Abstract
A greenhouse experiment was conducted to determine the potential of arbuscular mycorrhizal fungi (AMF) to mitigate the allelopathic effects of invasive Nicotiana glauca on the growth of Acacia gerrardii seedlings. Aqueous extracts from N. glauca leaves and flowers (at concentrations of 20, 60, and 100 g/L) and AMF treatment (±AMF) were applied to A. gerrardii seedlings arranged in a factorial experiment with four replicates. After 6 months, vegetative (plant fresh and dry weight, height, leaf number area), physiological (photosynthesis, stomatal conductance, transpiration rate), chlorophyll a and b, proline, and root (length, surface area, diameter, volume, tip number) parameters as well as root distribution in five root diameter classes (0–5 mm, in 1 mm increments) were examined. The presence of AMF increased the seedling growth parameters. The 20 g/L treatment stimulated most of the vegetative growth, root, chlorophyll, and photosynthesis parameters at both inoculant levels for all extract types and concentrations, and the 100 g/L treatment inhibited seedling growth whether inoculated with AMF or not. This decline was alleviated in AMF-treated seedlings. For both extract sources, root length decreased as extract concentration increased, and roots <1 mm accounted for the greatest proportion of total root length, surface area, and tip number, with higher values observed in AMF-treated seedlings compared to their untreated counterparts. Further research is required at the field level to identify AMF strains and their interaction effects in alleviating the allelopathic impact of N. glauca on A. gerrardii seedling growth.
1. Introduction
Nicotiana glauca Graham, which is also called tree tobacco, is an invasive plant that grows in semi-arid regions in different parts of the world [1]. N. glauca is a broad-based, fast-growing shrub native to northwest Argentina and Bolivia and widely adapted in warm ecological regions, and has extended across the world as a result of human activity [2]. This shrub has a great ability to naturalize and shows invasive behavior in arid and semi-arid environments [3]. Invasive plants not only threaten diversity and plant structure but also invade forests, rangelands, and inhabited areas. In Saudi Arabia, invasive plants such as N. glauca have profound effects on ecosystems, where they remove or dislocate many native plants (such as wild and native Acacia gerrardii, also known as Vachellia gerrardii (Benth.) P.J.H. Hurter) from their predominant habitats [1]. The A. gerrardii Benth tree is the most important wild native tree in Saudi Arabia because it provides nectar for honey bees as well as gums and tannins [4]. In Saudi Arabia, A. gerrardii trees are exposed to great pressure from humans as well as from invasive plants in general and invasive N. glauca in particular. In addition to human alterations imposed in forests and rangelands, biotic and abiotic stresses may also play critical roles in decreasing the number of acacia trees in the area [5].
N. glauca has been reported to produce poisonous and allelopathic compounds from the root and vegetative parts via a mechanism called allelopathy [6]. Allelopathy is a chemical process that increases a plant’s competitive power over a limited number of resources. According to the novel weapons hypothesis (NWH), invasive plants compete with native plants using various strategies. Among their strategies is allelochemical activity, which includes the use of chemicals as a weapon to dominate and compete. These allelochemical compounds can destroy indigenous plant species and facilitate colonization by invasive plants in a new environment [7]. Allelochemicals and phenolic compounds are considered to be the most important groups participating in allelopathy. According to [8], allelopathic substances affect the growth of individual plants, community structures, and plant invasion regimes. There is evidence suggesting that invasive plants interfere with native plants through the allelopathic process. The compounds produced by invasive plants may pave the way for new invasions and become a major tool for invading new habitats [9]. The compounds released from the different parts of the invasive N. glauca, mainly leaves, shoots, flowers, and roots, can have a negative impact on co-existing plant species [10]. These negative effects are more harmful to native plants and may be due to their lower resistance and possibly not having evolved any method of protection against these chemicals [11].
Allelopathic compounds can act indirectly by impacting soil microorganisms and suppressing beneficial mutualist fungi (such as AMF) or supporting antagonists of other plant species [12]. It is well known that AMF are very important to plants, as more than 80% of the plants on Earth form a symbiotic relationship with AMF [13]. AMF provide critical benefits to the plants with which they are associated. These benefits include increasing water and nutrient uptake, improving resistance to biotic and abiotic stresses by enhancing the antioxidant defense system of the recipient plant and protecting plants from chemical compounds [14]. Numerous studies have indicated that applying AMF affects the amount and concentration of phenolic compounds in plant root exudates [15]. Root colonization by AMF can increase plant resistance by prompting changes in host root exudates. For example, it was reported that AMF affect allelochemicals released from the roots of maize plants, whereby the released exudates of maize can inhibit the seedling growth of recipient plants, and AMF inoculants can alleviate this condition. After inoculation with AMF, the allelopathy indexes for the effect of maize root exudates on the growth of five vegetable crops decreased by 60.99% (radish), 70.19% (cucumber), 80.83% (lettuce), 36.26% (pepper), and 57.15% (ryegrass) [16]. Furthermore, AMF-associated plants are found to be more competitive and more capable of resisting biotic and abiotic stresses than plants that are not colonized or less colonized by AMF [17]. For example, extracts from invasive Alliaria petiolata had a strong inhibitory effect on the growth of Iris pallida, while the presence of AMF reduced the inhibitory effect [18]. Although AMF can cause changes in the root exudates and allelochemicals of plants, the released allelochemicals and allelopathic substances can also impact the growth and abundance of AMF and their spores in the plant rhizosphere [19]. For example, invasive Alliaria petiolata was found to inhibit AMF by producing allelopathic compounds that are toxic to AMF [20]. These invasive plants were found to have a generalist response to fungal symbionts, leading these species to successfully settle and establish seedlings [21]. In general, allelopathic compounds produced by invasive plants can impact rhizosphere microbes, and these microbes can also, to a certain degree, impact the plant allelopathic materials released in the soil [22]. Therefore, determining the relationship between allelopathic substances and soil fungi such as AMF is important in investigating allelopathy in invasive plants.
The potential of AMF to mitigate the allelopathic impact of invasive plants has received much research attention [16,23]. However, to our knowledge and based on the published literature, no information regarding the role of AMF in alleviating the allelopathic impact of invasive N. glauca on the growth of native A. gerrardii has been reported. We suppose that the presence or absence of AMF positively or negatively influences the survival and establishment of invasive plants that have been introduced in new habitats. Therefore, the aim of this study was to evaluate the allelopathic potential of extracts of aqueous leaves and flowers of invasive N. glauca on the growth of native A. gerrardii seedlings with and without AMF, as well as to explore the role of AMF in mitigating the effects of these substances on A. gerrardii seedling growth. This is the first trial to assess the effect of AMF and their interactions with allelopathic extracts of invasive N. glauca on A. gerrardii seedling growth.
2. Materials and Methods
2.1. Collection of A. gerrardii and N. glauca Materials
Wild A. gerrardii Benth trees and invasive N. glauca Graham shrubs grow in different parts of Saudi Arabia (particularly the southwest region). Viable seeds of A. gerrardi trees were collected from healthy growing plants in Saudi Arabia, Al-Aqee and Baljurashi (Al-Baha area; 19°39′48″ N, 41°21′45″ E) during October–December 2022. The seeds were gathered from healthy mature trees that naturally occurred in Al-Baha wadies. At the same sites, soil samples containing fine roots of A. gerrardi were collected beneath A. gerrardii and N. glauca rhizospheres and outside the plant canopy to determine the content of AMF. In addition, leaves and flowers of invasive N. glauca shrubs growing at the same sites were collected. The reason for using leaves and flowers in this experiment is that they contain higher amounts of inhibitory substances than other parts. Also, N. glauca produces an abundance of leaves and flowers, and these parts fall to the ground and decompose. Thus, allelopathic substances accumulate in forest areas, which can inhibit the germination and growth of acacia seedlings. The leaves and flowers were sun- and air-dried, then transported to the forestry laboratory of the Department of Plant Production, College of Food and Agriculture Sciences, King Saud University, for use in the experiment. The experiment was conducted in the greenhouse.
2.2. Inoculum Propagation
The collected soil was checked for the presence of AMF and spores, then propagated in a trap culture with sorghum (Sorghum bicolor). Original inocula were taken from the soil with fine roots collected beneath A. gerrardii and invasive N. glauca rhizospheres. The fine soil that contained AMF spores (100 spores/10 g soil) and fine roots was mixed with autoclaved sandy soil. Then, AMF inocula were grown in small pots (18 cm diameter, 30 cm height) with the fast-growing host plant species (Sorghum bicolor). Pots were irrigated with tap water as needed for 12 weeks until the host plants were well developed and ready to use as inocula in the experiment [24].
2.3. Aqueous Extract Preparation and Growth Conditions
Aqueous extract was prepared from the leaves and flowers of invasive N. glauca. The dried leaves and flowers were crushed separately using a grinding tool and then dipped separately in distilled water at concentrations of 20, 60, and 100 g/L. Tap water (0 g/L) was used as a control treatment. The suspension was refrigerated for 1 day. Then, the aqueous extracts were repeatedly filtered to eliminate fiber particles. Whatman filter paper No. 1 was used to filtrate the resulting supernatant. The final stock of N. glauca aqueous extract was refrigerated at 4 °C for continuous use in the experiment [25]. AMF treatment was applied to the pots (tubes) before seed germination: AMF inoculum (25 g inoculated soil containing about 250 AMF spores) was added to the top layer of soil (3 cm depth) in the pots. Thus, seedlings were established in the presence and absence of AMF. To establish A. gerrardii seedlings, 4 seeds of A. gerrardii were sown in each pot (16 cm diameter, 50 cm height) filled with a mixture of autoclaved sand and silt soil (3:1), with 4 replicates per treatment. The physical and chemical characteristics of the soil used in this experiment are presented in Table 1. Pots were irrigated as needed until seedlings germinated. After 7 days, germinated seedlings were thinned into 2 seedlings per pot. The aqueous extracts (20, 60, and 100 g/L) previously applied to each part were applied to seedlings separately after the emergence of true leaves in the amount of 250 mL/pot per week. Tap water (0 g/L) was used as a control. Hoagland Nutrient Solution (without phosphorus) was added monthly during the seedling growth stage. The experiment consisted of 2 aqueous extracts of plant parts (leaves and flowers) × 4 extraction concentrations × ± AMF × 4 replicates. All tubes (64) were placed in greenhouse conditions (30 °C, natural daylight). The experiment was arranged as a factorial experiment in a split-spit plot design for 6 months.

Table 1.
Pre-analysis of soil properties used in the experiment.
2.4. Status of AMF Colonization in Roots of A. gerrardii Seedlings
AMF were checked in root samples (25 g) from each treatment using the method described by [26] and modified by [27]. The roots were cleaned carefully in a pot using tap water to remove adhering soil, then cleaned with 10% KOH (Sigma-Aldrich, Taufkirchen, Germany) and stained with trypan blue in lactophenol as described by [28]. The stained root segments were tested using an optical microscope at 20 × magnification. Infection by AMF (mycelial, vesicular, arbuscular) in roots was calculated using the following formula:
2.5. Extraction and Identification of AMF Spores
After harvesting, AMF spores were extracted from each treatment using a wet sieving and decanting procedure [29]. Based on this method, a 100 g soil sample from each treatment was taken, air-dried, sieved, and placed in a bucket (1500 mL capacity), then 1000 mL of water was added. The soil sample was shaken and mixed with water to make a suspension. The soil–water suspension was left for 3 to 5 min. After that, the suspension was gradually sieved using sieves (ASTM-60, ASTM-100, and ASTM-240; Gilson Company, Lewis Center, OH, USA) for spore extraction [30]. The sieved supernatants were tested under the microscope to check for and observe AMF spores. The spore suspension was filtered using Whatman filter paper No. 1 (to enable easy counting). After filtration, the filter papers were examined under the microscope at 25 × magnification, and the number of spores was counted. Total spore number was computed for each sample per 100 g of dry soil. AMF spores and species were identified based on their morphological appearance under the microscope.
2.6. Parameter Measurements
2.6.1. Physiological Parameters
- Chlorophyll content: To measure chlorophyll content, samples of fresh leaves (0.5 g) were harvested (from different heights on the seedlings) and weighed on a digital balance scale. Leaf samples were taken after 4, 5, and 6 months of seedling growth. The leaf samples were crushed using a mortar and pestle and placed in glass tubes containing 5 mL of dimethyl formamide, then left for a day (24 h) at room temperature. The extracts were then filtered using Whatman filter paper No. 1. The filtrate was placed in a spectrophotometer cuvette (Shimadzu UV1800, Kyoto, Japan), and the absorption was read at 664 and 620 nm to determine chlorophyll a and b content, respectively [30].
- Total phenolic content: Total phenolic content in the leaves and flowers of invasive N. glauca used for extracts was identified in our previous work [31] following Ainsworth’s procedure using Folin–Ciocalteu reagent (Merck KGaA, Darmstadt, Germany) [32]. The extracts of leaf and flower samples (0.5 mL of 100 μg/mL) were mixed separately with 2 mL Folin–Ciocalteu reagent (diluted 1:10 with deionized water) and 4 mL liquid Na2CO3 (7.5% w/v). The resulting mixture was kept in an incubator for 30 min at room temperature. The absorbance was read at 765 nm by the spectrophotometer (Shimadzu UV1800, Kyoto, Japan). The equation of a standard curve produced with gallic acid was used to identify the final total phenolic content [32].
- Proline accumulation: To measure proline content, 0.5 g of leaf sample (from different heights of the plant) was immersed in 10 mL of 3% sulfosalicylic acid using a mortar and pestle. Samples were centrifuged at 3000× g for 10 minutes, and the resulting supernatant (2 mL) was mixed with glacial acetic acid and ninhydrin (2 mL) reagent (Sigma-Aldrich, Taufkirchen, Germany) and then incubated at 100 °C for an hour. The extract was incubated in a hot bath (100 °C) for 1 h, followed by an ice bath. After that, 4 mL of toluene was added to separate the proline, and the accumulated extract containing toluene was measured at 520 nm [33].
- Photosynthesis, stomatal conductance, and transpiration rate: Parameters of leaf gas exchange, net photosynthesis rate (Pn), stomatal conductance (C), and transpiration rate (E) were estimated using a portable handheld photosynthesis device (model CI-340, CID Bio-Science, Camas, WA, USA). These parameters were measured 3 months after applying aqueous extracts and AMF treatments. Monthly measurements were made of leaves located in the middle part of the plant between 10:00 and 12:00 on sunny days.
2.6.2. Growth Parameters
- Above-ground system: Plant height was measured from the cotyledon scars to the stem apex using a ruler. Leaf area (cm2) was measured using a portable laser leaf area meter (CI-202). Plant fresh and dry weight (g) were measured. To obtain fresh weight, whole plants were weighed on a digital balance scale. Plants were then dried at 70 °C for 48 h to obtain the dry weight. All parameters were measured at the end of the experiment.
- Root parameters: To measure length, area (cm2), volume, (cm3), and tip number, at the end of the experiment and after harvesting, seedling roots were cut and separated from the vegetative part and washed gently with tap water to remove adhered soil particles. Then, the seedling roots were dyed red. Later, roots were spread gently on the glass plate of a computer scanner for imaging and scanned at 600 dpi per inch. The images were saved in TIFF format and analyzed on a PC using WinRHIZO software (Regent Instruments Inc., Quebec City, QC, Canada) to identify total root length [25], total root surface area (cm2), root diameter (mm), and root tip number. The distributions of root length, root surface area, and root tips were measured in five diameter classes: 0–1, 1–2, 2–3, 3–4, and 4–5 mm.
2.7. Experimental Design and Statistical Analysis
The experiment was set up as a factorial experiment arranged in a split-split plot design encompassing 64 tubes. All other conditions and practices during the experimental period were kept constant. The data were arranged and subjected to analysis of variance [34], and means were separated at p < 0.05 using the least significant difference (LSD) test. Relationships between treatments (AMF, extract, and concentration) and studied parameters were determined using hierarchical clustered heat map analysis. Principle component analysis (PCA) of studied traits and variables was carried out based on the correlation matrix. Statistical analysis was conducted using Statistix 10.0 and JMP Pro. 16 software. Excel software was also used to produce graphs.
3. Results
3.1. AMF Colonization (%) in Roots of A. gerrarrdii Seedlings
All seedlings of A. gerrardii that were established in non-inoculated soil grown with all extract sources and concentrations were not infected with AMF during the experiment. However, AMF-treated seedlings showed clear AMF infection in the roots (Figure 1). Both aqueous extract source (leaf or flower) and concentration affected the percentage of AMF architecture colonization (mycelial, arbuscular, vesical) (Figure 2A–C). For the leaf aqueous extract, higher concentrations significantly reduced mycelial and arbuscular AMF (Figure 2A,B); however, the 20 g/L treatment stimulated arbuscular abundance (Figure 2B). For the flower aqueous extract, the 20 and 60 g/L treatments led to higher mycelial infection rates, while arbuscular was stimulated at 20 g/L and decreased at 60 and 100 g/L (Figure 2A,B). There was no significant effect (p < 0.05) of extract type or concentration on vesicular abundance in the roots of A. gerrardii seedlings (Figure 2C).
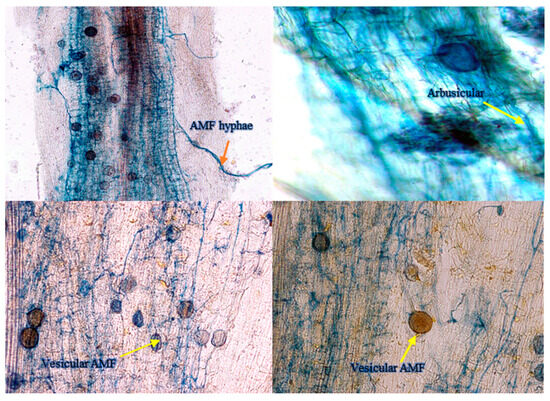
Figure 1.
AMF (mycelial, arbuscular, vesicular) colonization in roots of A. gerrardii seedlings grown with aqueous extracts of invasive N. glauca. Scale bar: 100 µm.
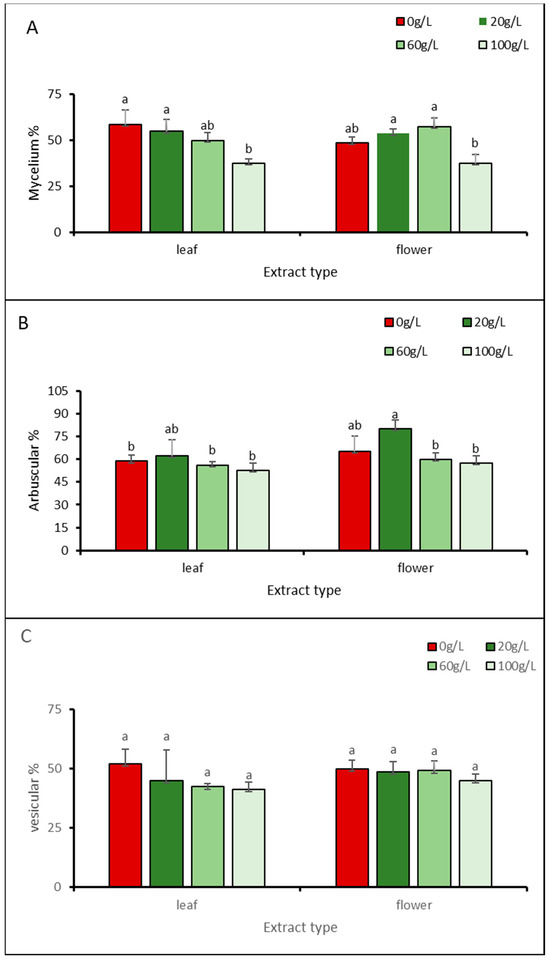
Figure 2.
Percentage of AMF infection on roots of A. gerrardii seedlings grown with different concentrations of allelopathic aqueous extracts of invasive N. glauca by architecture: (A) mycelial, (B) arbuscular, (C) vesicular. Different letters above columns indicate significant differences between means across treatments according to the least significant difference (LSD) test at p < 0.05.
In general, for the leaf aqueous extract, the highest mycelium colonization percentage was 58.7%, which was observed with the 0 g/L concentration, while the lowest was 37.5%, observed with the 100 g/L concentration. For the flower aqueous extract, the highest and lowest colonization rates were 57.5% and 37.5%, recorded with the 60 and 100 g/L concentrations, respectively (Figure 2A).
The highest values for arbuscular AMF on seedlings were recorded with the 20 g/L concentration of leaf and flower aqueous extracts at 62.5% and 80%, respectively. The lowest values were recorded with the 100 g/L treatment, at 52.5% (leaf) and 57.5% (flower) (Figure 2B).
For vesicular AMF, seedlings irrigated with 0 g/L showed the highest values with leaf (52%) and flower (50%) aqueous extracts, while seedlings treated with 100 g/L showed the lowest values (41.2% and 45%, respectively) (Figure 2C).
3.2. Spore Density
Several AMF spores were identified in the soil of A. gerrardii seedlings grown with extracts of invasive N. glauca. The identified species included Glomus ambisporum, Glomus ambisporum, Rhizophagus fasciculatus, Claroideoglomus eutinicatum, Glomus sp., Clomus eutinicatum, and Rhizophagus intraradices (Figure 3).
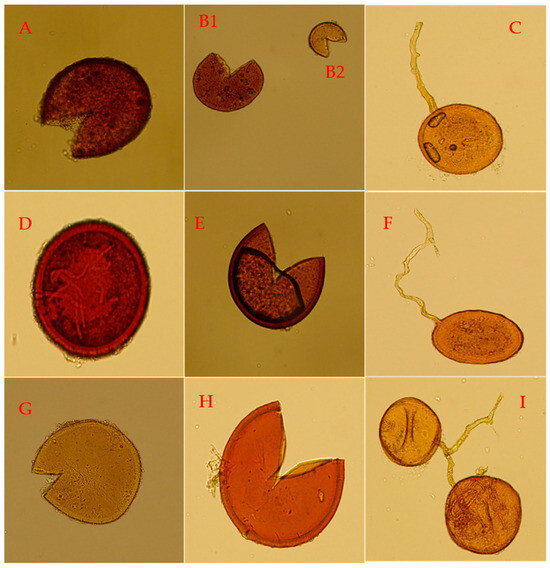
Figure 3.
AMF spores isolated from soil around A. gerrardii irrigated with leaf and flower aqueous extract of invasive N. glauca: (A) Glomus ambisporum, (B1) Glomus ambisporum, (B2) Claroideoglomus eutinicatum, (C) Claroideoglomus eutinicatum, (D) Rhizophagus fasciculatus, (E) Glomus ambisporum, (F) Claroideoglomus eutinicatum, (G) Glomus sp., (H) D. globifera, (I) Rhizophagus intraradices. Scale bar: 100 µm.
The concentration of aqueous extracts had a significant impact on the abundance of AMF spores (p = 0.0001). However, there was no significant interaction (p = 0.09) between the extract source and concentration, meaning that the extract sources had a similar impact on the number of AMF spores. The greatest number of spores was recorded in seedlings irrigated with tap water (0 g/L), followed by 20, 60, and 100 g/L treatments with both extract sources. For leaf aqueous extract, seedlings irrigated with 0 g/L (control) showed the highest spore number (145 100 g−1 soil), which was significantly different compared to 20, 60, and 100 g/L treatments. The lowest number of AMF spores (94.5 100 g−1 soil) was recorded in the soil of seedlings irrigated with 100 g/L, which was significantly different compared to 20 and 60 g/L. Similarly, with flower aqueous extract, higher AMF spore numbers were recorded in the soil of seedlings irrigated with 0 g/L (control) and lower numbers in the soil of seedlings irrigated with 100 g/L. It is obviously clear that for both extract sources when the extract concentration is increased, the number of AMF spores significantly decreases (Figure 4).
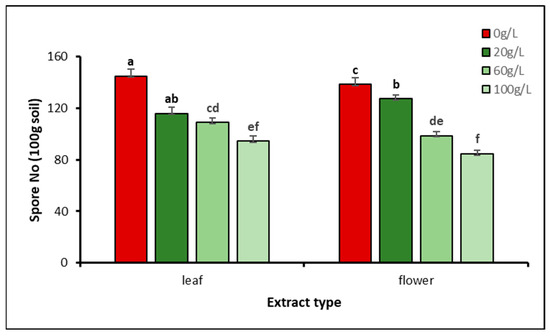
Figure 4.
Effect of aqueous extract source and concentration on AMF spore density in the soil around A. gerrardii. Lowercase letters above columns represent significant differences at p < 0.05 (LSD test).
3.3. Effects of AMF, Extract Source, and Concentration on Seedling Fresh and Dry Weight
AMF and extract concentration (p = 0.0001) and their interaction (p = 0.04) had significant effects on seedling fresh weight (FW) and dry weight (DW). Generally, the 20 g/L concentration of extract from both sources stimulated FW and DW of inoculated and non-inoculated seedlings. On the other hand, the highest concentration (100 g/L) of both extracts significantly reduced seedling growth in terms of FW and DW, but AMF inoculant ameliorated this decline (Table 2).

Table 2.
Effect of AMF and aqueous extracts of invasive N. glauca on fresh and dry weight of A. gerrardii seedlings.
Regarding the leaf extract, seedling FW and DW with the 20 g/L treatment showed the greatest mean values for AMF-inoculated seedlings (17.0 and 9.0 g, respectively) compared to seedlings without AMF (9.4 and 5.2 g, respectively). The 100 g/L treatment decreased seedling FW and DW, but AMF treatment enhanced FW and DW (120 and 6.1 g, respectively) compared to non-AMF-treated seedlings (8.4 and 4.9 g, respectively).
Regarding the flower extract, seedling FW and DW distinctly varied between AMF and extract treatments: AMF-treated seedlings had higher values compared to non-treated seedlings. With the 20 g/L concentration, AMF-inoculated seedlings had the highest FW (15.3 g) and DW (7.3 g) compared to seedlings without AMF (10.2 and 5.6 g, respectively). The 100 g/L concentration showed the lowest FW and DW, but AMF inoculant enhanced FW and DW (10.7 and 5.7 g, respectively) compared to non-treated seedlings (8.9 and 4.6 g, respectively) (Table 2). Also, with both extracts, AMF treatment increased shoot and root fresh and dry weight compared to non-AMF-treated seedlings (Tables S1).
3.4. Effects of AMF, Extract Source, and Concentration on Height, Leaf Number, Leaf Area, Collar Diameter, and Branch Number of A. gerrardii Seedlings
AMF inoculant, extract concentration, and their interaction had significant effects on the height, leaf number, and leaf area of A. gerrardii seedlings (Table 3).

Table 3.
Effects of extract and concentration on height, leaf number, and leaf area of A. gerrardii seedlings grown with and without AMF.
Leaf and flower extracts of invasive N. glauca decreased seedling height, particularly for non-AMF-treated seedlings, at concentrations of 60 and 100 g/L compared to control (0 g/L) (Figure 5 and Figure 6).

Figure 5.
Effects of AMF and different concentrations (0, 20, 60, and 100 g/L) of leaf aqueous extract of invasive N. glauca on vegetative growth of A. gerrardii seedlings.

Figure 6.
Effects of AMF and different concentrations (0, 20, 60, and 100 g/L) of flower aqueous extract of invasive N. glauca on vegetative growth of A. gerrardii seedlings.
Regardless of the extract concentration, AMF significantly affected seedling height (p = 0.0001). Seedling height was also affected by the interaction of AMF and extract concentration (p = 0.04). Without AMF treatment, seedling height decreased with increasing extract concentration. AMF inoculant enhanced seedling height compared to non-AMF-treated seedlings, even at the highest concentrations. Among AMF-treated seedlings, height was not significantly different for all concentrations, meaning that AMF alleviated the allelopathic effects in all treatments (Table 3). Flower extracts also affected the height of non-AMF-inoculated seedlings. There were significant differences between seedling heights for different extract concentrations. The highest concentration (100 g/L) had the strongest impact (Table 4). Similarly, the height of AMF-inoculated seedlings did not differ between extract concentrations, meaning that AMF enhanced seedling height even at the highest concentrations (Table 3).

Table 4.
Root length (RL) distribution per root diameter class (mm) in A. gerrardii seedlings.
AMF-inoculated seedlings showed higher values in terms of leaf number and area with the 20 and 60 g/L treatments compared to 100 g/L and control. Flower aqueous extract decreased leaf number and area at the 100 g/L concentration in both AMF and non-AMF seedlings compared to control. AMF-treated seedlings showed the highest leaf number and area with all concentrations compared to non-treated seedlings. The 20 g/L concentration with AMF treatment had a stimulatory effect on leaf number, while the highest leaf area was found with the control treatment (Table 3).
Regarding collar diameter, there was a significant variation between AMF-treated A. gerrardii seedlings compared to non-treated seedlings (p = 0.0001). No significant effect on seedling collar diameter was found for extract source, concentration, or their interaction with AMF. AMF-treated seedlings had the highest values for collar diameter with all extracts compared to non-AMF-treated seedlings (Table 3).
Regardless of the extract source or concentration, AMF was correlated with significant differences in branch number (p = 0.0001), but no interaction effect was found between AMF, concentration, and extract source. For all aqueous extracts, the 20 g/L concentration increased branch number (but not significantly, p = 0.09) with both inoculant levels in comparison to the control (0 g/L) treatment. Also, for the leaf and flower extracts, concentration had a varied effect on branch number in non-AMF-treated seedlings; the 60 and 100 g/L treatments significantly decreased the branch number compared to the control. AMF-treated seedlings showed an increase compared to non-AMF-treated counterparts. In most cases, the 20 g/L concentration stimulated branch number in both AMF and non-AMF seedlings compared to control (Table 3).
3.5. Effects of AMF, Extract, and Their Concentration on A. gerrardii Root Growth
3.5.1. Root Length (RL)
Extract concentration was found to have different effects (p = 0.04) on total root length, with no significant difference between extract sources (p = 0.78). This means that both extract sources had similar patterns of effect. Regardless of the extract source or concentration, AMF treatment significantly increased total root length (p = 0.015). For the leaf extract, there was an obvious increase in root length with increased concentrations of up to 60 g/L in all seedlings. However, the 100 g/L treatment reduced the total root length in AMF and non-AMF seedlings. AMF alleviated the allelopathic effect of aqueous extracts at 100 g/L concentration compared to seedlings without AMF. For both extract sources, the maximum root length was observed in AMF-treated seedlings compared to their counterparts with all concentrations (Figure 7A).
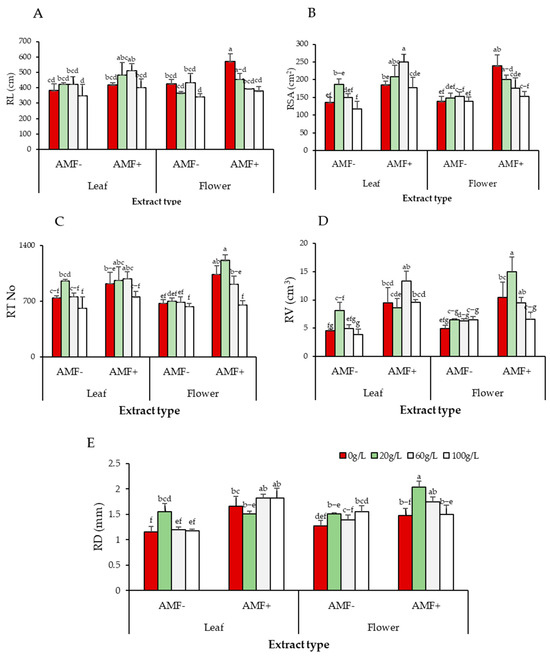
Figure 7.
Effect of AMF, extract type, and concentration on (A) RL, (B) RSA, (C) RT, (D) RV, and (E) RD of A. gerrardii seedlings. Lowercase letters in columns represent significant differences at p < 0.05 (LSD test).
3.5.2. Root Surface Area (cm2)
Root surface area values were high and significantly different between seedlings with AMF inoculant (p = 0.0001) and without AMF across extract sources and concentrations (p = 0.03). There were significant differences in root surface area in seedlings with leaf and flower aqueous extract treatment subjected to AMF inoculant. Non-AMF-treated seedlings grown with 100 g/L of either leaf or flower extract had the smallest root surface area. The 20 and 60 g/L concentrations of leaf extract increased root surface area either with or without AMF, with more pronounced stimulation in AMF-treated seedlings (Figure 7B).
3.5.3. Root Tip Number (RT)
Root tip numbers were high in seedlings inoculated with AMF compared to non-AMF-treated seedlings (p = 0.0001). The maximum root tips were observed in seedlings grown with the 20 g/L concentration of flower extract treated with AMF. However, root tips of A. gerrardii seedlings did not vary significantly between extract sources (p = 0.63). For both extract sources, concentration had a significant effect on root tip number (p = 0.0003): root tip number obviously decreased with the 100 g/L treatment and increased with the 20 and 60 g/L treatments, and the lowest number of root tips appeared in seedlings grown with 100 g/L of leaf aqueous extract without AMF treatment. Generally, AMF enhanced root tip numbers with both extracts and all concentrations compared to no AMF treatment (Figure 7C).
3.5.4. Root Volume (RV)
AMF significantly affected the root volume of A. gerrardii irrigated with different extract sources and concentrations (p = 0.00001). Seedlings grown with flower extract at 20 g/L and treated with AMF had the largest root volume, which varied significantly from that observed with other concentrations. For both extract sources, root volume in non-AMF-treated seedlings decreased with increasing extract concentration, particularly at 60 and 100 g/L. Seedlings treated with AMF had the largest root volume compared to non-treated seedlings across extract sources and concentrations. Without AMF treatment, seedlings treated with 100 g/L of leaf extract exhibited considerably reduced root volume compared to control (0 g/L) and other treatments (Figure 7D, Table S1).
3.5.5. Root Diameter (RD)
Regardless of the aqueous extract source, AMF treatment increased root diameter significantly compared to no AMF treatment (p = 0.0001). Increased extract concentration had a significant negative impact on root diameter, particularly in seedlings without AMF (p = 0.03). The largest root diameter was obtained in AMF-treated seedlings grown in 20 g/L flower extract. For the leaf extract, AMF-inoculated seedlings showed a significant difference from non-inoculated seedlings across concentrations, except 20 g/L (Figure 7E).
In general, and regardless of whether seedlings were irrigated with leaf or flower extract, the root system of A. gerrardii seedlings was larger for seedlings grown with AMF inoculant compared to seedlings without AMF (Figure 8).
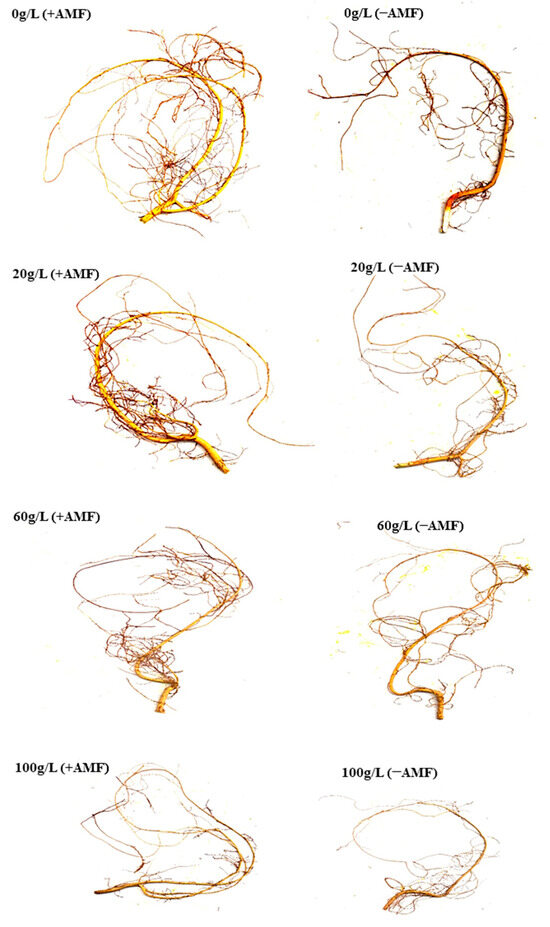
Figure 8.
Effects of flower aqueous extract of invasive N. glauca at various concentrations (0−100 g/L) and AMF inoculant on the root system of A. gerrardii seedlings.
3.6. Effects of AMF, Extract Source, and Concentration on Root Length per Diameter Class
Root length decreased with increased root diameter (Table 4). Most root lengths were within the 0–1 mm diameter class, and root lengths were greater (but not significantly) in seedlings treated with AMF than seedlings without AMF. Increased extract concentration significantly affected root length in the 0–1 mm diameter class (p = 0.01). Both extract sources had a similar impact on all diameter classes. AMF significantly affected root length in the 1–2, 2–3, 3–4, and 4–5 mm diameter classes. With the leaf extract, the longest root lengths were observed in the 0–1 mm class of seedlings grown with 20 and 60 g/L concentrations and treated with AMF, while the shortest root lengths were recorded for the 3–4 mm diameter class of seedlings grown with 100 g/L extract without AMF. Similarly, AMF-treated seedlings grown with 20 and 60 g/L flower extract in the 0–1 mm class had the longest root lengths, whereas the shortest root lengths were observed for non-AMF-treated seedlings grown with the 20, 60, and 100 g/L concentrations in the 3–4 mm class. Generally, seedlings with AMF treatment had enhanced root length and volume in all diameter classes compared to seedlings without AMF. Also, with all concentrations of both aqueous extract sources, most AMF-treated seedlings had the longest root length and volume in all diameter classes compared to non-treated seedlings (Table 4, Table S2).
3.7. Effects of Aqueous Extract Type, AMF, and Concentration on Root Surface Area per Diameter Class
Root surface area decreased with increased root diameter except in the 4–5 mm root diameter class, which had the largest root surface area (Table 5). AMF inoculant significantly enhanced seedling root surface area in all diameter classes, with p = 0.04, 0.0002, 0.01, 0.0005, and 0.0004 for 0–1, 1–2, 2–3, 3–4, 4–5 mm, respectively. In general, the largest root surface areas were recorded for the 0–1 and 4–5 mm diameter classes for seedlings grown with all aqueous extracts. Seedlings treated with AMF had the largest root surface area in the 0–1 and 4–5 mm diameter classes. In addition, AMF-treated seedlings grown with all extracts at all concentrations had the largest surface area. Seedlings grown with 60 g/L leaf aqueous extract and inoculated with AMF had the highest and most significant root surface area, which varied significantly from the other concentrations. Seedlings irrigated with 100 g/L leaf or flower aqueous extract that was not treated with AMF had a smaller root surface area in all diameter classes. In contrast, with the same extract concentration, there was an increase in root surface area for AMF-treated seedlings. In comparison to control seedlings (irrigated with 0 g/L), seedlings grown with 20 and 60 g/L extract showed a positive increase in root surface area both with and without AMF treatment. However, the 100 g/L concentration negatively affected root surface area, particularly in non-AMF-treated seedlings, across aqueous extract sources and concentrations (Table 5).

Table 5.
Distribution of root surface area (RSA, cm3) of A. gerrardii seedlings per root diameter class (mm).
3.8. Effects of Extract Source, AMF, and Concentration on Number of Root Tips per Diameter Class
Table 6 presents the distribution of root tip numbers across the five root diameter classes. Generally, the number of root tips per diameter decreased with increased root diameter with both types of extract. AMF inoculant significantly affected the root tip number across the five diameter classes. However, treatment concentration affected root tip number in the 0–1, 3–4, and 4–5 mm diameter classes. The highest values for root tips (more than 95%) were mostly in the 0–1 mm diameter class. In this diameter class, AMF-treated seedlings showed high numbers of root tips across extract sources and treatment concentrations compared to non-AMF-treated seedlings. Differences were observed in the number of root tips in non-AMF-treated seedlings irrigated with 0, 20, 60, and 100 g/L concentrations of both extracts. The number of root tips decreased significantly with an increased concentration of 100 g/L, but the 20 and 60 g/L concentrations had a stimulatory effect on root tips compared to the control (0 g/L) and 100 g/L. This means that the 20 and 60 g/L extracts increased the root tip number in AMF-treated seedlings.

Table 6.
Distribution of root tip (RT) number in A. gerrardii seedlings per root diameter class (mm).
3.9. Effects of AMF, Extract Source, and Concentration on Chlorophyll a and b in A. gerrardii Seedlings
Irrespective of extract source and concentration, AMF treatment had a significant effect on the content of chlorophyll a (p = 0.0003) and chlorophyll b (p = 0.0036). All AMF-inoculated seedlings grown with extracts from different sources and at different concentrations had higher values of chlorophyll a and b compared to non-inoculated seedlings. Regardless of AMF treatment, extract source, and concentration had significant effects on chlorophyll a and b contents. For chlorophyll a, the effect of the extract increased with increasing concentration. On the other hand, the 20 and 60 g/L concentrations enhanced chlorophyll b compared to 100 g/L. For the leaf extract, the 100 g/L concentration decreased chlorophyll b in non-AMF-treated seedlings compared to control (non-treated seedlings with 0 g/L). For the flower extract, seedlings grown with the 100 g/L concentration without AMF had reduced chlorophyll b compared to control; however, AMF-treated seedlings grown with the 100 g/L extract had higher chlorophyll b content (Figure 9A,B).
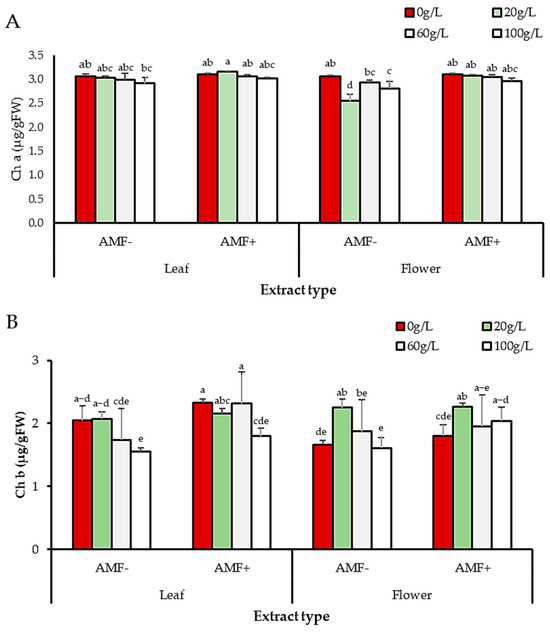
Figure 9.
Effects of extract source, AMF, and concentration on (A) chlorophyll a and (B) chlorophyll b content. Lowercase letters in columns represent significant differences at p < 0.05 (LSD test).
Regardless of AMF or extract concentration, treatment with extracts from different sources (leaf and flower) had different effects on the content of chlorophyll a, but not on chlorophyll b. Flower extract treatment had the lowest mean values of chlorophyll a, while leaf extract treatment had the highest values. Seedlings irrigated with flower extract had the lowest chlorophyll b content, while seedlings irrigated with leaf extract had the highest chlorophyll b content (Figure 9A,B).
3.10. Effects of AMF, Aqueous Extract Source, and Concentration on Photosynthesis (Pn), Stomatal Conductance (C), and Transpiration Rate (E) in A. gerrardii Seedlings
Among non-inoculated plants, seedlings grown with 100 g/L of leaf aqueous extract showed a reduction in Pn by −51.12%, C by −42.24%, and E by −17.60% compared to seedlings without AMF grown with 0 g/L (control), but those grown with 20 and 60 g/L treatment did not vary significantly from the control. The 100 g/L extract decreased photosynthesis in A. gerrardii seedlings treated or not treated with AMF, but in inoculated seedlings Pn, C, and E, it increased by 45.26%, 20.74%, and 21.98%, respectively, compared to non-inoculated seedlings (Table 7).

Table 7.
Effects of AMF inoculant and extracts on gas exchange parameters in A. gerrardii seedlings.
In seedlings grown with flower aqueous extract, the 100 g/L treatment decreased Pn by −6.86%, C by −25.57%, and E by −28.57% compared to the control (no AMF, 0 g/L). In AMF-inoculated seedlings, the effect of 20 and 60 g/L treatments did not vary significantly compared to seedlings grown with AMF and 0 g/L. Furthermore, significant increases in C and E were found in AMF-inoculated seedlings grown with 100 g/L extract compared to non-inoculated seedlings, whereas there was decreased Pn, though not significant, compared to the control (Table 7).
3.11. Effects of AMF, Aqueous Extract Source, and Concentration on Proline Accumulation in A. gerrardii Seedlings
AMF inoculant, extract source, and treatment concentration had significant effects on proline accumulation (p = 0.0001) (Figure 10). A significant interaction was observed between concentration and AMF treatment (p = 0.0001). Also, AMF, extract source, and treatment concentration significantly varied regarding proline accumulation (p = 0.0002). The 20, 60, and 100 g/L treatment concentrations significantly increased proline content with both inoculant levels and extract sources compared to the control (0 g/L). Nonetheless, AMF-inoculated seedlings grown in all extract concentrations, except 60 g/L, had the lowest proline content (Figure 10).
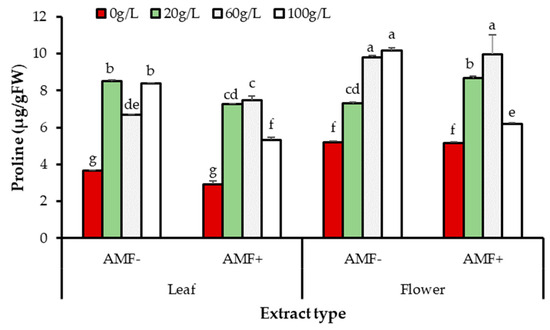
Figure 10.
Effects of extract type, AMF, and concentration on proline accumulation in A. gerrardii seedlings. Lowercase letters in columns represent significant differences at p < 0.05 (LSD test).
The results of statistical analysis show that there was an interaction between extract type and AMF (p = 0.023). Seedlings treated with leaf extract at all concentrations had higher proline content compared to seedlings treated with flower extract. It was observed that AMF had an effective role in reducing proline content in inoculated seedlings compared to non-inoculated seedlings grown with both extract sources at all concentrations (Figure 10).
The interactive effect of AMF, extract source, and their concentration was significant for proline accumulation (p = 0.0002); AMF-treated seedlings had the lowest proline content with both types of extracts at all concentrations. AMF treatment reduced proline content in seedlings grown with extract at all concentrations, even 100 g/L, compared to non-AMF-treated seedlings (Figure 10).
Furthermore, the findings obtained from the hierarchically clustered heat map show that most of the studied parameters (root system, vegetative, chlorophyll, photosynthesis) increased in A. gerrardii seedlings treated with AMF and irrigated with leaf and flower extract at all concentrations. On the other hand, most of the seedlings without AMF grown with different extracts and concentrations clustered into one group (blue and light blue) and recorded the lowest values for most of the studied traits except proline accumulation (Figure 11).
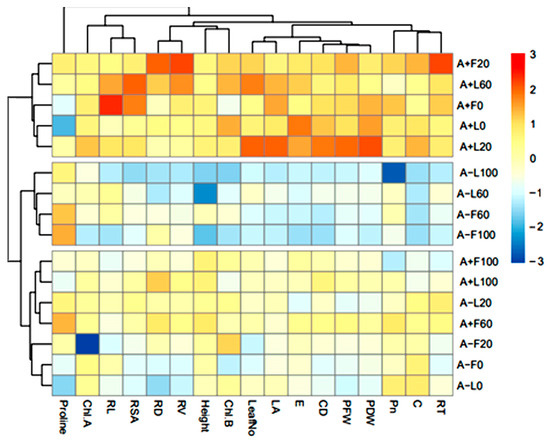
Figure 11.
Hierarchical clustered heat map indicating similarities and dissimilarities between studied parameters for AMF, extract source, and treatment concentration. Red indicates similar traits, blue indicates dissimilar traits; color intensity indicates the amount of similarity or dissimilarity. A+L0-100 = seedlings treated with AMF and irrigated with leaf extract at concentrations of 0–100 g/L; A-L0-100 = non-AMF-treated seedlings irrigated with leaf aqueous extract at concentrations of 0–100 g/L; A+F0-100 = seedlings treated with AMF and irrigated with flower extract at concentrations of 0–100 g/L; A-F0-100 = non-AMF-treated seedlings irrigated with flower aqueous extract at concentrations of 0–100 g/L. RT, root tips; C, stomatal conductance; Pn, photosynthesis; PDW, plant dry weight; PFW, plant fresh weight; CD, collar diameter; E, transpiration rate; LA, leaf area; Chl. B, chlorophyll b; RV, root volume; RD, root diameter; RSA, root surface area; RL, root length.
Principal component analysis (PCA) was carried out based on a correlation matrix for the vegetative, root, and physiological parameters and AMF, extract source, and treatment concentration (Figure 12). The findings indicate that the first two PCs describe about 74.2% of the variation. A positive correlation is found between vegetative growth (PFW, PDW, height, leaf number, leaf area, collar diameter), root (RSA, RT, RV, RD), and physiological (Ch a, Ch b, Pn, C) traits, and are related to the first PC, which describes 64.2% of the variation. Proline accumulation is related to the second PC, which describes 10.0% of the variation. Two main clusters can be recognized: The first cluster includes AMF-treated seedlings and those grown with different concentrations of extract from different sources, which are related to most of the root, vegetative, and physiological parameters. The second cluster contains seedlings without AMF grown with different concentrations of extract from different sources and is linked to proline accumulation (Figure 12).
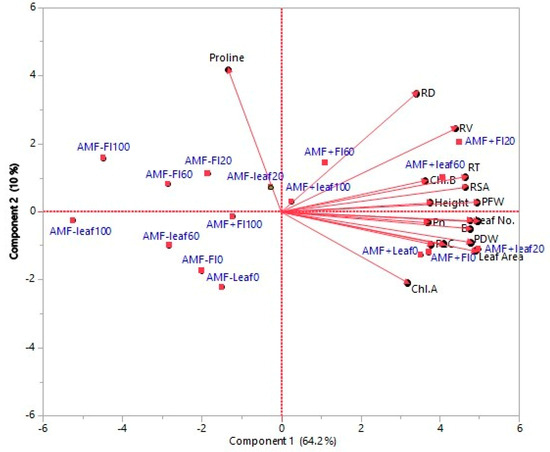
Figure 12.
PCA of vegetative, root, physiological parameters, and extract sources and concentrations with and without AMF treatment. AMF-leaf0-100 = non-AMF-treated seedlings irrigated with leaf aqueous extract at concentrations of 0–100 g/L; AMF-Fl0-100 = non-AMF-treated seedlings irrigated with flower aqueous extract at concentrations of 0–100 g/L; AMF+leaf0-100 = AMF-treated seedlings irrigated with leaf aqueous extract at concentrations of 0–100 g/L; AMF-Fl0+100 = AMF-treated seedlings irrigated with flower aqueous extract at concentrations of 0–100 g/L.
4. Discussion
4.1. Response of AMF Root Colonization and Spore Abundance in A. gerrardii Soil to Aqueous Extracts of Invasive N. glauca
The findings of this study indicate various infection rates for AMF in A. gerrardii roots among aqueous extract sources and concentrations, with inhibitory effects at the highest concentration (100 g/L) and a stimulatory effect (in a few cases) at the lowest concentration (20 g/L) for both extract treatments. The inhibitory effects of allopathic substances on AMF colonization can be ascribed to toxic and allelopathic substances, such as phenolic acids, tannins, and alkaloid compounds, which can affect AMF. Higher concentrations of allopathic materials could affect soil nutrient cycling, which in turn will affect AMF growth. There is evidence that allelopathic compounds released from invasive plants can inhibit AMF growth and decrease root infection rates in neighboring plant species [35]. Allelopathic substances from the roots of Stellera chamaejasme L. were found to decrease the infection rate and mycelial growth of AMF associated with different plants [36]. On the other hand, many studies have reported the positive impact of allelopathic compounds (particularly at low concentrations) on AMF root colonization. The results from the literature show that root exudates from invasive Triadica sebifera significantly increased AMF root colonization of several native plant species [37].
This study shows that the abundance of AMF spores in A. gerrardii soils varied across aqueous extract sources and treatment concentrations, with spores being negatively affected by increasing concentrations. The lower numbers of AMF spores in soils treated with allelopathic substances could be attributed to changes in the soil physiochemical properties (such as pH, porosity, and moisture content) as a result of the presence of allelopathic toxic compounds. This is in line with the findings of [36], in which the authors reported that allelopathic substances from Stellera chamaejasme L. had a significant negative effect on the abundance and density of AMF spores. The litter of that plant was reported to have a negative effect on AMF spore density [35,38]. The results of a study on allelopathy and its effect on AMF spores indicated that allelopathic substances resulting from exotic plant species inhibit the germination of AMF spores, resulting in significant changes in native AMF communities [39]. Another study reported that allelopathic compounds released from garlic mustard had a significant inhibitory effect on the spore germination of forest AMF (Glomus clarum), even at the lowest concentration [40].
4.2. Role of AMF in Alleviating the Effects of Aqueous Extracts of Invasive N. glauca on the Vegetative Growth of A. gerrardii Seedlings
Our findings provide clear evidence that AMF can alleviate the effects of allelopathic aqueous extracts of invasive N. glauca on the growth of A. gerrardii seedlings. In natural conditions, the amount of allelopathic substances varies in different soil layers. For example, in one study [41], the amounts of allelochemicals in litter, humus, and soil layers were 688.81, 479.13, and 328.93 ng/g, respectively. The negative effects of the aqueous extracts can be reduced by AMF by enhancing plant vegetative growth, which in turn positively influences plant tolerance to allelochemical compounds and the stress they induce. In this regard, studies have reported that soil microbes such as AMF are among the most important agents against allelopathic effects and significantly decrease the harmful allelopathic effects of leaf leachates of different invasive plant species. Many studies have indicated that AMF may gradually adapt to the allelochemicals of invasive plants and alleviate their allelopathic effects [42,43]. AMF root colonization can alleviate the biotic and abiotic stresses experienced by plants, as plant roots secrete allelopathic substances when they come in contact with soil-borne pathogens [44]. It is well known that allelopathic compounds can change soil chemical properties, which can result in stress on native plants and affect their growth. This stress can be alleviated by AMF, which assists plants with acquiring nutrients and water from the soil. AMF colonization increases the ability of plants to absorb water and nutrients and enhances their ability to tolerate stress conditions and pathogen attacks [45]. Colonization of many plant species by beneficial AMF appears to alleviate the effects of allelochemicals on plant growth and development [37]. The stresses that result from allelopathic compounds can be significantly decreased by symbiotic AMF. Soil microorganisms such as AMF can degrade allelopathic substances or increase plant tolerance to allelopathic compounds and decrease allelochemical substances that are produced by allelopathic plant species [46,47]. AMF provide direct and indirect support for associated plants to counteract biotic and abiotic stress. The direct support includes chemical and physical interactions between AMF and plants that alleviate stress. In this case, AMF hyphae sense the environment and create a physical fence, which reduces stress and controls the protection mechanism in the plant, allowing for a suitable stress response [8].
The results of the present study show that all aqueous extracts of invasive N. glauca decreased the growth parameters (plant fresh and dry weight, height, leaf number, leaf area) of A. gerrardii seedlings, particularly at the highest concentration (100 g/L). The effects were more pronounced in non-AMF-inoculated seedlings. In this regard, another study found that the aqueous extracts, leachates, root exudates, and decomposing residues of Imperata cylindrica plants suppressed the growth of several plants, including woody plant species, and reduced their rhizobium nodulation and AMF colonization [48]. In another study, extracts prepared from vegetative parts of Nicotiana tabacum had an inhibitory effect on vegetative parameters and biomass production of Zea mays L. seedlings under laboratory conditions [49]. The results of the present study also show that at low concentrations, both extracts had little stimulatory effect on the vegetative growth parameters of Acacia gerrardii seedlings, and in most cases, the enhancement did not significantly differ from the control treatment (0 g/L). This result is in accordance with the findings of [50], in which the authors reported that applying powder and aqueous extracts of leaves and flowers of invasive N. glauca on soil in which tomato plants were growing was effective at stimulating their growth. However, the same extracts had an inhibitory effect on the growth of Cynodon dactylon plants.
4.3. Role of AMF in Alleviating the Effects of Allelopathic Extracts of Invasive N. glauca on Root Parameters of A. gerrardii Seedlings
In the present study, seedlings treated with AMF had superior root traits across extract sources and treatment concentrations. This can be ascribed to the positive benefits provided by AMF hyphae. Most AMF studies state that AMF is associated with more than 80% of the plants in the world, and they improve plant growth by enhancing nutrient and water uptake by plants. This improvement is very clear under stress conditions. One study reported that AMF-treated seedlings had higher values for root length, root surface area, root volume, root average diameter, and root tip number than non-AMF-treated seedlings under different stress conditions [51]. For example, there is evidence in the published literature that applying allelopathic extracts of Alliaria petiolata to Impatiens pallida inhibited root and vegetative growth in the absence of AMF. However, the presence of AMF actually protected the I. pallida plants from the effects of the allelopathic compounds [23]. Allelochemical compounds can interfere with indole-3-acetic acid (the most important regulator auxin for root elongation), resulting in less root development [52]. Also, allopathic substances change the apical meristems of roots, leading to severe effects on water absorption and root growth [53]. Under biotic/abiotic stress, AMF was found to stimulate greater root-hair growth in trifoliate orange, be associated with mycorrhiza-modulated auxin synthesis and transport, and enhance plant stress tolerance [54,55]. Improved growth parameters, particularly in seedlings inoculated with AMF under allelopathic stress, can be associated with the ability of AMF to enhance the water absorption capacity of plants by increasing root hydraulic conductivity, the absorptive surface area of the root system, and the plant’s ability to access moisture from very fine soil pores [56].
The increased root length in AMF-treated seedlings that were grown with leaf and flower allelopathic extract reflects the response of seedlings to optimize tissues that acquire growth-limiting resources, with plants allocating more resources to the development of fine roots. The capacity of roots to acquire water and nutrients is greatly influenced by root length [57]. In a study on Quercus suber, root length gradually decreased with increased diameter [58]. Gradual changes in root length based on diameter could influence seedling performance since root length is linked to root longevity and the capacity of seedlings to acquire and transport soil resources [59]. The results of another study demonstrated that smaller diameters and more root tips per unit of root length enable plants to increase the volume of exploited soil [60].
An increased number of root tips in seedlings grown with higher concentrations of allelopathic extracts and treated with AMF compared to non-AMF-treated seedlings supports the role of AMF in plant resistance to allelopathic stress conditions, which is partly consistent with the findings reported by [47]. It was also reported that AMF can result in positive changes in the architecture of the plant root system and modulate reactive oxygen species (ROS) in the plant, resulting in decreased plant stress [61]. Under the 20 and 60 g/L treatments, a high root biomass was observed in seedlings inoculated with mycorrhiza compared to non-AMF seedlings. This could be due to the acquisition of more plant nutrients by the roots. Similarly, another study reported an increase in root growth in I. pallida following inoculation with AMF and exposure to allelopathic stress compared to plants grown in the same condition but without AMF [23].
Moreover, AMF has an important role in modifying root exudates [62] and changing soil biological processes [63]. In addition, inoculation with AMF was found to be significant in alleviating and inhibiting the impact on the relative amount of allelopathic substances containing phenolic compounds, sterols, and benzoic acid [64]. Likewise, other soil microbes in the plant rhizosphere [65] can be useful and positively enhance nutrient uptake in plants exposed to biotic or abiotic stress conditions [66].
4.4. Role of AMF in Alleviating the Effects of Aqueous Extracts of Invasive N. glauca on Chlorophyll a and b Content in A. gerrardii Seedlings
In this study, the content of chlorophyll a and b in A. gerrardii seedlings was higher in the AMF-treated seedlings than the non-AMF-treated seedlings across aqueous extract sources and treatment concentrations. In a study conducted on Ferula haussknechtii H. Wolff ex Rech. F., it was found that allelopathic extracts decreased the chlorophyll concentration in recipient plants that were exposed to allelopathic extracts without AMF inoculant, but AMF-inoculated plants were capable of sustaining their pigment content at a high level because the seedlings were able to gain more moisture via AMF hyphae [67]. In addition, AMF can help plants gain more nutrients from the soil, particularly phosphorus, resulting in a good vigor index and growth that is positively reflected in the chlorophyll content [68]. Furthermore, varying chlorophyll content under different stress conditions in plants inoculated with AMF has been previously reported in eggplant [69]. The authors reported an increase in the chlorophyll content of Thymus daenensis and Thymus vulgaris after being inoculated with different AMF strains [70].
4.5. Role of AMF in Alleviating the Effects of Aqueous Extracts of Invasive N. glauca on Gas Exchange Parameters in A. gerrardii Seedlings
Stimulation and increased growth in AMF-inoculated seedlings are directly controlled by the photosynthetic role of AMF. Nonetheless, all concentrations of both types of extracts at both stages (−AMF, +AMF) significantly decreased the photosynthetic attributes because of the allelopathic extract concentration, but AMF inoculant had a stimulatory role in avoiding the photosynthetic reduction. AMF are known to be symbiotic with most plants around the world and are widely distributed in different environments, and they may considerably improve plant stress tolerance [71]. The enhanced gas exchange in AMF-treated seedlings positively impacted the photosynthetic rate across extract sources and treatment concentrations for non-inoculated seedlings. The enhanced photosynthesis rate in AMF-treated seedlings compared to non-treated seedlings indicates the beneficial role of AMF in plants grown in stressful growth conditions [72]. Similarly, a higher photosynthesis rate was reported in AMF-inoculated plants under allelopathic stress compared to non-inoculated plants [73]. Plant stomatal conductance and non-stomatal measurements are involved in photosynthesis rate organization, and in the present study, AMF obviously enhanced gas exchange traits, causing significantly higher levels of photosynthesis in AMF-treated seedlings. AMF play a vital role in controlling stomatal improvement, density, and conductance by enhancing abscisic acid production and genes in plants under stress [74]. A published study reported that photosynthesis and transpiration rates were higher in different cultivars of soybean plants in the presence of AMF under stress conditions, meaning that AMF treatment can enhance water uptake and improve the water use efficiency of plants [75]. Moreover, the association of many plant species with AMF under different biotic stresses (such as allelopathic stress) leads to improved photosynthesis rate and stomatal conductance [76].
4.6. Role of AMF in Mitigating the Effects of Allelopathic Aqueous Extracts on Proline Accumulation in A. gerrardii Seedlings
Several researchers have reported decreased proline accumulation in AMF-treated plants [77]. This could be because proline accumulation is an indicator of abiotic or biotic stress, which plants are exposed to in certain conditions. Therefore, the lower proline accumulation in AMF-inoculated plants, which was observed in our study, could be due to the role of AMF in organizing plant growth, which clearly reduced the allelopathic stress on AMF-inoculated seedlings over non-inoculated seedlings with all concentrations of both extracts. Another study [78] explained that soil microbes usually identify transportation processes and amounts of allelochemical compounds in the below-ground system, which in turn may alleviate or increase them in the plant. Another study indicated the important role of rhizosphere microorganisms in the maintenance of eucalyptus plant species, which may decrease the allelopathic impact on Eucalyptus grandisis [79]. AMF associated with roots probably exploit eucalyptus allelochemicals such as phenols as a carbon source for decomposition, which ultimately decreases the allelopathic effect on eucalyptus plants [55,80].
In general, our results may potentially prove that AMF increase the possibility of alleviating the effects of allelopathic aqueous extracts of invasive N. glauca on A. gerrardii growth, providing a perspective to recognize their positive role in forest health.
5. Conclusions
In this study, applying AMF alleviated the allelopathic effects of leaf and flower aqueous extracts of invasive N. glauca on the growth of native A. gerrardii seedlings. The highest concentration (100 g/L) of extract decreased the AMF infection rate in the roots of A. gerrardii seedlings and the abundance of AMF spores in the soil. In addition, the highest concentration inhibited the growth parameters (vegetative part and root system) of A. gerrardii seedlings. However, AMF colonization alleviated and reduced the inhibitory effect of the aqueous extracts. On the other hand, a low concentration (20 g/L) of both extract types (leaf and flower) mostly stimulated the above- and below-ground parameters of inoculated and non-inoculated A. gerrardii seedlings. Also, AMF enhanced the photosynthesis and chlorophyll parameters of A. gerrardii seedlings grown with the allelopathic aqueous extracts. AMF also reduced the proline accumulation in AMF-treated seedlings compared to seedlings without AMF. Our results may potentially provide a theoretical basis for studies of AMF and their interactive role in alleviating the allelopathic effects of invasive plants on the growth of native plant species.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f15081447/s1, Table S1. Effect of AMF and extract types on shoot fresh (SFW) and shoot dry (SDW) weights of A. gerrardii seedlings; Table S2. Effect of AMF and extract types on root volume distribution per root diameter class (mm) in A. gerrardii seedlings.
Author Contributions
Conceptualization, A.M.A., T.S.A., A.A.A., A.M.A.-E., E.M.A., and S.N.S.; Data curation, A.M.A.; Formal analysis, A.M.A. and E.M.A.; Funding acquisition, T.S.A. and A.M.A.-E.; Investigation, A.M.A., T.S.A., and A.A.A.; Methodology, A.M.A., T.S.A., A.A.A., and E.M.A.; Project administration, T.S.A.; Resources, A.M.A., T.S.A., and A.A.A.; Software, A.M.A. and T.S.A.; Supervision, T.S.A. and A.A.A.; Validation, A.M.A. and T.S.A.; Visualization, A.M.A., T.S.A., A.A.A., and S.N.S.; Writing—original draft, A.M.A.; Writing—review and editing, A.M.A., T.S.A., and A.M.A.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Researchers Supporting Project, number RSPD2024R676, King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
All data used in the present study are published in the paper.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project, number RSPD2024R676, King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thomas, J.; El-Sheikh, M.A.; Alfarhan, A.H.; Alatar, A.A.; Sivadasan, M.; Basahi, M.; Al-Obaid, S.; Rajakrishnan, R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid. Environ. 2016, 127, 53–65. [Google Scholar] [CrossRef]
- Issaly, E.A.; Sersic, A.N.; Pauw, A.; Cocucci, A.A.; Traveset, A.; Benitez-Vieyra, S.M.; Paiaro, V. Reproductive ecology of the bird-pollinated Nicotiana glauca across native and introduced ranges with contrasting pollination environments. Biol. Invasions. 2020, 22, 485–498. [Google Scholar] [CrossRef]
- Ollerton, J.; Watts, S.; Connerty, S.; Lock, J.; Parker, L.; Wilson, I.; Schueller, S.; Nattero, J.; Cocucci, A.A.; Izhaki, I.; et al. Pollination ecology of the invasive tree tobacco Nicotiana glauca: Comparisons across native and non-native ranges. J. Pollinat. Ecol. 2012, 9, 85–95. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Al-Huqail, A.A.; AbdAllah, E.F. Biodiversity of arbuscular mycorrhizal fungi associated with Acacia gerrardii Benth in different habitats of Saudi Arabia. Pak. J. Bot. 2018, 50, 1211–1217. [Google Scholar]
- Alatar, A.A.; El-Sheikh, M.A.; Thomas, J.; Hegazy, A.K.; El Adawy, H.A. Vegetation, floristic diversity, and size-classes of Acacia gerrardii in an arid wadi ecosystem. Arid. Land Res. Manag. 2015, 29, 335–359. [Google Scholar] [CrossRef]
- Inderjit; Cahill, J.F. Linkages of plant–soil feedbacks and underlying invasion mechanisms. AoB Plants 2015, 7, plv022. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, P.; Potts, D.; Pettibone, G.; Warren, R. Do novel weapons that degrade mycorrhizal mutualisms promote species invasion? Plant Ecol. 2018, 219, 539–548. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Djurdjević, L.; Gajić, G.; Kostić, O.; Jarić, S.; Pavlović, M.; Mitrović, M.; Pavlović, P. Distribution, Functional Ecology of Plants. Seasonal dynamics of allelopathically significant phenolic compounds in globally successful invader Conyza canadensis L. plants and associated sandy soil. Flora 2012, 207, 812–820. [Google Scholar] [CrossRef]
- Qiao, Y.-J.; Gu, C.-Z.; Zhu, H.-T.; Wang, D.; Zhang, M.-Y.; Zhang, Y.-X.; Yang, C.-R.; Zhang, Y.-J. Allelochemicals of Panax notoginseng and their effects on various plants and rhizosphere microorganisms. Plant Divers. 2020, 42, 323–333. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Mangla, S.; Inderjit; Callaway, R.M. Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J. Ecol. 2008, 96, 58–67. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Raza, W.; Wang, X.; Ran, W.; Shen, Q. Systemic modification of cotton root exudates induced by arbuscular mycorrhizal fungi and Bacillus vallismortis HJ-5 and their effects on Verticillium wilt disease. Appl. Soil Ecol. 2012, 61, 85–91. [Google Scholar] [CrossRef]
- Ma, J.; Xie, Y.; Yang, Y.; Jing, C.; You, X.; Yang, J.; Sun, C.; Qin, S.; Chen, J.; Cao, K.; et al. AMF colonization affects allelopathic effects of Zea mays L. root exudates and community structure of rhizosphere bacteria. Front. Plant Sci. 2022, 13, 1050104. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Duchesneau, K.; Golemiec, A.; Colautti, R.I.; Antunes, P.M. Functional shifts of soil microbial communities associated with Alliaria petiolata invasion. Pedobiologia 2021, 84, 150700. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds Jr, D.J.M.r. Separated components of root exudate and cytosol stimulate different morphologically identifiable types of branching responses by arbuscular mycorrhizal fungi. Mycol. Res. 2007, 111, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Pickett, B.; Maltz, M.; Aronson, E. Impacts of invasive plants on soil fungi and implications for restoration. Divers. Ecol. Invasive Plants 2018, 45. [Google Scholar]
- Řezáčová, V.; Řezáč, M.; Gryndler, M.; Hršelová, H.; Gryndlerova, H.; Michalova, T. Plant invasion alters community structure and decreases diversity of arbuscular mycorrhizal fungal communities. Appl. Soil Ecol. 2021, 167, 104039. [Google Scholar] [CrossRef]
- Lu, F.; Zheng, L.; Chen, Y.; Li, D.; Zeng, R.; Li, H. Soil microorganisms alleviate the allelopathic effect of Eucalyptus grandis× E. urophylla leachates on Brassica chinensis. J. For. Res. 2017, 28, 1203–1207. [Google Scholar] [CrossRef]
- Barto, K.; Friese, C.; Cipollini, D. Arbuscular mycorrhizal fungi protect a native plant from allelopathic effects of an invader. J. Chem. Ecol. 2010, 36, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Struble, J.; Skipper, H.D. Soil. Vesicular-arbuscular mycorrhizal fungal spore production as influenced by plant species. Plant Soil 1988, 109, 277–280. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Peraza-Echeverría, L.; Soto-Hernández, R.; San Miguel-Chávez, R.; Pérez-Brito, D.; Tapia-Tussell, R.; Ortiz-Vázquez, E.; Rodríguez-García, C. Diospyros cuneata inhibition of Fusarium oxysporum: Aqueous extract and its encapsulation by ionic gelation. J. Plant Pathol. Microbiol. 2016, 7, 332. [Google Scholar]
- Daniels, B.A.; Skipper, H.D. Method for the Recovery and Quantitative Estimation of Propagules from Soil; American Phytopathological Society: Saint Paul, MN, USA, 1982; pp. 29–36. [Google Scholar]
- Utobo, E.B.; Ogbodo, E.N.; Nwogbaga, A.C. Techniques for extraction and quantification of arbuscular mycorrhizal fungi. Libyan Agric. Res. Cent. J. Int. 2011, 2, 68–78. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, IN16–IN18. [Google Scholar] [CrossRef]
- Gerdemann, J.; Nicolson, T. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Abdelmalik, A.M.; Alshahrani, T.S.; Alqarawi, A.A.; Ahmed, E.M.J.D. Allelopathic Potential of Nicotiana glauca Aqueous Extract on Seed Germination and Seedlings of Acacia gerrardii. Diversity 2023, 16, 26. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.a.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Voegele, A.; Graeber, K.; Oracz, K.; Tarkowská, D.; Jacquemoud, D.; Turečková, V.; Urbanová, T.; Strnad, M.; Leubner-Metzger, G.J.J.o.E.B. Embryo growth, testa permeability, and endosperm weakening are major targets for the environmentally regulated inhibition of Lepidium sativum seed germination by myrigalone A. J. Exp. Bot. 2012, 63, 5337–5350. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.-Y.; Jiang, S.-Y.; Guo, S.-X.; Wang, J.-F.; Hu, Y.; Li, S.-M.; Li, H.-M.; Wang, T.; Sun, Y.-K.; et al. Allelopathy and arbuscular mycorrhizal fungi interactions shape plant invasion outcomes. NeoBiota 2023, 89, 187–207. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Xing, F.; Chen, C.; Huang, G.; Gao, Y. Interaction between root exudates of the poisonous plant Stellera chamaejasme L. and arbuscular mycorrhizal fungi on the growth of Leymus Chinensis (Trin.) Tzvel. Microorg. 2020, 8, 364. [Google Scholar] [CrossRef]
- Tian, L.; Lin, X.; Tian, J.; Ji, L.; Chen, Y.; Tran, L.-S.P.; Tian, C. Research advances of beneficial microbiota associated with crop plants. Int. J. Mol. Sci. 2020, 21, 1792. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, R.-H.; Li, X.-L.; Liu, X.-W.; Li, Y.-N.; Xing, F. Nitrogen addition overrides the effects of Stellera chamaejasme litter on the growth of Leymus chinensis and its associated mycorrhizal fungi. J. Plant Ecol. 2022, 15, 1007–1020. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, J.; Leng, D.; Hu, S.; Yong, J.W.; Chen, X. An invasive plant promotes its arbuscular mycorrhizal symbioses and competitiveness through its secondary metabolites: Indirect evidence from activated carbon. PLoS ONE 2014, 9, e97163. [Google Scholar] [CrossRef]
- Cantor, A.; Hale, A.; Aaron, J.; Traw, M.B.; Kalisz, S. Low allelochemical concentrations detected in garlic mustard-invaded forest soils inhibit fungal growth and AMF spore germination. Biol. Invasions 2011, 13, 3015–3025. [Google Scholar] [CrossRef]
- Quan, W.; Wang, A.; Li, C.; Xie, L. Evolution. Allelopathic potential and allelochemical composition in different soil layers of Rhododendron delavayi forest, southwest China. Front. Ecol. Evol. 2022, 10, 963116. [Google Scholar] [CrossRef]
- ud din Khanday, M.; Bhat, R.A.; Haq, S.; Dervash, M.A.; Bhatti, A.A.; Nissa, M.; Mir, M.R. Arbuscular mycorrhizal fungi boon for plant nutrition and soil health. In Soil Science: Agricultural and Environmental Prospectives; Springer International Publishing: Cham, Switzerland, 2016; pp. 317–332. [Google Scholar]
- Li, Y.-P.; Feng, Y.-L.; Chen, Y.-J.; Tian, Y.-H. Soil microbes alleviate allelopathy of invasive plants. Sci. Bull. 2015, 60, 1083–1091. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, N.; Wu, P.; Huo, H.; Xu, G.; Wu, G. Arbuscular mycorrhizal colonization alleviates Fusarium wilt in watermelon and modulates the composition of root exudates. Plant Growth Regul. 2015, 77, 77–85. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Cipollini, K.; Titus, K.; Wagner, C. Allelopathic effects of invasive species (Alliaria petiolata, Lonicera maackii, Ranunculus ficaria) in the Midwestern United States. Allelopath. J. 2012, 29, 63–76. [Google Scholar]
- Cheng, J.K.; Cao, M.Y.; Yang, H.R.; Yue, M.F.; Xin, G.R.; Chen, B. Interactive effects of allelopathy and arbuscular mycorrhizal fungi on the competition between the invasive species Bidens alba and its native congener Bidens biternata. Weed Res. 2022, 62, 268–276. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
- Khoshkharam, M.; Shahrajabian, M.; Sun, W.; Cheng, Q. Survey the allelopathic effects of tobacco (Nicotiana tabacum L.) on corn (Zea mays L.) growth and germination. Cercet. Agron. Mold. 2020. [Google Scholar] [CrossRef]
- Jmii, G.; Sayari, M.; Mars, M.; Gharsallaoui, S.; Haouala, R. Nicotiana glauca, a Key Plant for Tomato Growth Enhancement and for the Weed Cynodon dactylon Control. Tunis. J. Plant Prot. 2022, 17, 77–96. [Google Scholar] [CrossRef]
- Zuo, S.P.; Ma, Y.Q.; Ye, L.T. In vitro assessment of allelopathic effects of wheat on potato. Allelopath. J. 2012, 30, 1–10. [Google Scholar]
- Šoln, K.; Koce, J.D. Allelopathic root inhibition and its mechanisms. Allelopath. J. 2021, 52, 181–198. [Google Scholar] [CrossRef]
- Šoln, K.; Klemenčič, M.; Koce, J.D. Plant cell responses to allelopathy: From oxidative stress to programmed cell death. Protoplasma 2022, 259, 1111–1124. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P.; et al. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Liu, S.; Qin, F.; Yu, S. Eucalyptus urophylla root-associated fungi can counteract the negative influence of phenolic acid allelochemicals. Appl. Soil Ecol. 2018, 127, 1–7. [Google Scholar] [CrossRef]
- Džafić, E.; Pongrac, P.; Likar, M.; Regvar, M.; Vogel-Mikuš, K. The arbuscular mycorrhizal fungus Glomus mosseae alleviates autotoxic effects in maize (Zea mays L.). Eur. J. Soil Biol. 2013, 58, 59–65. [Google Scholar] [CrossRef]
- Kuyper, T.W.; Wang, X.; Muchane, M.N. The interplay between roots and arbuscular mycorrhizal fungi influencing water and nutrient acquisition and use efficiency. In The Root Systems in Sustainable Agricultural Intensification; Wageningen University: Wageningen, The Netherlands, 2021; pp. 193–220. [Google Scholar]
- Disante, K.B.; Fuentes, D.; Cortina, J. Sensitivity to zinc of Mediterranean woody species important for restoration. Sci. Total. Environ. 2010, 408, 2216–2225. [Google Scholar] [CrossRef]
- Eissenstat, D.; Wells, C.; Yanai, R.; Whitbeck, J.L. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef]
- Kashyap, P.; Sharma, I.; Kashyap, S.; Agarwala, N. Arbuscular Mycorrhizal Fungi (AMF)-Mediated Control of Foliar Fungal Diseases. In Arbuscular Mycorrhizal Fungi and Higher Plants: Fundamentals and Applications; Springer: Singapore, 2024; pp. 193–223. [Google Scholar]
- Frew, A.; Powell, J.R.; Johnson, S.N. Soil. Aboveground resource allocation in response to root herbivory as affected by the arbuscular mycorrhizal symbiosis. Plant Soil 2020, 447, 463–473. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Lǚ, L.-H.; Zou, Y.-N.; Wu, Q.-S. Mycorrhizas mitigate soil replant disease of peach through regulating root exudates, soil microbial population, and soil aggregate stability. Soil Sci. Plant Anal. 2019, 50, 909–921. [Google Scholar] [CrossRef]
- Emmett, B.D.; Lévesque-Tremblay, V.; Harrison, M.J. Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J. 2021, 15, 2276–2288. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Akhzari, D.; Shayganfar, A. The Interaction of Artemisia persica Allelopathy, Drought and Arbuscular Mycorrhizal Fungi on Growth and Physiological Indices of Ferula haussknechtii H. Wolff ex Rech. f. Ecopersia 2019, 7, 203–210. [Google Scholar]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Ali, M.; Amin, B.; Cheng, Z. Arbuscular mycorrhizal fungi and dry raw garlic stalk amendment alleviate continuous monocropping growth and photosynthetic declines in eggplant by bolstering its antioxidant system and accumulation of osmolytes and secondary metabolites. Front. Plant Sci. 2022, 13, 849521. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, B.; Yu, Y.; Wang, S.; Wei, M.; Wang, C.; Du, D. The allelopathy of horseweed with different invasion degrees in three provinces along the Yangtze River in China. Physiol. Mol. Biol. Plants 2021, 27, 483–495. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The multifaceted roles of Arbuscular Mycorrhizal Fungi in peanut responses to salt, drought, and cold stress. Plants Biol. 2023, 23, 36. [Google Scholar] [CrossRef]
- Rashidi, S.; Yousefi, A.R.; Pouryousef, M.; Goicoechea, N. Effect of arbuscular mycorrhizal fungi on the accumulation of secondary metabolites in roots and reproductive organs of Solanum nigrum, Digitaria sanguinalis and Ipomoea purpurea. Chem. Biol. Technol. Agric. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral, J.S.R.; Santana, L.R.; Tavares, G.G.; Santos, L.D.S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L.; et al. The arbuscular mycorrhizal fungus Rhizophagus clarus improves physiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, T.; Zhang, D.; Ding, K.; Li, C.; Ma, F. Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Doubková, P.; Vlasáková, E.; Sudová, R. Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 2013, 370, 149–161. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Alías, J.C.; Lobón, N.C. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Merilä, P.; Malmivaara-Lämsä, M.; Spetz, P.; Stark, S.; Vierikko, K.; Derome, J.; Fritze, H. Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl. Soil Ecol. 2010, 46, 259–267. [Google Scholar] [CrossRef]
- Qin, F.; Yu, S. Arbuscular mycorrhizal fungi protect native woody species from novel weapons. Plant Soil 2019, 440, 39–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).