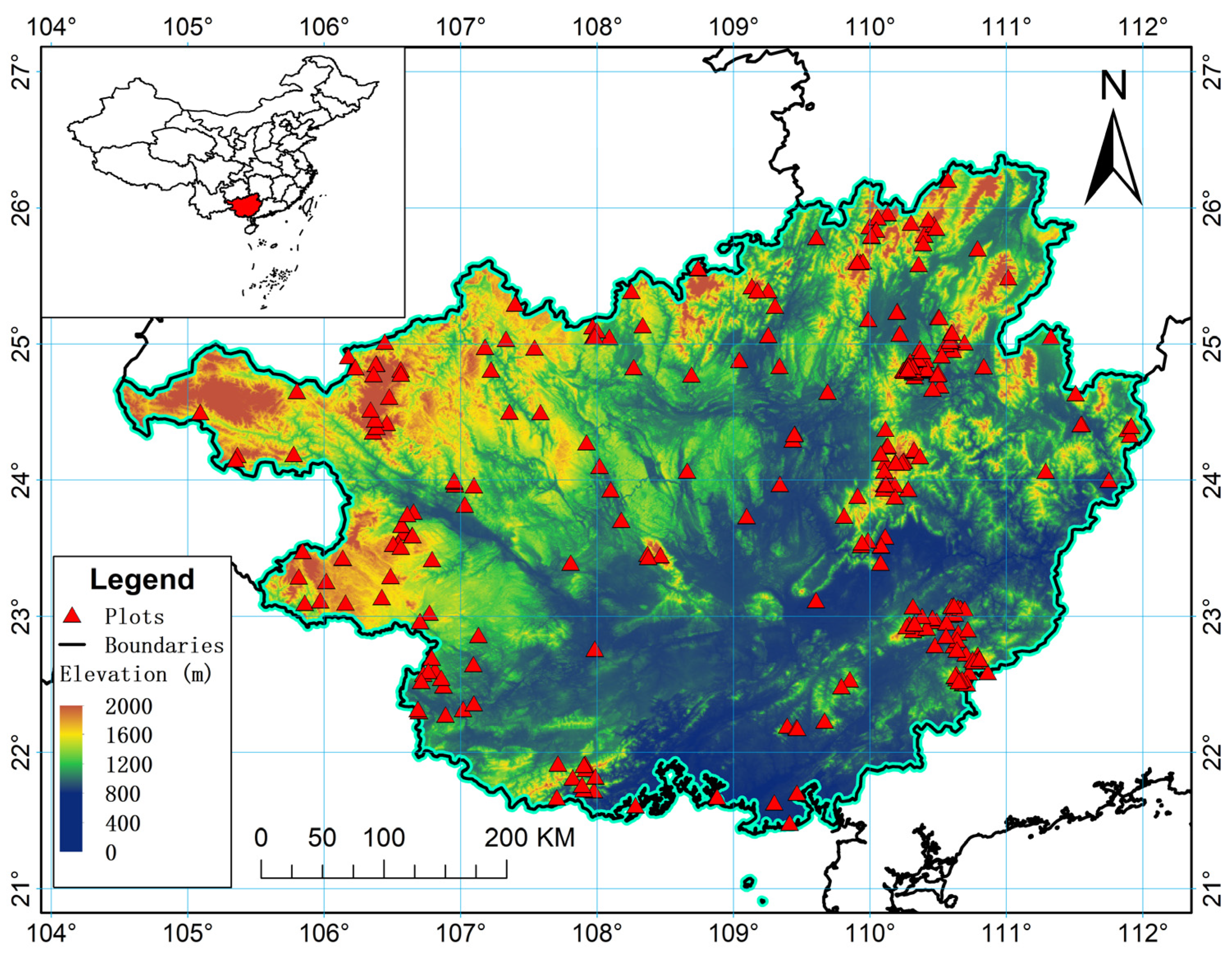
2.1. Study Area and Data Source
2.2. Environmental Predictors
2.3. Modeling Procedure
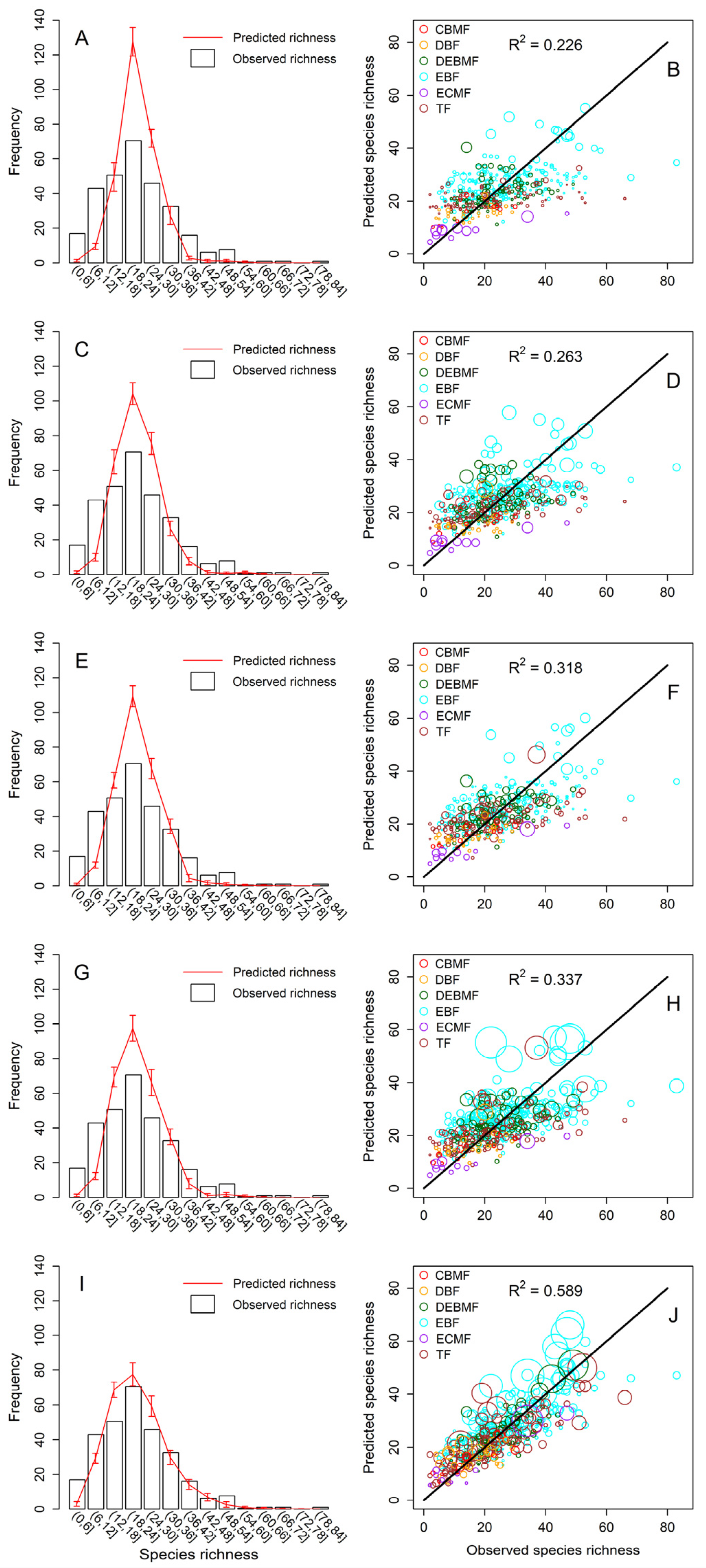
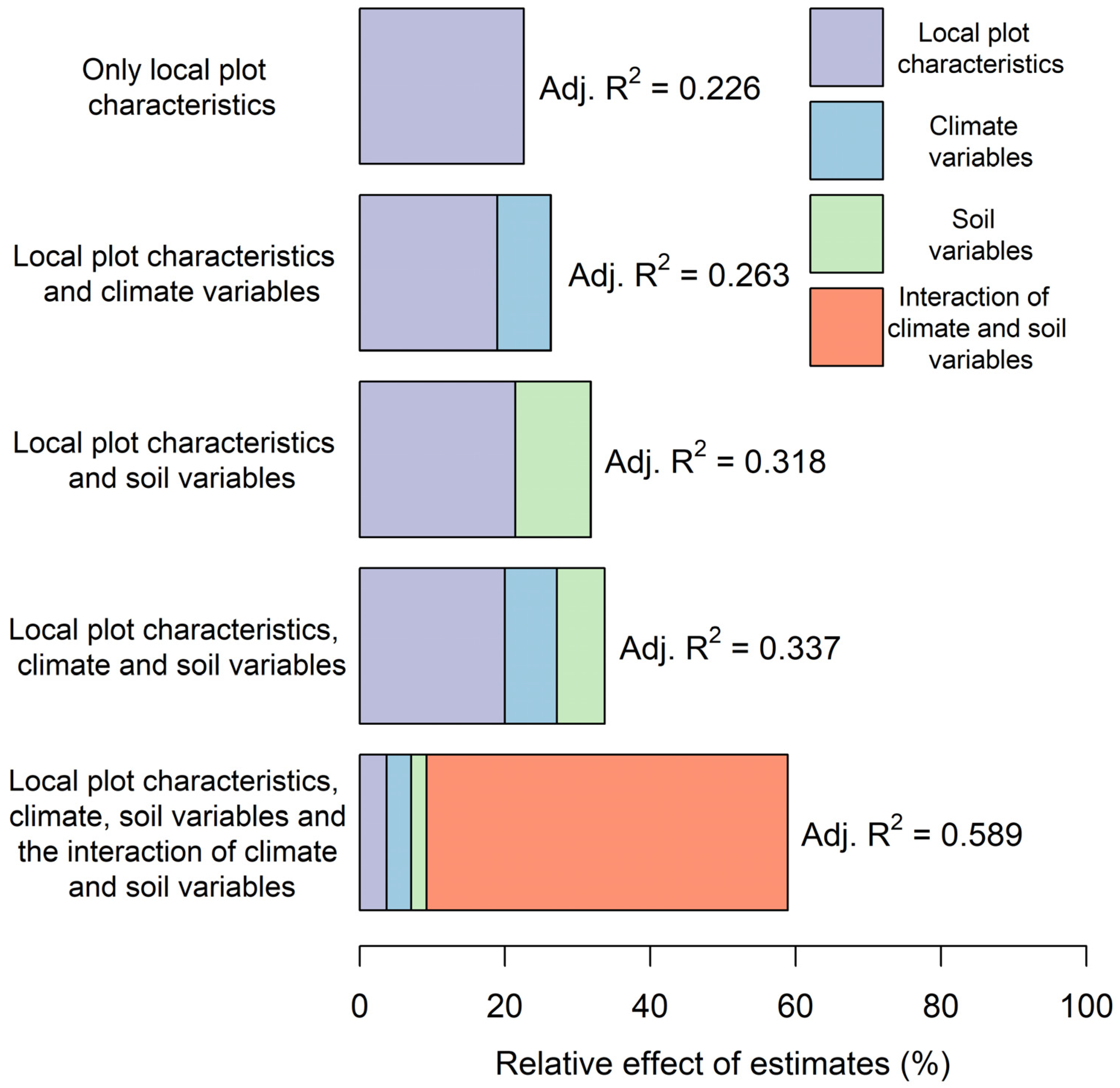
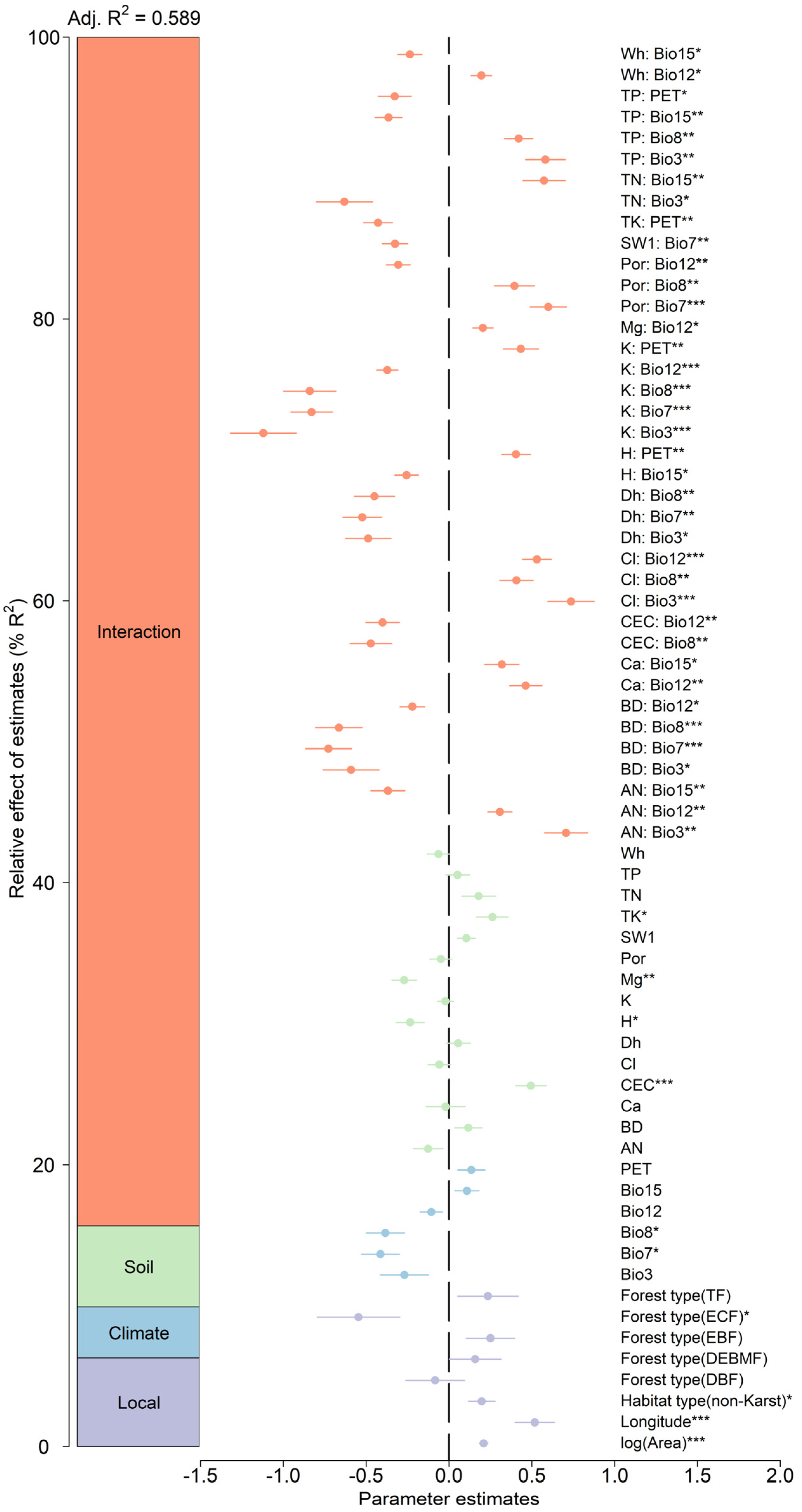
3. Results
3.1. Performance of Five Combinations of Predictors
3.2. Effects of Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Xue, K.; Xie, J.; Deng, Y.; Wu, L.; Cheng, X.; Fei, S.; Deng, S.; He, Z.; Van Nostrand, J.D.; et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Chang. 2012, 2, 106–110. [Google Scholar] [CrossRef]
- De Laender, F.; Rohr, J.R.; Ashauer, R.; Baird, D.J.; Berger, U.; Eisenhauer, N.; Grimm, V.; Hommen, U.; Maltby, L.; Meliàn, C.J.; et al. Reintroducing environmental change drivers in biodiversity-ecosystem functioning research. Trends Ecol. Evol. 2016, 31, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wan, S.; Piao, S.; Knapp, A.K.; Classen, A.T.; Vicca, S.; Ciais, P.; Hovenden, M.J.; Leuzinger, S.; Beier, C.; et al. A meta-analysis of 1119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat. Ecol. Evol. 2019, 3, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Schmid, B.; De Laender, F.; Eisenhauer, N.; Zhang, X.; Chen, H.; Craven, D.; De Boeck, H.J.; Hautier, Y.; Petchey, O.L.; et al. Biodiversity promotes ecosystem functioning despite environmental change. Ecol. Lett. 2021, 25, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar] [CrossRef]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Chen, S.; Mao, L.; Ouyang, Z. Drivers of β-diversity along latitudinal gradients revisited. Glob. Ecol. Biogeogr. 2013, 22, 659–670. [Google Scholar] [CrossRef]
- Smyčka, J.; Roquet, C.; Renaud, J.; Thuiller, W.; Zimmermann, N.; Lavergne, S. Disentangling drivers of plant endemism and diversification in the European Alps-a phylogenetic and spatially explicit approach. Perspect. Plant Ecol. Evol. Syst. 2017, 28, 19–27. [Google Scholar] [CrossRef]
- Tripathi, P.; Behera, M.D.; Roy, P.S. Spatial heterogeneity of climate explains plant richness distribution at the regional scale in India. PLoS ONE 2019, 14, e0218322. [Google Scholar] [CrossRef] [PubMed]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Schaepman, M.E.; et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef]
- Thomas, H.J.D. Environmental drivers of plant form and function. Nat. Ecol. Evol. 2022, 6, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Iozia, L.M.; Crisafulli, V.; Varone, L. Climatic variations along an aridity gradient drive significant trait intraspecific variability in Mediterranean plant species. J. Arid. Environ. 2023, 217, 105042. [Google Scholar] [CrossRef]
- Huang, E.; Chen, Y.; Fang, M.; Zheng, Y.; Yu, S. Environmental drivers of plant distributions at global and regional scales. Global Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar] [CrossRef]
- Huang, E.; Chen, Y.; Yu, S. Climate factors drive plant distributions at higher taxonomic scales and larger spatial scales. Front. Ecol. Evol. 2024, 11, 1233936. [Google Scholar] [CrossRef]
- Liang, M.; Baiser, B.; Hallett, L.M.; Hautier, Y.; Jiang, L.; Loreau, M.; Record, S.; Sokol, E.R.; Zarnetske, P.L.; Wang, S. Consistent stabilizing effects of plant diversity across spatial scales and climatic gradients. Nat. Ecol. Evol. 2022, 6, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Distribution, speciation, and bioaccumulation of potentially toxic element in the grey mangroves at India Sundarbans, in relation to vessel movement. Mar. Environ. Res. 2023, 189, 106042. [Google Scholar] [CrossRef]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Variations in soil blue carbon sequestration between natural mangrove metapopulations and a mixed mangrove plantation: A case study from the world’s largest contiguous mangrove forest. Life 2023, 13, 271. [Google Scholar] [CrossRef]
- Zhang, W.-P.; Fornara, D.; Yang, H.; Yu, R.-P.; Callaway, R.M.; Li, L. Plant litter strengthens positive biodiversity-ecosystem functioning relationships over time. Trends Ecol. Evol. 2023, 28, 473–484. [Google Scholar] [CrossRef]
- Trifković, V.; Bončina, A.; Ficko, A. Density-dependent mortality models for mono- and multi-species uneven-aged stands: The role of species mixture. For. Ecol. Manage. 2023, 545, 121260. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Bertrand, R.; Perez, V.; Gégout, J.C. Disregarding the edaphic dimension in species distribution models leads to the omission of crucial spatial information under climate change: The case of Quercus pubescens in France. Global Chang. Biol. 2012, 18, 2648–2660. [Google Scholar] [CrossRef]
- Dubuis, A.; Giovanettina, S.; Pellissier, L.; Pottier, J.; Vittoz, P.; Guisan, A. Improving the prediction of plant species distribution and community composition by adding edaphic to topo-climatic variables. J. Veg. Sci. 2013, 24, 593–606. [Google Scholar] [CrossRef]
- Scherrer, D.; Guisan, A. Ecological indicator values reveal missing predictors of species distributions. Sci. Rep. 2019, 9, 3061. [Google Scholar] [CrossRef] [PubMed]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Davies, S.J.; Chuyong, G.B.; Kenfack, D.; et al. Soil resources and topography shape local tree community structure in tropical forests. Proc. R. Soc. B 2012, 280, 20122532. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.M.S.; Scharfer, C.E.G.R.; Silva, J.O.; Ferreira Júnior, W.G.; dos Santos, R.M.; Neri, A.V. The influence of soil on vegetation structure and plant diversity in different tropical savannic and forest habitats. J. Plant Ecol. 2016, 11, 226–236. [Google Scholar] [CrossRef]
- Conti, L.; de Bello, F.; Lepš, J.; Acosta, A.T.R.; Carboni, M. Environmental gradients and micro-heterogeneity shape fine-scale plant community assembly on coastal dunes. J. Veg. Sci. 2017, 28, 762–773. [Google Scholar] [CrossRef]
- Walthert, L.; Meier, E.S. Tree species distribution in temperate forests is more influenced by soil than by climate. Ecol. Evol. 2017, 7, 9473–9484. [Google Scholar] [CrossRef]
- Buri, A.; Grand, S.; Yashiro, E.; Adatte, T.; Spangenberg, J.E.; Pinto-figueroa, E.; Verrecchia, E.; Guisan, A. What are the most crucial soil variables for predicting the distribution of mountain plant species? A comprehensive study in the Swiss Alps. J. Biogeogr. 2020, 47, 1143–1153. [Google Scholar] [CrossRef]
- Chauvier, Y.; Thuiller, W.; Brun, P.; Lavergne, S.; Descombes, P.; Karger, D.N.; Renaud, J.; Zimmermann, N.E. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 2021, 91, e01433. [Google Scholar] [CrossRef]
- Wiens, J.A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- McGill, B.J. Matters of scale. Science 2010, 328, 575–576. [Google Scholar] [CrossRef]
- Chang, L.-W.; Zelený, D.; Li, C.-F.; Chiu, S.-T.; Hsieh, C.-H. Better environmental data may reverse conclusions about niche- and dispersal-based processes in community assembly. Ecology 2013, 94, 2145–2151. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.P.; Usinowicz, J.; Levine, J.M. The spatial scales of species coexistence. Nat. Ecol. Evol. 2017, 1, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. Community diversity: Relative roles of local and regional processes. Science 1987, 235, 167–171. [Google Scholar] [CrossRef]
- White, E.P.; Hurlbert, A.H. The combined influence of the local environment and regional enrichment on bird species richness. Am. Nat. 2010, 175, E35–E43. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Mod, H.K.; Chevalier, M.; Luoto, M.; Guisan, A. Scale dependence of ecological assembly rules: Insights from empirical datasets and joint species distribution modelling. J. Ecol. 2020, 108, 1967–1977. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Jiménez, I.; Jørgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef]
- Xing, D.; He, F. Environmental filtering explains a U-shape latitudinal pattern in regional β-deviation for eastern North American trees. Ecol. Lett. 2019, 22, 284–291. [Google Scholar] [CrossRef]
- Zhang, C.; He, F.; Zhang, Z.; Zhao, X.; von Gadow, K. Latitudinal gradients and ecological drivers of β-diversity vary across spatial scales in a temperate forest region. Global Ecol. Biogeogr. 2020, 29, 1257–1264. [Google Scholar] [CrossRef]
- Veryard, R.; Wu, J.; O’Brien, M.J.; Anthony, R.; Both, S.; Burslem, D.F.R.P.; Chen, B.; Cagigal, E.F.M.; Godfray, H.C.J.; Godoong, E.; et al. Positive effects of tree diversity on tropical forest restoration in a field-scale experiment. Sci. Adv. 2023, 9, eadf0938. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P.; Zhu, H. The tropical-subtropical evergreen forest transition in east Asia: An exploration. Plant Divers. 2020, 42, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Brummitt, N.; Araújo, A.C.; Harris, T. Areas of plant diversity-What do we know? Plant People Planet 2021, 3, 33–44. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsson region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef]
- Su, Z.; Li, X.; Ding, T.; Ning, S.; Chen, W.; Mo, X. The Vegetation of Guangxi; China Forestry Publishing House: Beijing, China, 2014; ISBN 9787503876257. [Google Scholar]
- Wang, X.; Guo, K.; Wen, Y. Vegetation of Guangxi (2 Volumes); Higher Education Press: Beijing, China, 2014; ISBN 978-7-04-041564-3. [Google Scholar]
- Zheng, W.; Zeng, W.; Tang, Y.; Shi, W.; Cao, K. Species diversity and biogeographical patterns of Lauraceae and Fagaceae in northern tropical and subtropical regions of China. Acta Ecol. Sin. 2018, 34, 8676–8687. [Google Scholar] [CrossRef]
- Tang, Z.; Qiao, X.; Fang, J. Species-area relationship in biological communities. Biodivers. Sci. 2009, 17, 549–559. [Google Scholar] [CrossRef]
- Ricklefs, E.R.; He, F. Region effects influence local tree species diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, W.; Dai, Y.; Liu, B.; Zhu, A.; Duan, Q.; Wu, L.; Ji, D.; Ye, A.; Yuan, H.; Zhang, Q.; et al. A China Dataset of Soil Properties for Land Surface Modeling. J. Adv. Model. Earth Syst. 2013, 5, 212–224. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.-Q.; Xiang, W.-S.; Li, X.-K.; Cao, K.-F. Environmental filtering and dispersal limitation jointly shaped the taxonomic and phylogenetic beta diversity of natural forests in southern China. Ecol. Evol. 2021, 11, 8783–8794. [Google Scholar] [CrossRef]
- Xu, T.; Hutchinson, M.F. ANUCLIM, Version 6.1; Fenner School of Environment and Society, Australian National University: Acton, Australia, 2011. [Google Scholar]
- Xu, T.; Hutchinson, M.F. New developments and applications in the ANUCLIM spatial climatic and bioclimatic modelling package. Environ. Modell. Softw. 2013, 40, 267–279. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Cao, B.; Bai, C.; Zhang, M.; Lu, Y.; Gao, P.; Yang, J.; Xue, Y.; Li, G. Future landscape of renewable fuel resources: Current and future conservation and utilization of main biofuel crops in China. Sci. Total Environ. 2022, 806, 150946. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yuan, X.; Sun, Y.; Liu, Y. Species distribution modeling based on MaxEnt to inform biodiversity conservation in the central urban area of Chongqing municipality. Ecol. Indic. 2024, 158, 111491. [Google Scholar] [CrossRef]
- Le Provost, G.; Badenhausser, I.; Le Bagousse-Pinguet, Y.; Clough, Y.; Henckel, L.; Violle, C.; Bretagnolle, V.; Roncoroni, M.; Manning, P.; Gross, N. Land-use history impacts functional diversity across multiple trophic groups. Proc. Natl. Acad. Sci. USA 2020, 117, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 0132. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Gross, N.; Baillod, A.B.; Bertrand, C.; Carrié, R.; Hass, R.; Henckel, L.; Miguet, P.; Vuillot, C.; Alignier, A.; et al. Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. USA 2019, 116, 16442–16447. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lutz, J.A.; Král, K.; Vrška, T.; Yin, X.; Myers, J.A.; Abiem, I.; Alonso, A.L.; Bourg, N.; Burslem, D.F.R.P.; et al. Direct and indirect effects of climate on richness drive the latitudinal diversity gradient in forest trees. Ecol. Lett. 2018, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; van der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystem. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef]
- Coelho, M.T.P.; Barreto, E.; Rangel, T.F.; Diniz-Filho, J.A.F.; Wüest, R.O.; Bach, W.; Skeels, A.; McFadden, I.R.; Roberts, D.W.; Pellissier, L.; et al. The geography of climate and the global patterns of species diversity. Nature 2023, 622, 537–544. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, F.; Wu, Q.; Heděnec, P.; Peng, Y.; Li, Z.; Yuan, C.; Zhao, Z.; Jin, X.; Tan, S.; et al. Climate and soil properties regulate the initial concentrations of potassium, calcium and magnesium in plant litter on a global scale. Funct. Ecol. 2024, 38, 1378–1390. [Google Scholar] [CrossRef]
- Tuomisto, H.; Zuquim, G.; Cárdenas, G. Species richness and diversity along edaphic and climatic gradients in Amazonia. Ecography 2014, 37, 1034–1046. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, W.P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Simpson, A.H.; Richardson, S.J.; Laughlin, D.C. Soil-climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Global Ecol. Biogeogr. 2016, 25, 964–978. [Google Scholar] [CrossRef]
- Pennington, V.E.; Palmquist, K.A.; Bradford, J.B.; Lauenroth, W.K. Climate and soil texture influence patterns of forb species richness and composition in big sagebrush plant communities across their spatial extent in the western U.S. Plant Ecolog. 2017, 218, 957–970. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Bison, M.; Duparc, A.; Garel, M.; Loison, A.; Renaud, J.; Poggiato, G. Combining point-process and landscape vegetation models to predict large herbivore distributions in space and time—A case study of Rupicapra rupicapra. Divers. Distrib. 2018, 24, 352–362. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 3, 315–323. [Google Scholar] [CrossRef]
- Schuur, E.A.; Matson, P.A. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 2001, 128, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zhang, S.; Chen, H.; Li, T.; Zhang, C.; Xu, X.; Mao, Z.; Gong, G.; Deng, O.; Deng, L. The influence of climate, topography, parent material and vegetation on soil nitrogen fractions. CATENA 2019, 175, 329–338. [Google Scholar] [CrossRef]
- Teuling, A.J.; Hupet, F.; Uijlenhoet, R.; Troch, P.A. Climate variability effects on spatial soil moisture dynamics. Geophys. Res. Lett. 2007, 34, L06406. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture-climate interactions in a changing climate: A review. Earth Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Palpurina, S.; Wagner, V.; von Wehrden, H.; Hájek, M.; Horsák, M.; Brinkert, A.; Hölzel, N.; Wesche, K.; Kamp, J.; Hájková, P.; et al. The relationship between plant species richness and soil pH vanishes with increasing aridity across Eurasian dry grasslands. Global Ecol. Biogeogr. 2016, 26, 425–434. [Google Scholar] [CrossRef]
- Ding, J.; Eldridge, D.J. Climate and plants regulate the spatial variation in soil multifunctionality across a climatic gradient. CATENA 2021, 201, 105233. [Google Scholar] [CrossRef]
- García-García, A.; Cuesta-Valero, F.J.; Miralles, D.G.; Mahecha, M.D.; Quaas, J.; Reichstein, M.; Zscheischler, J.; Peng, J. Soil heat extremes can outpace air temperature extremes. Nat. Clim. Chang. 2023, 13, 1237–1241. [Google Scholar] [CrossRef]
- Cong, W.; van Ruijven, J.; Mommer, L.; de Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Eisenhauer, N.; Ferlian, O.; Zhang, J.; Zhou, G.; Lu, X.; Liu, C.; Zhang, D. Species richness promotes ecosystem carbon storage: Evidence from biodiversity-ecosystem functioning experiments. Proc. R. Soc. B 2020, 287, 20202063. [Google Scholar] [CrossRef]
- Spohn, M.; Bagchi, S.; Biederman, L.A.; Borer, E.T.; Bråthen, K.A.; Bugalho, M.N.; Caldeira, M.C.; Catford, J.A.; Collins, S.L.; Eisenhauer, N. The positive effect of plant diversity on soil carbon depends on climate. Nat. Commun. 2023, 14, 6624. [Google Scholar] [CrossRef]
- Kirschbaum, M.U. Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry 2000, 48, 21–51. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Wang, Y.-P.; Vitousek, P.M.; Field, C.B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 2008, 454, 327–330. [Google Scholar] [CrossRef]
- Larsen, M.J.; Jurgensen, M.F.; Harvey, A.E. N2-fixation associated with wood decayed by some common fungi in western Montana. Can. J. For. Res. 1978, 8, 341–345. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol. Biochem. 2013, 61, 69–75. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Bales, R.C.; Hopmans, J.W.; O’Geen, A.T.; Meadows, M.; Hartsough, P.C.; Kirchner, P.; Hunsaker, C.T.; Beaudette, D. Soil moisture response to snowmelt and rainfall in a Sierra Nevada mixed-conifer forest. Vadose Zone J. 2011, 10, 786–799. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats: Vegetation Ecology of Central Europe; Springer: Berlin, Germany, 2017; Volume 2, ISBN 978-3-319-43046-1. [Google Scholar]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.; Kitajima, K. Plant ecology of tropical and subtropical karst ecosystems. Biotropica 2019, 51, 626–640. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Hu, G.; Zhu, J.-D.; Luo, D.-H.; Ni, J. Spatial patterns and interspecific associations of dominant tree species in two old-growth karst forests, SW China. Ecol. Res. 2010, 25, 1151–1160. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Hu, G.; Ni, J. Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, southwestern China. J. Mt. Sci. 2013, 10, 95–104. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Mallik, A.U.; Huang, F.; Xiang, W.; Ding, T.; Wen, S.; Lu, D.; Li, D.; He, H.; et al. Topographic species–habitat associations of tree species in a heterogeneous tropical karst seasonal rain forest, China. J. Plant Ecol. 2017, 10, 450–460. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, K.; Zhang, B.; Chen, Z.; Jiao, Q.; Liu, B.; Chen, H. Exploring the relationship between vegetation spectra and eco-geo-environmental conditions in karst region, Southwest China. Environ. Monit. Assess. 2010, 160, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Jin, Y.; Ricklefs, R. Phylogenetic diversity anomaly in angiosperms between eastern Asia and eastern North America. Proc. Natl. Acad. Sci. USA 2017, 114, 11452–11457. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Ricklefs, R.E. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 2000, 407, 180–182. [Google Scholar] [CrossRef]
- Qian, H. Environmental determinants of woody plant diversity at a regional scale in China. PLoS ONE 2013, 8, e75832. [Google Scholar] [CrossRef]
- Zhu, H. The tropical forests of southern China and conservation of biodiversity. Bot. Rev. 2017, 83, 87–105. [Google Scholar] [CrossRef]
- O’ Brien, E.M. Climatic gradients in woody plant species richness: Towards an explanation based on an analysis of southern Africa’s woody flora. J. Biogeogr. 1993, 20, 181–198. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, Y.; Hu, W.; Lai, J. Application of “rdacca.hp” R package in ecological data analysis: Case and progress. Chin. J. Plant. Ecol. 2023, 47, 134–144. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 027–046. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Cornell, H. Toward a better understanding of the regional causes of local community richness. Ecol. Lett. 2008, 11, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Weiher, E.; Clarke, G.D.P.; Keddy, P.A. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 1998, 81, 309–322. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Maire, V.; Gross, N.; Börger, L.; Proulx, R.; Wirth, C.; Pontes, L.D.S.; Soussana, J.-F.; Louault, F. Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol. 2012, 196, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Q.; Sui, X.; Li, B.; He, F.; Chu, C. The effects of habitat filtering and non-habitat processes on species spatial distribution vary across life stages. Am. J. Bot. 2018, 105, 1469–1476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Zeng, W.; Aritsara, A.N.A.; Yi, Y.; Zhu, S.; Cao, K. The Interaction between Climate and Soil Properties Influences Tree Species Richness in Tropical and Subtropical Forests of Southern China. Forests 2024, 15, 1441. https://doi.org/10.3390/f15081441
Shi W, Zeng W, Aritsara ANA, Yi Y, Zhu S, Cao K. The Interaction between Climate and Soil Properties Influences Tree Species Richness in Tropical and Subtropical Forests of Southern China. Forests. 2024; 15(8):1441. https://doi.org/10.3390/f15081441
Chicago/Turabian StyleShi, Wei, Wenhao Zeng, Amy Ny Aina Aritsara, Yin Yi, Shidan Zhu, and Kunfang Cao. 2024. "The Interaction between Climate and Soil Properties Influences Tree Species Richness in Tropical and Subtropical Forests of Southern China" Forests 15, no. 8: 1441. https://doi.org/10.3390/f15081441
APA StyleShi, W., Zeng, W., Aritsara, A. N. A., Yi, Y., Zhu, S., & Cao, K. (2024). The Interaction between Climate and Soil Properties Influences Tree Species Richness in Tropical and Subtropical Forests of Southern China. Forests, 15(8), 1441. https://doi.org/10.3390/f15081441




