Impacts of Downed Dead Wood Poplar Trees on Forest Regeneration in the Semi-Arid Region of Northern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Experimental Design
2.3. Field Survey
2.3.1. Field Investigation Methods
- ①
- The downed dead wood reserves were surveyed and recorded according to the standards of international downed dead wood investigation and research: each downed dead wood with a diameter ≥ 10 cm in the sample plot was surveyed. We used a breast diameter ruler to measure the diameter of the big and small heads (the big head was not more than 10 cm away from the roots, and the small head was not more than 10 cm from the crown of the tree). The diameters of the large and small heads of the tree were measured using a diameter gauge. A laser rangefinder was used to measure the length of downed dead wood, and we measured the X and Y coordinates of the wood within the sample plot, where X represents the east–west coordinates and Y represents the north–south coordinates.
- ②
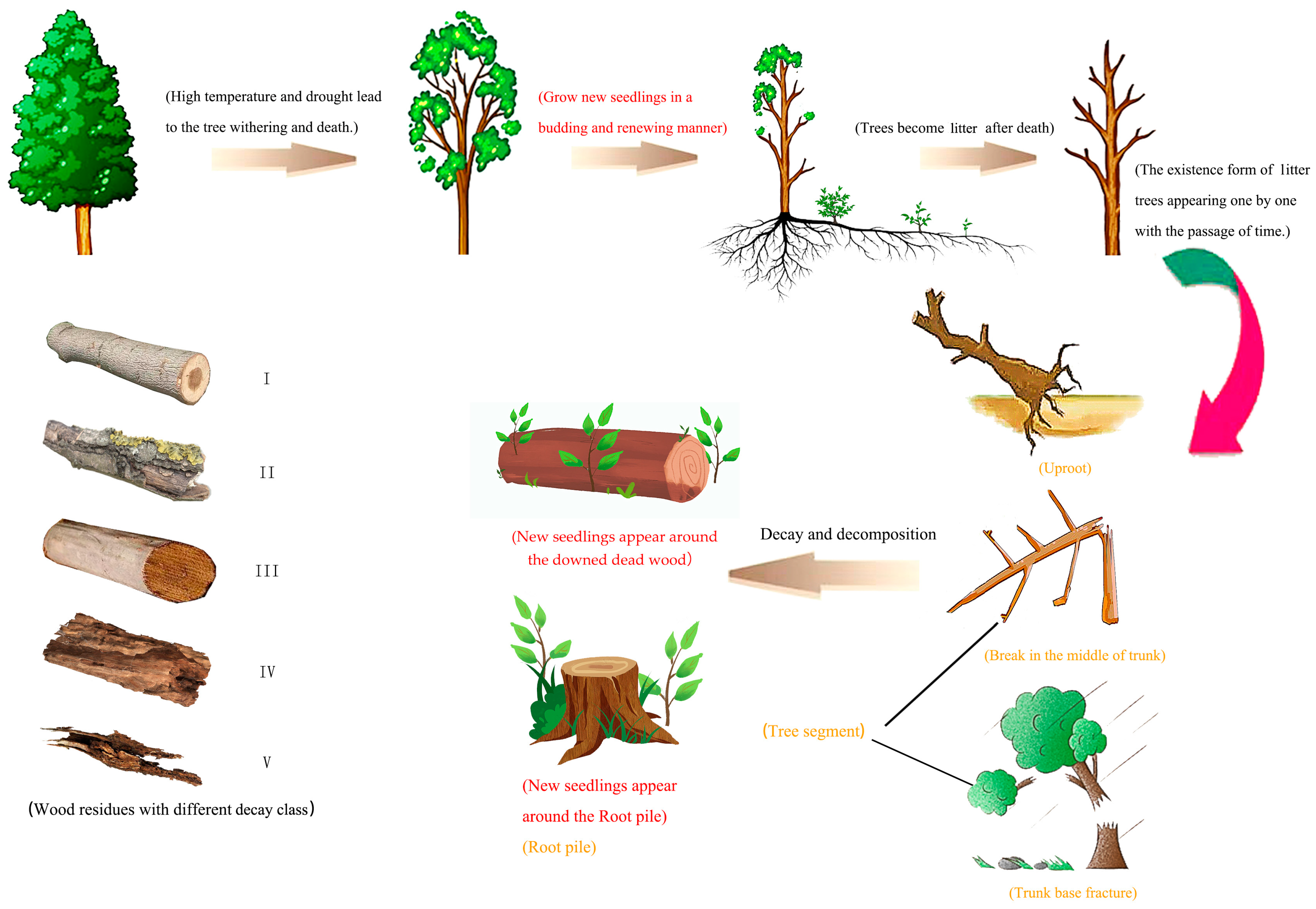
- The existing forms of downed dead wood were investigated and divided into five forms: uprooted (the downed dead wood is pulled out entirely from soil, with its roots attached to it), break in the middle of the trunk, break in the base of the trunk (also called trunk base fracture), tree segments, and root piles (Table 2, Figure 2, orange font).
- ③
- Investigation of the decay class of downed dead wood was based on a five-level classification (from I to V, indicating slightly decomposed to heavily decomposed), referring to suitable indicators used in other studies [23]. The conditions of organisms attached to the downed dead wood, seedling growth, and root invasion were combined to determine the class, and a small knife was inserted into the downed dead wood debris to test its mechanical hardness (Table 3, Figure 2).
- ④
- The forest renewal density and regeneration mode or approach were investigated, and the species and quantity of seedlings were surveyed using the full sample plot method [24]. The soil cutting method was used to determine and count the renewal mode of the new seedlings. The surface soil was cut open to directly observe whether there was a connection point between the mother plant root system and the young plant root system. A young plant connected to the mother plant root system in a “┴” shape is called a clone plant (asexual reproductive ramet), while a young plant without a connection point with the mother plant root system and with its main root growing vertically into the soil is called a seedling plant (sexual reproductive ramet). Finally, by calculating the proportion of seedlings with different renewal modes through survey data, the proportion of asexual reproduction can be used to infer the vitality of the root system of withered trees.
2.3.2. Indoor Testing and Data Processing
2.3.3. Data Analysis
3. Results
3.1. Distribution Characteristics of Downed Dead Wood in the Poplar Forest of Saihanwula Nature Reserve
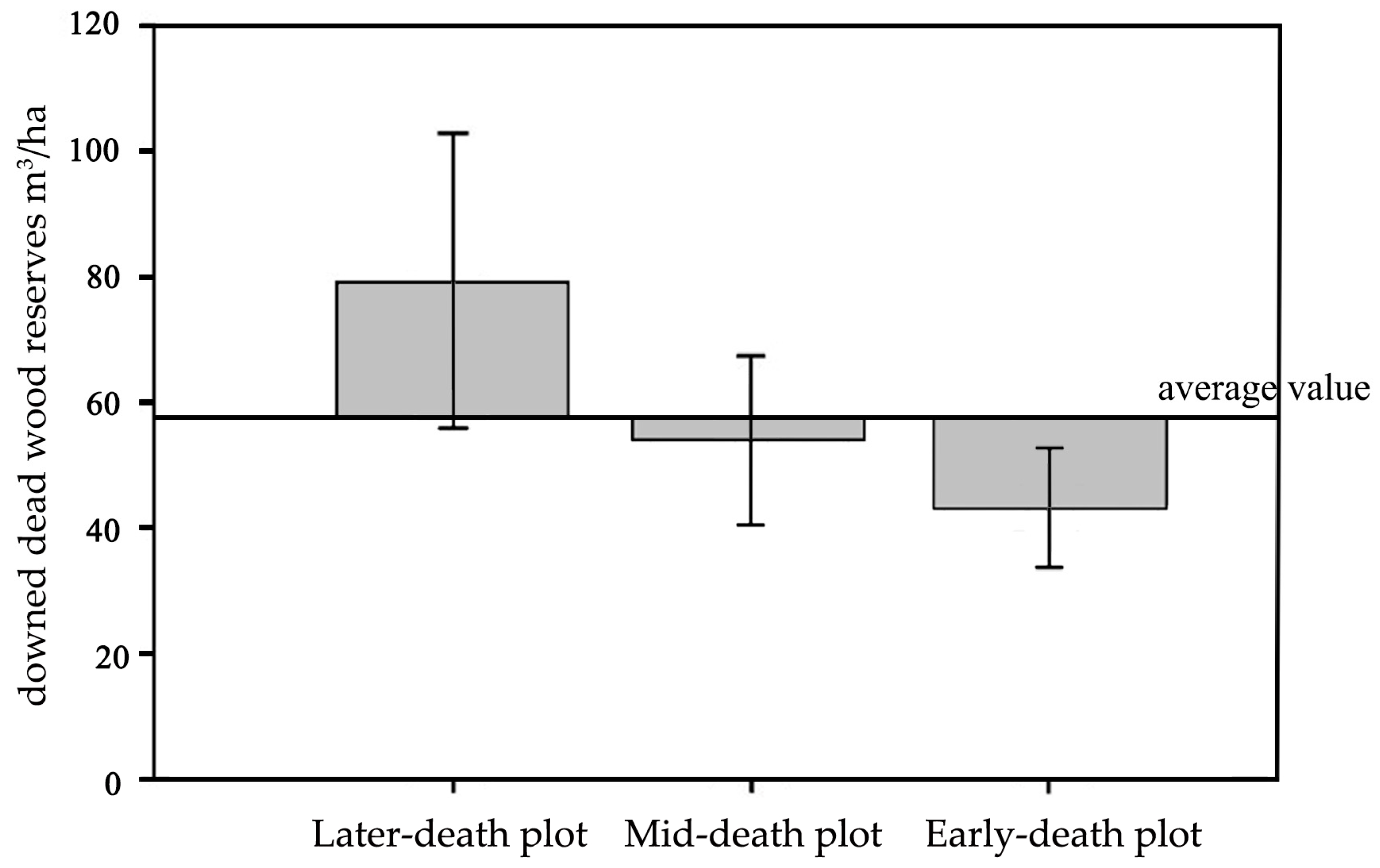
3.1.1. Downed Dead Wood Reserves
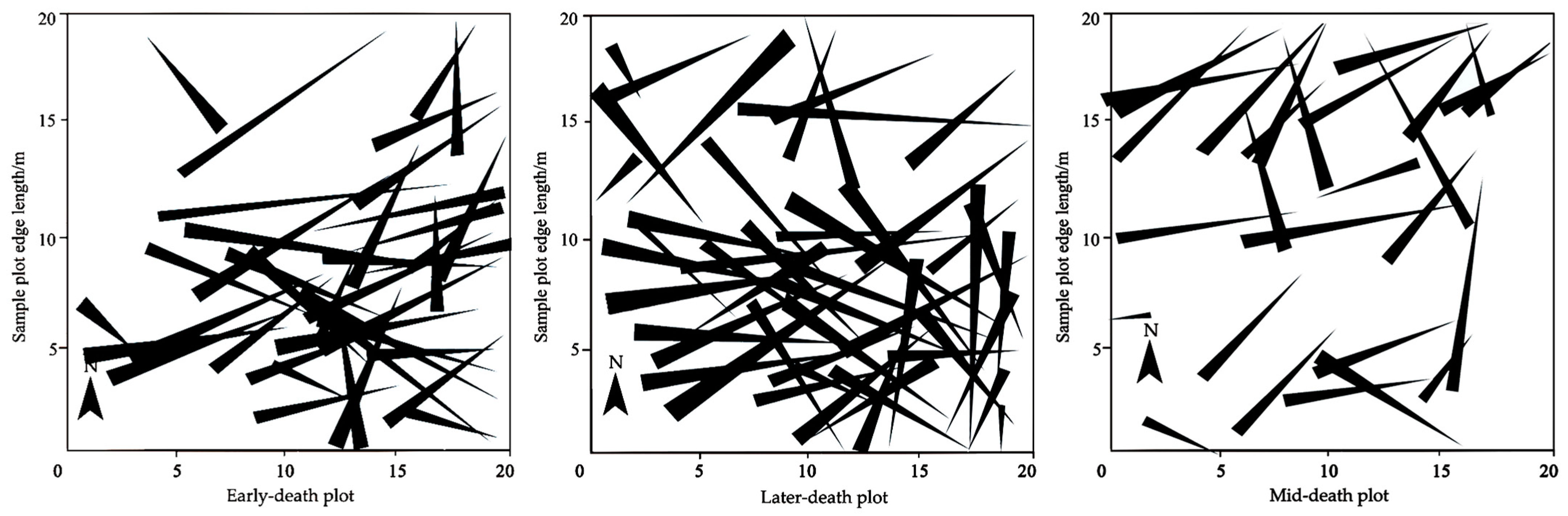
3.1.2. Spatial Distribution Pattern of Downed Dead Wood
3.1.3. Existing Form of Downed Dead Wood
3.1.4. The Main Factors Contributing to the Death of a Large Number of Trees
3.2. Regeneration Characteristics of Poplar Forest
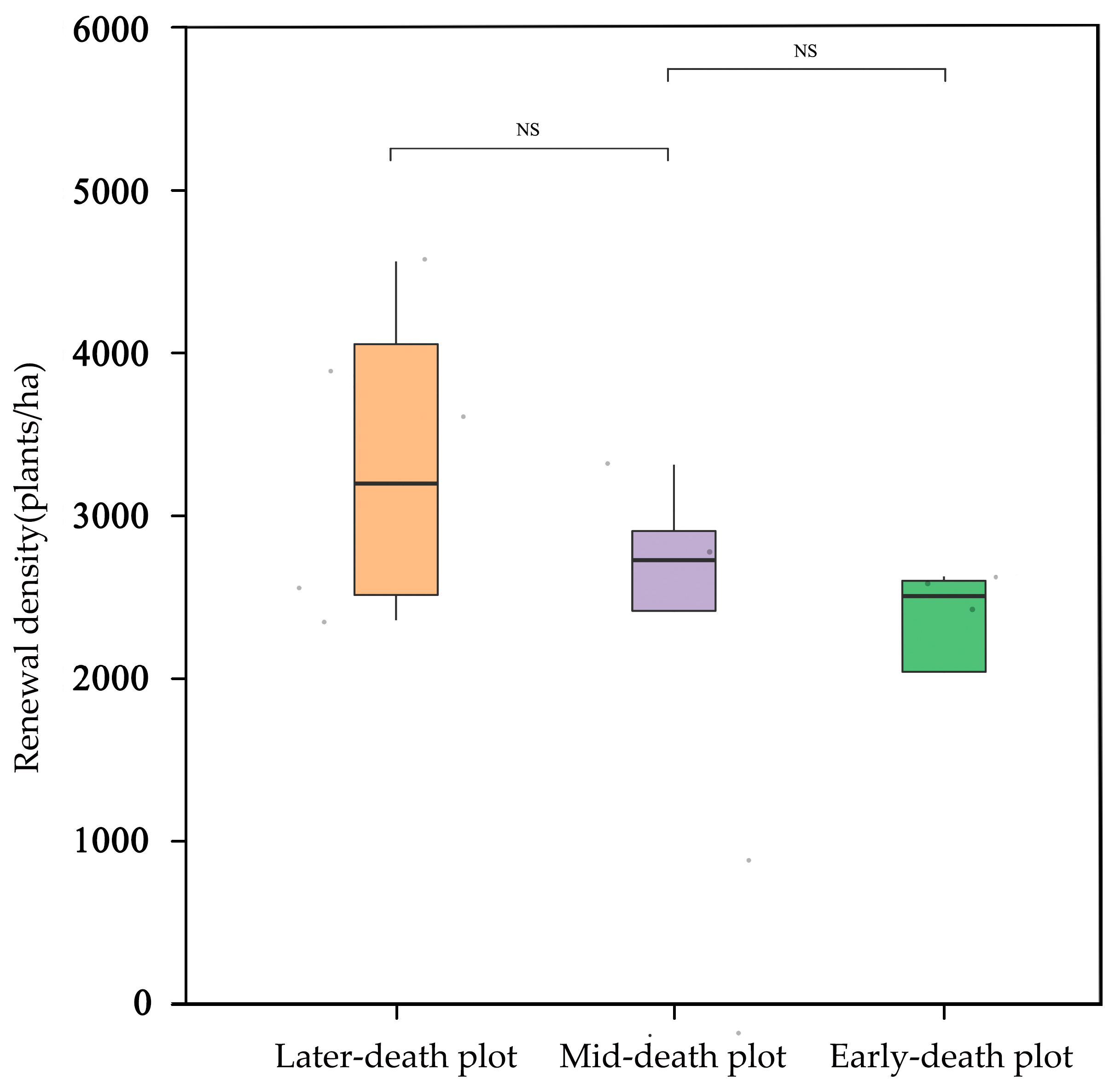
3.2.1. Forest Regeneration Density after Death
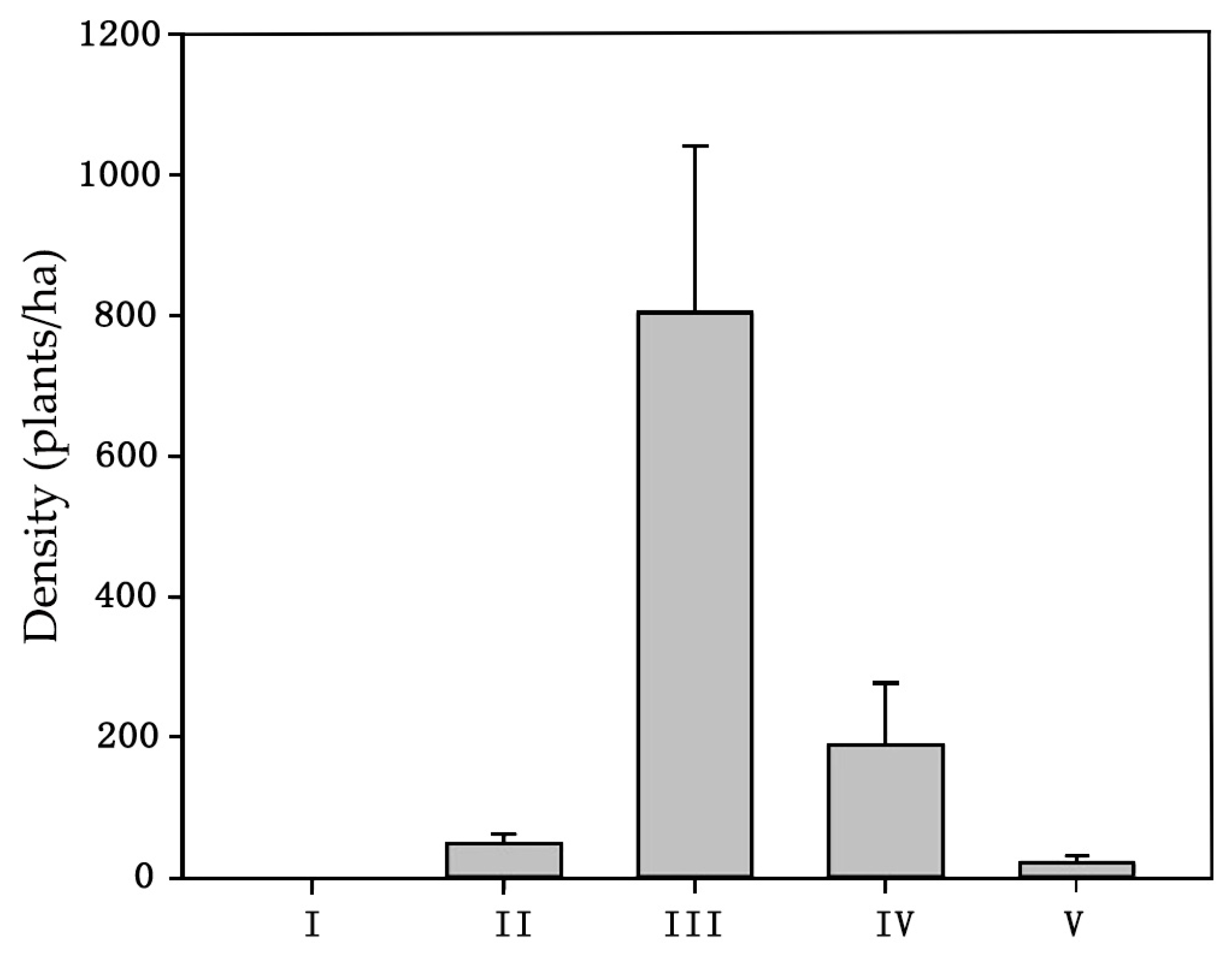
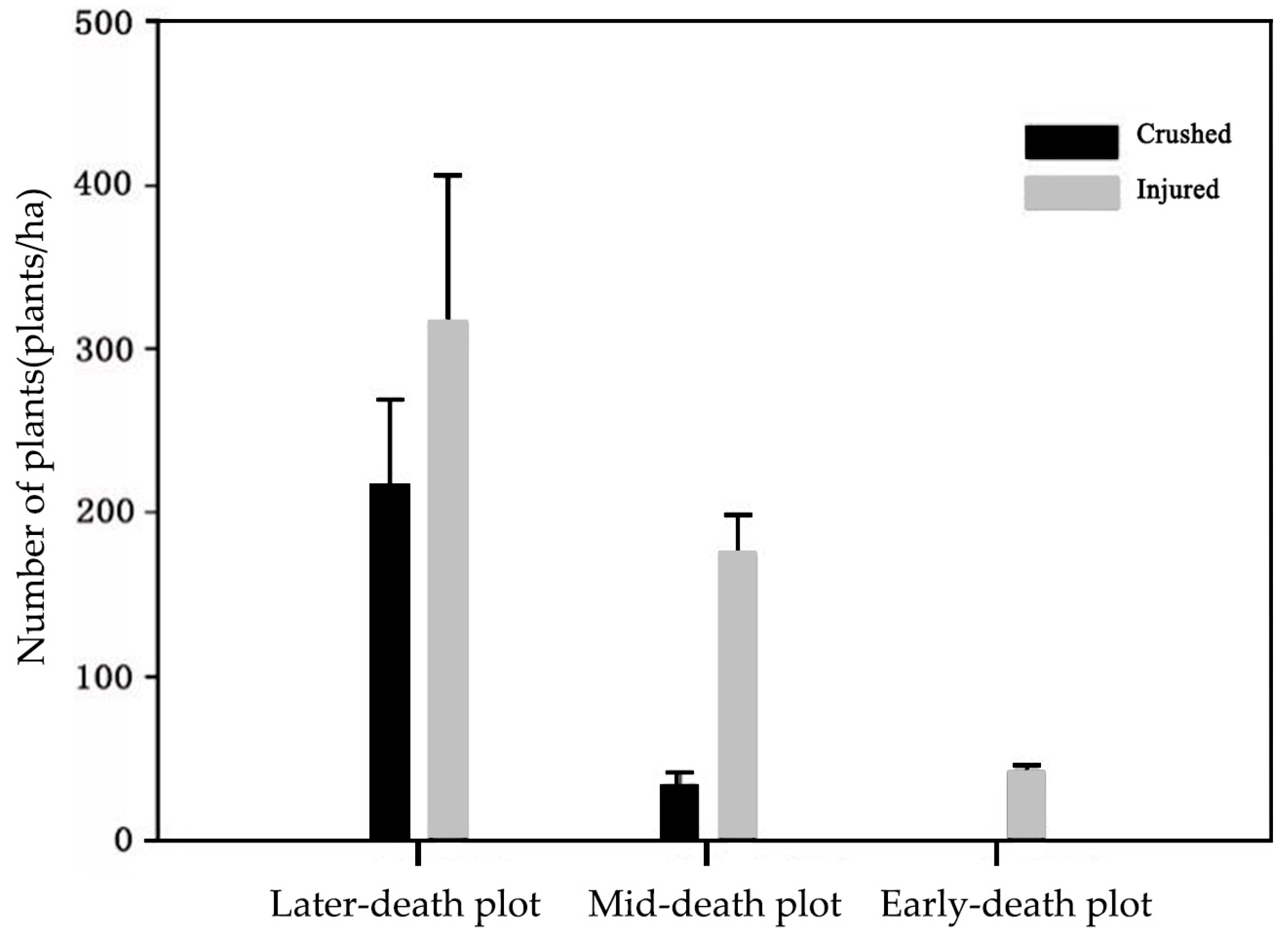
3.2.2. Impact of Downed Dead Wood on Forest Regeneration
4. Discussion
4.1. A Large Number of Downed Dead Wood Occurred in the Poplar Forest after Abnormal Death
4.2. Inhibition of Forest Renewal by Downed Dead Wood in the Secondary Forest
4.3. Secondary Forests Have the Ability to Quickly Renew after Death
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Q.C.; Zhao, P.W.; Bao, B.X.; Guan, L.J.; Bao, H. Study on the characteristics of regenerated seedlings in Populus sylvestris forest in Hanshan Nature Reserve, Inner Mongolia. J. Northwest For. Univ. 2023, 38, 113–118. [Google Scholar]
- Spies, T.A.; Franklin, J.P.; Thomas, T.B. Coarse woody debris in Douglas-fir forests of westem Oregon and Washington. Ecology 1988, 69, 1689–1702. [Google Scholar] [CrossRef]
- He, D.J.; He, X.J.; Hong, W. Research progress on crude dead wood residues in forest ecosystems. For. Sci. Res. 2009, 22, 715–721. [Google Scholar]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Cummins, K.W. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Davis, C.J.; Castleberry, B.S.; Kilgo, C.J. Influence of coarse woody debris on herpetofaunal communities in upland pine stands of the southeastern Coastal Plain. For. Ecol. Manag. 2009, 259, 1111–1117. [Google Scholar] [CrossRef]
- Phillip, S. Input and decay of coarse woody debris in coniferous stands in western Oregon and Washington. Can. J. For. Res. 1982, 12, 18–28. [Google Scholar]
- Yu, F.; Shingo, K.; Akira, S.M.; Takashi, O.; Hiroshi, T. Accumulation and decay dynamics of coarse woody debris in a Japanese old-growth subalpine coniferous forest. Ecol. Res. 2014, 29, 257–269. [Google Scholar]
- Yang, X.H.; Ning, P.; Gao, R.M. Natural regeneration characteristics and influencing factors of typical forest communities in ecological public welfare forests in the middle section of Zhongtiao Mountain. Chin. Sci. Soil Water Conserv. 2024, 1, 1–10. [Google Scholar]
- Donald, M.; Munesh, K.; Kumar, U.S.; Stephen, S. Editorial: Effect of forest disturbances on natural forest regeneration in a changing tropical environment. Front. For. Glob. Chang. 2023, 6, 1289315. [Google Scholar]
- Shorohova, E.; Kapitsa, E. The decomposition rate of non-stem components of coarse woody debris (CWD) in European boreal forests mainly depends on site moisture and tree species. Eur. J. For. Res. 2016, 135, 593–606. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhou, G.Y.; Zhao, H.B. Basic characteristics of crude woody residues in the Lingnan Chenopodium and Luofu persimmon formation. For. Sci. Res. 2016, 29, 448–454. [Google Scholar]
- Guan, L.J.; Zhao, P.W.; Zhou, M.; Shu, Y.; Wu, Y.H.; Chen, J.J. Study on the characteristics of woody residue reserves in the secondary forest area of Hanshan, Inner Mongolia. J. Southwest For. Univ. 2022, 42, 133–140. [Google Scholar]
- Wei, S.J.; Sun, L.; Wei, S.W.; Hu, H.Q. Research progress on crude woody residues in forest ecosystems. J. Zhejiang AF Univ. 2013, 30, 585–598. [Google Scholar]
- Zhao, P.W.; Guan, L.J.; Zhou, M.; Shu, Y.; Wu, Y.H.; Chen, J.J. Plant diversity characteristics under forest death gradient in the northern section of the secondary forest in Hanshan, Inner Mongolia. J. Zhejiang AF Univ. 2024, 41, 41–48. [Google Scholar]
- Liu, X.D. Analysis of Specific Measures for Forestry Ecological Protection and Natural Forest Protection. New Agric. 2021, 2, 100. [Google Scholar]
- Ando, Y.; Fukasawa, Y.; Oishi, Y. Interactive effects of wood decomposer fungal activities and bryophytes on spruce seedling regeneration on coarse woody debris. Ecol. Res. 2017, 32, 173–182. [Google Scholar] [CrossRef]
- He, M.; Wei, J.S.; Shi, L. Response of radial growth and mortality of Populus davidiana in the southern section of the Daxing’an Mountains to regional climate change. J. Ecol. 2018, 37, 3237–3244. [Google Scholar]
- Xu, C.; Liu, H.; Zhou, M. Enhanced sprout-regeneration offsets warming-induced forest mortality through shortening the generation time in semiarid birch forest. For. Ecol. Manag. 2018, 409, 298–306. [Google Scholar] [CrossRef]
- Zeng, N.; Yao, H.; Zhou, M. Species-specific determinants of mortality and recruitment in the forest-steppe ecotone of northeast China. For. Chron. 2016, 92, 336–344. [Google Scholar] [CrossRef]
- Hély, C.; Bergeron, Y.; Flannigan, D.M. Coarse woody debris in the southeastern Canadian boreal forest: Composition and load variations in relation to stand replacement. Can. J. For. Res. 2000, 30, 674–687. [Google Scholar] [CrossRef]
- Zou, P.W.; Xu, F. Construction of Ecological Security Pattern and Prediction of Landscape Ecological Risks: A Case Study of Saihanwula National Nature Reserve. J. Ecol. 2023, 43, 9981–9993. [Google Scholar]
- Wu, C.; Yuan, X.; Yang, G.; Dehe, N.; Zhang, Y.; Liu, Y.Q.; Wang, G.G. How does position affect the decomposition of fine woody debris in subtropical forest? For. Ecol. Manag. 2024, 560, 121829. [Google Scholar] [CrossRef]
- Díaz Villa, M.V.E.; Cristiano, P.M.; De Diego, M.S.; Rodríguez, S.A.; Efron, S.T.; Bucci, S.J.; Scholz, F.; Goldstein, G. Do selective logging and pine plantations in humid subtropical forests affect aboveground primary productivity as well as carbon and nutrients transfer to soil? For. Ecol. Manag. 2022, 503, 119736. [Google Scholar] [CrossRef]
- Chen, Q.H.; Wen, Y.G.; Zhou, X.G.; Wang, L.; Sun, D.J.; Huang, X.G.; Deng, S.H. The impact of different regeneration methods on understory plant diversity after eucalyptus forests replace Pinus massoniana forests. Guangxi Sci. 2023, 30, 504–512. [Google Scholar]
- Zhao, P.W.; Xu, C.Y.; Zhou, M.; Zhang, B.; Ge, P.; Zeng, N.; Liu, H.Y. Rapid regeneration offsets losses from warming-induced tree mortality in an aspen-dominated broad-leaved forest in northern China. PLoS ONE 2018, 13, 0195630. [Google Scholar] [CrossRef]
- Shi, L.; Li, G.; Liu, H.; Dech, J.P.; Zhou, M.; Zhao, P.; Ren, Z. Dendrochronological Reconstruction of June Drought (PDSI) from 1731–2016 for the Western Mongolian Plateau. Atmosphere 2020, 11, 839. [Google Scholar] [CrossRef]
- Jonsson, G.B.; Hofgaard, A. The structure and regeneration of high-altitude Norway spruce forests: A review of Arnborg (1942, 1943). Scand. J. For. Res. 2011, 26 (Suppl. 10), 17–24. [Google Scholar] [CrossRef]
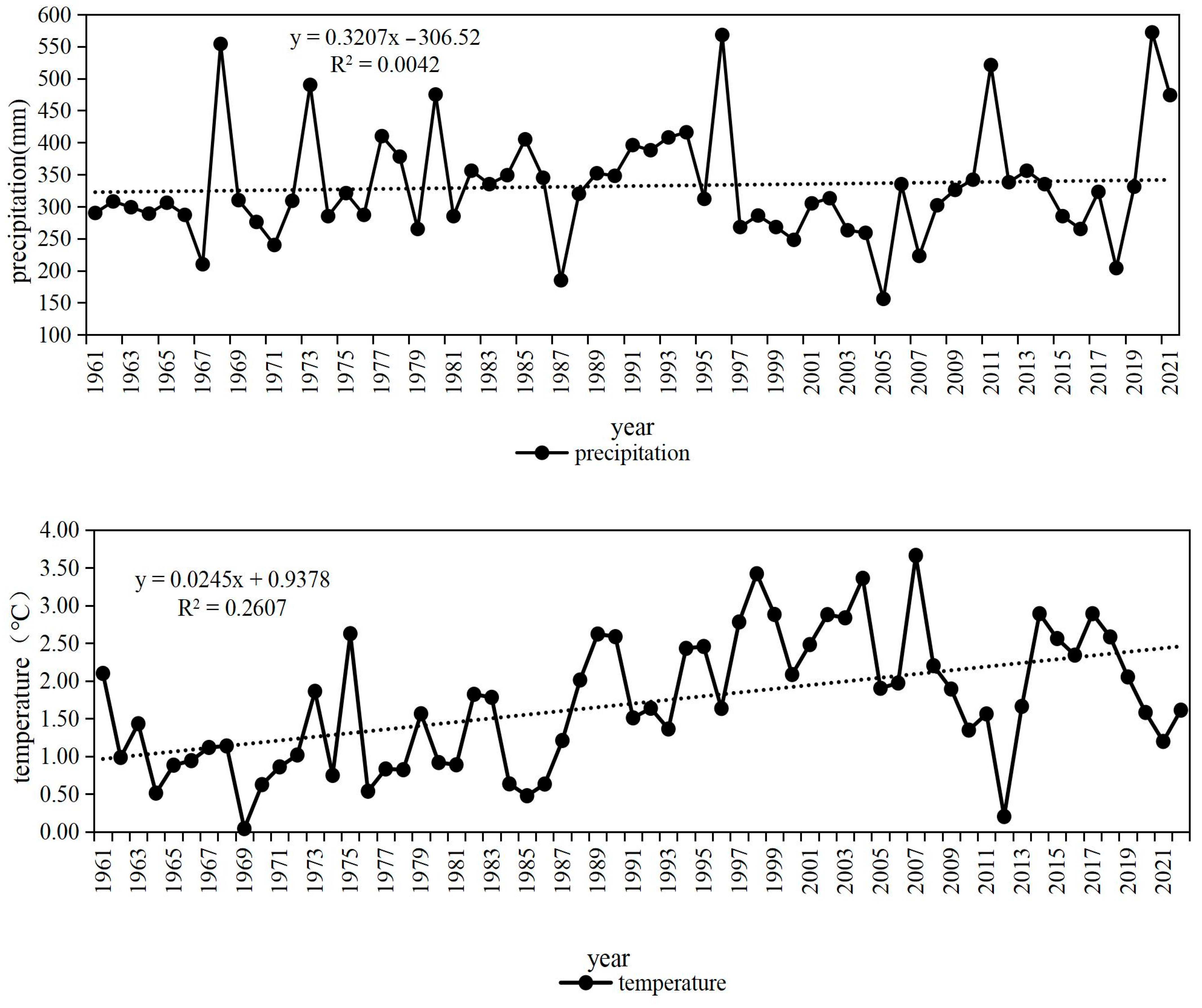
- Wang, Z.C.; Chen, S.H.; Li, C. Analysis of Climate Change Characteristics in the West Liaohe River Basin in the Past 57 Years. J. Inn. Mong. Agric. Univ. 2020, 41, 42–48. [Google Scholar]
- Liang, H.M.; Wei, J.S.; He, M.; Zhou, M.; Zhao, P.W. Response of Radial Growth of North China Larch Artificial Forest in the Southern Section of Daxing’an Mountains to Climate Change. Temp. For. Res. 2019, 2, 31–36+62. [Google Scholar]
- Zhao, P.W.; Guan, L.J.; Liu, B.B.; Zhou, M.; Shu, Y. Current status and prospects of forest dynamics research in the eastern section of semi-arid regions in China. World For. Res. 2021, 34, 74–79. [Google Scholar]
- Zeng, N.; Zhou, M.; Zhao, P.W. Spatial pattern and interspecific relationships of broad-leaved secondary forests in the southern section of the Greater Khingan Mountains. J. Northeast For. Univ. 2014, 42, 37–41. [Google Scholar]
- Mori, A.; Mizumachi, E.; Osono, T.; Yusuke, D. Substrate-associated seedling recruitment and establishment of major conifer species in an old-growth subalpine forest in central Japan. For. Ecol. Manag. 2004, 196, 287–297. [Google Scholar] [CrossRef]
- Zhao, P.W.; Guan, L.J.; Zhou, M.; Zou, J.M.; Bao, H.; Guo, J.Y.; Zhou, L.W. Basic characteristics of woody debris in the secondary forest area of Hanshan, Inner Mongolia. Resour. Environ. Arid Areas 2021, 35, 155–161. [Google Scholar]
- Guan, L.J.; Zhao, P.W.; Zhou, M. The impact of fallen trees on forest regeneration in secondary forest areas in the southern section of the Greater Khingan Mountains. For. Sci. Res. 2022, 35, 97–103. [Google Scholar]
- Yifan, S.; Ge, Y.; Guangfu, Z. Light Competition Contributes to the Death of Masson Pines of Coniferous-Broadleaf Mixed Forests in Subtropical China. Forests 2022, 13, 85. [Google Scholar] [CrossRef]
- Tuomas, A.; Juha, H. Harmonized decay classification for dead wood in Nordic national forest inventories. Scand. J. For. Res. 2024, 39, 1–7. [Google Scholar]
- Xiao, T.X.; Hong, Y.L.; Zhao, L.S. Response of aboveground biomass and diversity to nitrogen addition along a degradation gradient in the Inner Mongolian steppe, China. Sci. Rep. 2015, 5, 10284. [Google Scholar]
- Zhang, S.L. Research on Plant Diversity and Protection in Saihanwula Nature Reserve, Inner Mongolia; Beijing Forestry University: Beijing, China, 2007. [Google Scholar]
- Wu, X.C.; Pei, T.T.; Li, X.Y. Research progress on the response of tree growth to climate change. J. Beijing Norm. Univ. Nat. Sci. Ed. 2016, 52, 109–116. [Google Scholar]
- Feng, Q.Q.; Zhou, M.; Zhao, P.W. Study on Seed Rain and Surface Seed Bank of Betula platyphylla at Different Ages in the Southern Section of the Greater Khingan Mountains. For. Resour. Manag. 2019, 4, 74–79. [Google Scholar]
- Chen, J.Y. The Effect of Coarse Woody Residues on Seedling Regeneration in Typical Broad-Leaved Korean Pine Forests; Northeast Forestry University: Harbin, China, 2016. [Google Scholar]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki, M.M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef]

| Plot | Longitude | Latitude | Altitude (m) | Aspect | Slope (°) | Canopy Closure | Downed Dead Wood Diameter |
|---|---|---|---|---|---|---|---|
| E1 | 118°43′47.19″ | 44°12′59.36″ | 1217.2 | WN | 12° | 0.83 | 12.83 ± 3.05 |
| E2 | 118°43′47.39″ | 44°12′58.30″ | 1227.6 | WN | 10° | 0.76 | 12.24 ± 1.15 |
| E3 | 118°43′46.27″ | 44°12′56.90″ | 1237.2 | WN | 13° | 0.60 | 10.69 ± 1.98 |
| M1 | 118°43′46.33″ | 44°13′00.37″ | 1212.8 | WN | 16° | 0.68 | 9.32 ± 2.89 |
| M2 | 118°43′48.10″ | 44°12′57.74″ | 1231.8 | WN | 19° | 0.51 | 8.75 ± 3.46 |
| M3 | 118°43′47.38″ | 44°12′57.20″ | 1234.1 | WN | 12° | 0.78 | 8.91 ± 1.93 |
| L1 | 118°44′08.53″ | 44°12′56.46″ | 1227.2 | WN | 18° | 0.65 | 10.64 ± 3.59 |
| L2 | 118°44′10.55″ | 44°12′56.65″ | 1232.9 | WN | 17° | 0.56 | 9.69 ± 3.41 |
| L3 | 118°44′07.52″ | 44°12′55.62″ | 1239.2 | WN | 23° | 0.66 | 9.84 ± 2.32 |
| The Existence of Downed Dead Wood | Features |
|---|---|
| Uproot | Uprooted death wood |
| Trunk base fracture | The height of the broken pole < 1 m |
| Break in the middle of trunk | The height of the broken pole ≥ 1 m |
| Root pile | Standing dead trees, length < 1 m |
| Tree segment | Large dead branches and dead wood without head and tail, length > 1 m |
| Features | Decay class | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Leaves | Exist | None | None | None | None |
| Branches | All twigs exist | Big branch exists | Big, thick branches exist | The branches have fallen off, and the trunk is still there | None |
| Bark | Exist | Exist | Mostly exist | Mostly fallen off | None |
| Backbone shape | Round | Round | Round | Round to oval | Oval to flat |
| Invaded by the root | None | None | Sapwood area | Invade all | Invade all |
| Plant growth | None | Little plant growth | Few shrub seedlings and moss | Large area of moss | Bush moss and big tree |
| Plot | Uproot | Percentage | Trunk Base Fracture | Percentage | Break in the Middle of Trunk | Percentage | Tree Segment | Percentage |
|---|---|---|---|---|---|---|---|---|
| Later-death plot | 26 ± 5 | 6.38% | 281 ± 15 | 69.04% | 96 ± 7 | 23.58% | 4 ± 1.2 | 0.98% |
| Mid-death plot | 13 ± 2 | 3.96% | 209 ± 12 | 63.71% | 104 ± 6 | 31.71% | 2 ± 0.5 | 0.61% |
| Early-death plot | 17 ± 4 | 4.91% | 245 ± 13 | 70.81% | 83 ± 11 | 23.98% | 1 ± 0.3 | 0.28% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Guan, L.; Yao, H.; Shu, Y.; Yue, Y.; Liu, F.; Zheng, Y.; Hao, L.; Xiang, C.; Zhou, L. Impacts of Downed Dead Wood Poplar Trees on Forest Regeneration in the Semi-Arid Region of Northern China. Forests 2024, 15, 1460. https://doi.org/10.3390/f15081460
Zhao P, Guan L, Yao H, Shu Y, Yue Y, Liu F, Zheng Y, Hao L, Xiang C, Zhou L. Impacts of Downed Dead Wood Poplar Trees on Forest Regeneration in the Semi-Arid Region of Northern China. Forests. 2024; 15(8):1460. https://doi.org/10.3390/f15081460
Chicago/Turabian StyleZhao, Pengwu, Lijuan Guan, Huaxia Yao, Yang Shu, Yongjie Yue, Furen Liu, Yaxiong Zheng, Longfei Hao, Changlin Xiang, and Liwen Zhou. 2024. "Impacts of Downed Dead Wood Poplar Trees on Forest Regeneration in the Semi-Arid Region of Northern China" Forests 15, no. 8: 1460. https://doi.org/10.3390/f15081460
APA StyleZhao, P., Guan, L., Yao, H., Shu, Y., Yue, Y., Liu, F., Zheng, Y., Hao, L., Xiang, C., & Zhou, L. (2024). Impacts of Downed Dead Wood Poplar Trees on Forest Regeneration in the Semi-Arid Region of Northern China. Forests, 15(8), 1460. https://doi.org/10.3390/f15081460







