Accumulation of Glomalin-Related Soil Protein Regulated by Plantation Types and Vertical Distribution of Soil Characteristics in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Soil Sampling
2.2. Determination of Soil Physicochemical Properties
2.3. Extraction and Determination of GRSP in Soil
2.4. Data Analysis
3. Results
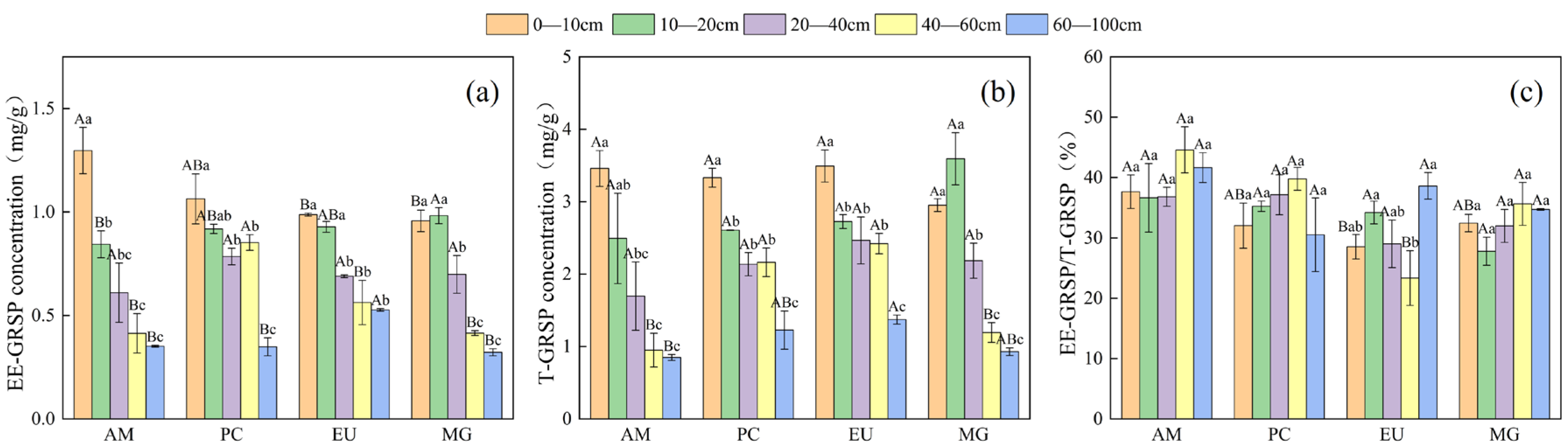
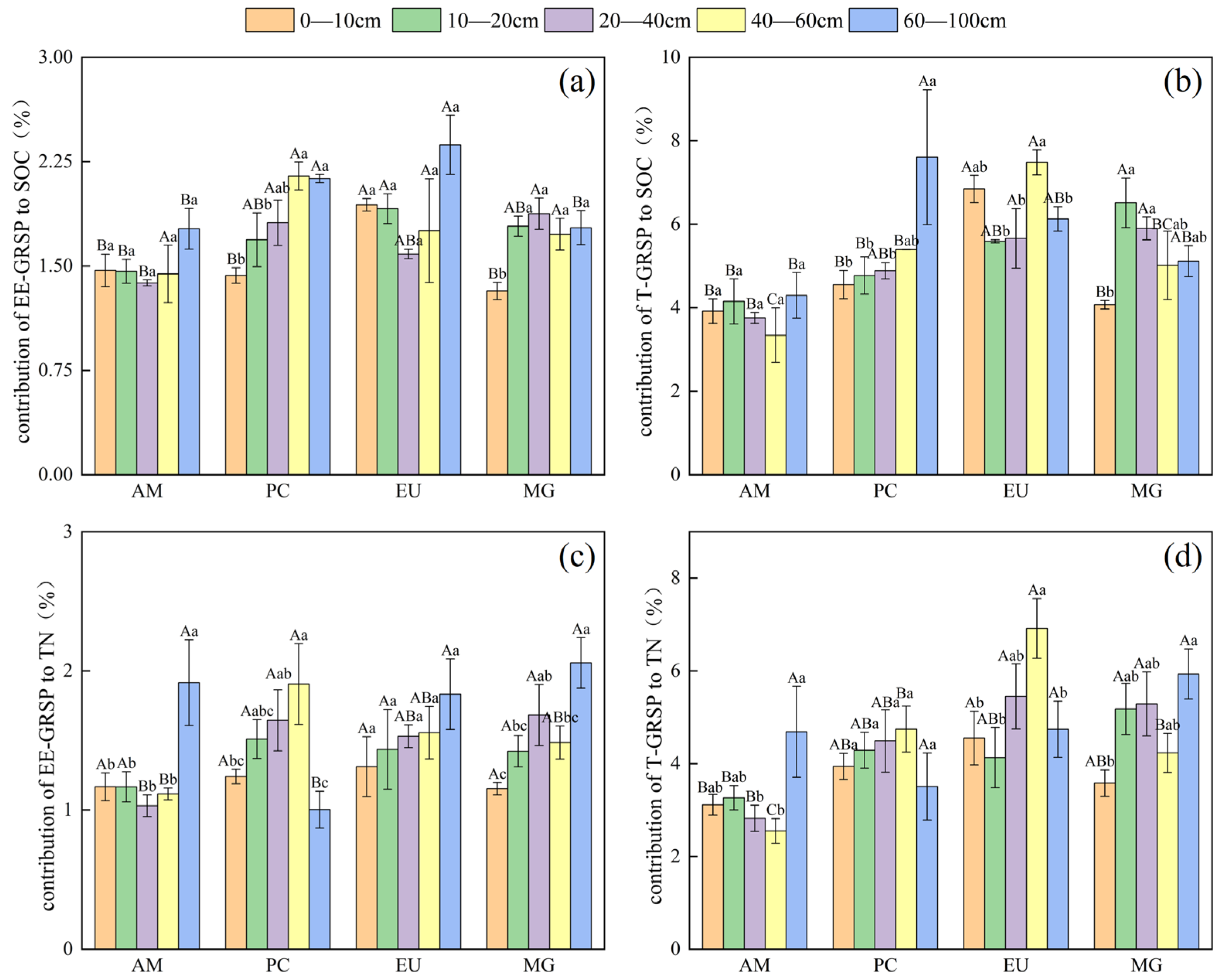
3.1. Effects of Tree Species and Soil Depth on GRSP Content and Distribution
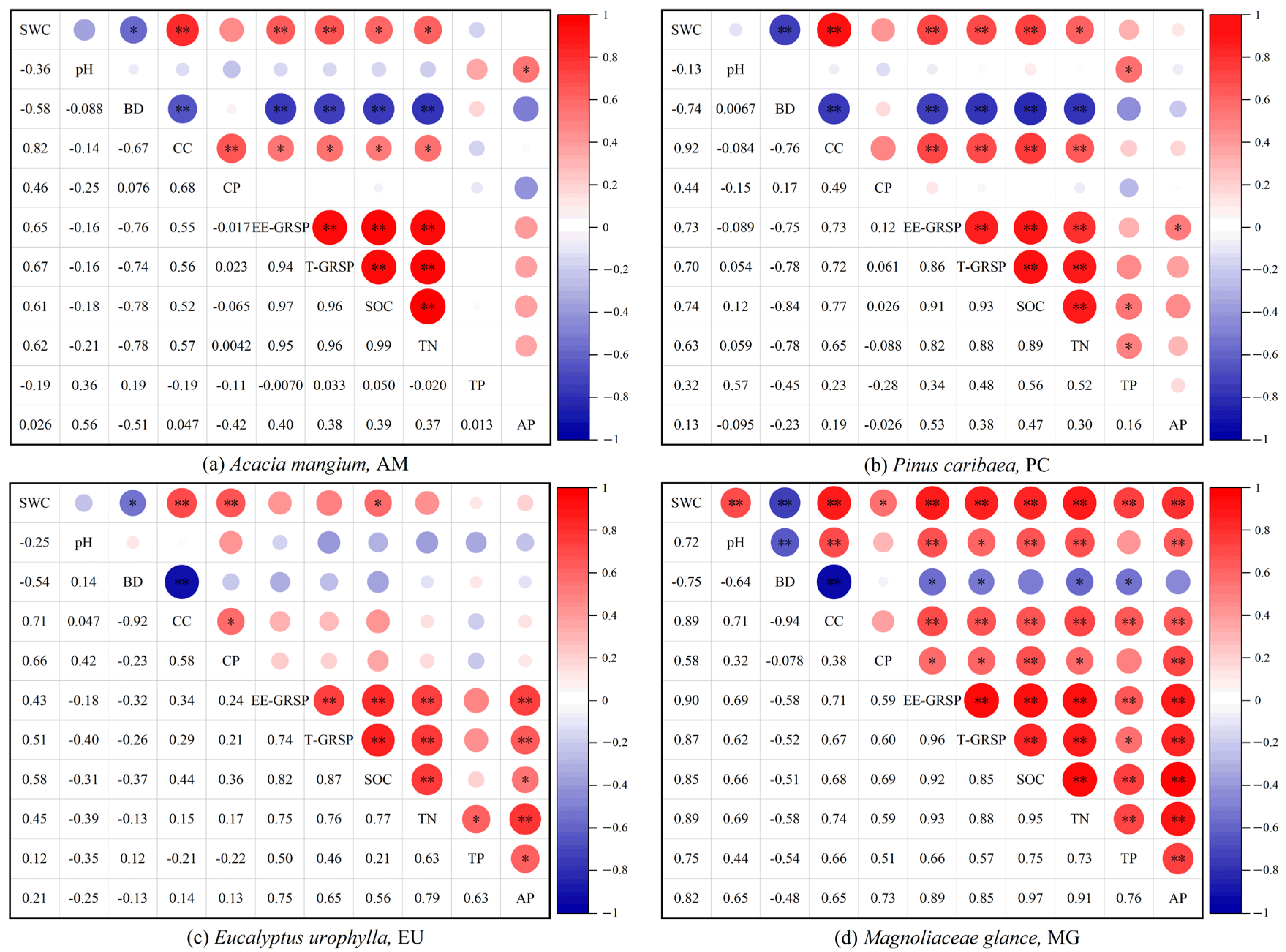
3.2. Correlation between Soil Physicochemical Properties and GRSP
4. Discussion
4.1. Effects of Tree Species on the Accumulation of GRSP in Soil
4.2. Variation in Concentration and Contribution to Carbon and Nitrogen of GRSP in Deep Soil
4.3. Effects of Soil Properties on the Vertical Distribution of GRSP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, S.F.; Upadhyaya, A. Extraction of an Abundant and Unusual Protein from Soil and Comparison with Hyphal Protein of Arbuscular Mycorrhizal Fungi. Soil Sci. 1996, 161, 575. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A Survey of Soils for Aggregate Stability and Glomalin, a Glycoprotein Produced by Hyphae of Arbuscular Mycorrhizal Fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular Mycorrhizae, Glomalin, and Soil Aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Wright, S.F.; Clark, D.A.; Ruess, R.W. Soil Stocks of Glomalin Produced by Arbuscular Mycorrhizal Fungi across a Tropical Rain Forest Landscape. J. Ecol. 2004, 92, 278–287. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large Contribution of Arbuscular Mycorrhizal Fungi to Soil Carbon Pools in Tropical Forest Soils. Plant Soil 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Barbosa, M.V.; Pedroso, D.D.F.; Curi, N.; Carneiro, M.A.C. Do Different Arbuscular Mycorrhizal Fungi Affect the Formation and Stability of Soil Aggregates? Ciênc. Agrotec. 2019, 43, e003519. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Zhong, S.; Yin, G.; Gao, Y.; He, X. Recalcitrant Carbon Components in Glomalin-Related Soil Protein Facilitate Soil Organic Carbon Preservation in Tropical Forests. Sci. Rep. 2017, 7, 2391. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The Effects of Arbuscular Mycorrhizal Fungi on Glomalin-Related Soil Protein Distribution, Aggregate Stability and Their Relationships with Soil Properties at Different Soil Depths in Lead-Zinc Contaminated Area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef]
- Singh, A.K.; Rai, A.; Singh, N. Effect of Long Term Land Use Systems on Fractions of Glomalin and Soil Organic Carbon in the Indo-Gangetic Plain. Geoderma 2016, 277, 41–50. [Google Scholar] [CrossRef]
- Gao, W.-Q.; Wang, P.; Wu, Q.-S. Functions and Application of Glomalin-Related Soil Proteins: A Review. JSM 2019, 48, 111–119. [Google Scholar] [CrossRef]
- Wu, F.; Dong, M.; Liu, Y.; Ma, X.; An, L.; Young, J.P.W.; Feng, H. Effects of Long-Term Fertilization on AM Fungal Community Structure and Glomalin-Related Soil Protein in the Loess Plateau of China. Plant Soil 2011, 342, 233–247. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Yang, W.; Zhu, X. Spatial Changes in Glomalin-Related Soil Protein and Their Correlation with Soil Properties in the Black Soil Region of Northeast China. Agronomy 2022, 12, 2165. [Google Scholar] [CrossRef]
- Staunton, S.; Saby, N.P.A.; Arrouays, D.; Quiquampoix, H. Can Soil Properties and Land Use Explain Glomalin-Related Soil Protein (GRSP) Accumulation? A Nationwide Survey in France. Catena 2020, 193, 104620. [Google Scholar] [CrossRef]
- Li, T.; Yuan, Y.; Mou, Z.; Li, Y.; Kuang, L.; Zhang, J.; Wu, W.; Wang, F.; Wang, J.; Lambers, H.; et al. Faster Accumulation and Greater Contribution of Glomalin to the Soil Organic Carbon Pool than Amino Sugars Do under Tropical Coastal Forest Restoration. Glob. Chang. Biol. 2023, 29, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Preger, A.C.; Rillig, M.C.; Johns, A.R.; Du Preez, C.C.; Lobe, I.; Amelung, W. Losses of Glomalin-Related Soil Protein under Prolonged Arable Cropping: A Chronosequence Study in Sandy Soils of the South African Highveld. Soil Biol. Biochem. 2007, 39, 445–453. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Z.; Wang, Q.; Wang, H.; Fu, Y.; He, X. Glomalin Contributed More to Carbon, Nutrients in Deeper Soils, and Differently Associated with Climates and Soil Properties in Vertical Profiles. Sci. Rep. 2017, 7, 13003. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, Z.; Wang, H.; Fu, Y. Variation in Glomalin in Soil Profiles and Its Association with Climatic Conditions, Shelterbelt Characteristics, and Soil Properties in Poplar Shelterbelts of Northeast China. J. For. Res. 2020, 31, 279–290. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration China Forest Resources Report (2014–2018); Chinese Forestry Publishing House: Beijing, China, 2019.
- Gu, R.; Xiao, K.; Zhu, Z.; He, X.; Li, D. Afforestation Enhances Glomalin-Related Soil Protein Content but Decreases Its Contribution to Soil Organic Carbon in a Subtropical Karst Area. J. Environ. Manag. 2024, 356, 120754. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Fang, C.; Chen, J.; Li, B. The Effects of Plantation Practice on Soil Properties Based on the Comparison between Natural and Planted Forests: A Meta-Analysis. Glob. Ecol. Biogeogr. 2012, 21, 318–327. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, Q.; Cheng, G.; Zhu, M.; Zhang, Z.; Jing, L.; Wang, L.; Li, Q.; Tao, Q.; Zhang, X.; et al. Tree Growth and Density Enhanced, While Diversity and Spatial Clustering Reduced Soil Mycorrhizal C and N Sequestration: Strong Interaction with Soil Properties in Northeastern China. Sci. Total Environ. 2024, 912, 169131. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The Role of Glomalin in Mitigation of Multiple Soil Degradation Problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638. [Google Scholar] [CrossRef]
- Rotter, P.; Malý, S.; Sáňka, O.; Sáňka, M.; Čižmár, D.; Zbíral, J.; Čechmánková, J.; Kalábová, T. Is Glomalin an Appropriate Indicator of Forest Soil Reactive Nitrogen Status? J. Plant Nutr. Soil Sci. 2017, 180, 694–704. [Google Scholar] [CrossRef]
- Singh, A.K.; Rai, A.; Banyal, R.; Chauhan, P.S.; Singh, N. Plant Community Regulates Soil Multifunctionality in a Tropical Dry Forest. Ecol. Indic. 2018, 95, 953–963. [Google Scholar] [CrossRef]
- LY/T 1215–1999; Forestry Industry Standards of China. Determination of Forest Soil Water-Related Physical Properties. Chinese Academy of Forestry Sciences: Beijing, China, 1999.
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agricultural Publishing House: Beijing, China, 2000. [Google Scholar]
- Cissé, G.; Essi, M.; Kedi, B.; Nicolas, M.; Staunton, S. Accumulation and Vertical Distribution of Glomalin-Related Soil Protein in French Temperate Forest Soils as a Function of Tree Type, Climate and Soil Properties. Catena 2023, 220, 106635. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M. Glomalin in Ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The Role of Arbuscular Mycorrhizal Fungi and Glomalin in Soil Aggregation: Comparing Effects of Five Plant Species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G. Transformation of a Podocarpus Falcatus Dominated Natural Forest into a Monoculture Eucalyptus Globulus Plantation at Munesa, Ethiopia: Soil Organic C, N and S Dynamics in Primary Particle and Aggregate-Size Fractions. Agric. Ecosyst. Environ. 2005, 106, 89–98. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of Broadleaf and Needle Litter in Forests of British Columbia: Influences of Litter Type, Forest Type, and Litter Mixtures. Can. J. For. Res. 2011, 30, 1742–1750. [Google Scholar] [CrossRef]
- Singh, A.K.; Jiang, X.J.; Yang, B.; Li, H.; Liu, W.; Singh, N. Effect of Root-Glomalin on Soil Carbon Storage in Trees’ Rhizosphere and Interspace of a Tropical Dry Forest. Land Degrad. Dev. 2021, 32, 5281–5291. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; He, X.; Liu, J. Glomalin-Related Soil Protein Responses to Elevated CO2 and Nitrogen Addition in a Subtropical Forest: Potential Consequences for Soil Carbon Accumulation. Soil Biol. Biochem. 2015, 83, 142–149. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Xia, H.; Zou, B.; Li, N.; Liu, J.; Zhu, W. Effects of Nitrogen-Fixing and Non-Nitrogen-Fixing Tree Species on Soil Properties and Nitrogen Transformation during Forest Restoration in Southern China. Soil Sci. Plant Nutr. 2010, 56, 297–306. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.; Yu, X.; Gu, H.; Zheng, X.; Wang, C.; Xiao, F.; Shu, L.; et al. Co-Symbiosis of Arbuscular Mycorrhizal Fungi (AMF) and Diazotrophs Promote Biological Nitrogen Fixation in Mangrove Ecosystems. Soil Biol. Biochem. 2021, 161, 108382. [Google Scholar] [CrossRef]
- Frater, P.N.; Borer, E.T.; Fay, P.A.; Jin, V.; Knaeble, B.; Seabloom, E.; Sullivan, L.; Wedin, D.A.; Harpole, W.S. Nutrients and Environment Influence Arbuscular Mycorrhizal Colonization Both Independently and Interactively in Schizachyrium scoparium. Plant Soil 2018, 425, 493–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Xu, L.; Ma, S.; Cui, D.; Zhu, K.; Feng, W. Mixed Conifer-Broadleaf Trees on Arbuscular Mycorrhizal and Ectomycorrhizal Communities in Rhizosphere Soil of Different Plantation Stands in the Temperate Zone, Northeast China. Front. Microbiol. 2022, 13, 986515. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, X.; Lin, W.; Zheng, Y.; Li, J.; Hui, D.; Guo, J. Decreased Glomalin-Related Soil Protein with Nitrogen Deposition in a 3-Year-Old Cunninghamia Lanceolata Plantation. J. Soils Sediments 2022, 22, 931–941. [Google Scholar] [CrossRef]
- Cheng, G.; Zhu, M.; Zhang, X.; Guo, Y.; Yang, Y.; Yun, C.; Wu, Y.; Wang, Q.; Wang, W.; Wang, H. Northeastern China Shelterbelt-Farmland Glomalin Differences Depend on Geo-Climates, Soil Depth, and Microbial Interaction: Carbon Sequestration, Nutrient Retention and Implication. Appl. Soil Ecol. 2023, 191, 105068. [Google Scholar] [CrossRef]
- Sun, X.; Xing, Y.; Yan, G.; Liu, G.; Wang, X.; Wang, Q. Dynamics of Glomalin-Related Soil Protein and Soil Aggregates during Secondary Succession in the Temperate Forest. Catena 2024, 234, 107602. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep Soil Organic Matter—A Key but Poorly Understood Component of Terrestrial C Cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. The Depth Distribution of Soil Organic Carbon in Relation to Land Use and Management and the Potential of Carbon Sequestration in Subsoil Horizons. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2005; Volume 88, pp. 35–66. [Google Scholar]
- Ahrens, B.; Guggenberger, G.; Rethemeyer, J.; John, S.; Marschner, B.; Heinze, S.; Angst, G.; Mueller, C.W.; Kögel-Knabner, I.; Leuschner, C.; et al. Combination of Energy Limitation and Sorption Capacity Explains 14C Depth Gradients. Soil Biol. Biochem. 2020, 148, 107912. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Wang, D.; Wang, M.; Liao, C.; Yang, X.; Liu, F. Decoupled Linkage between Soil Carbon and Nitrogen Mineralization among Soil Depths in a Subtropical Mixed Forest. Soil Biol. Biochem. 2017, 109, 135–144. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; He, X.; Zhang, W.; Song, K.; Han, S. Role and Variation of the Amount and Composition of Glomalin in Soil Properties in Farmland and Adjacent Plantations with Reference to a Primary Forest in North-Eastern China. PLoS ONE 2015, 10, e0139623. [Google Scholar] [CrossRef]
- Gałązka, A.; Niedźwiecki, J.; Grządziel, J.; Gawryjołek, K. Evaluation of Changes in Glomalin-Related Soil Proteins (GRSP) Content, Microbial Diversity and Physical Properties Depending on the Type of Soil as the Important Biotic Determinants of Soil Quality. Agronomy 2020, 10, 1279. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, W.; Wang, Q.; Wu, Y.; Wang, H.; Pei, Z. Glomalin Amount and Compositional Variation, and Their Associations with Soil Properties in Farmland, Northeastern China. J. Plant Nutr. Soil Sci. 2017, 180, 563–575. [Google Scholar] [CrossRef]
- Chojnacka, A.; Jonczak, J.; Oktaba, L.; Pawłowicz, E.; Regulska, E.; Słowińska, S.; Olejniczak, I.; Oktaba, J.; Kruczkowska, B.; Jankiewicz, U. Dynamics of Fungal Community Structure in a Silver Birch (Betula Pendula Roth) Succession Chronosequence on Poor-Quality Post-Arable Soil. Agric. Ecosyst. Environ. 2023, 342, 108225. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Wang, H.; Nie, S.; Liang, Z. Effects of Soil Salinity on the Content, Composition, and Ion Binding Capacity of Glomalin-Related Soil Protein (GRSP). Sci. Total Environ. 2017, 581–582, 657–665. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Wang, W.; Zhong, Z.; Pei, Z.; Ren, J.; Wang, H.; Zu, Y. Spatial Variations in Concentration, Compositions of Glomalin Related Soil Protein in Poplar Plantations in Northeastern China, and Possible Relations with Soil Physicochemical Properties. Sci. World J. 2014, 2014, 160403. [Google Scholar] [CrossRef]
- Nautiyal, P.; Rajput, R.; Pandey, D.; Arunachalam, K.; Arunachalam, A. Role of Glomalin in Soil Carbon Storage and Its Variation across Land Uses in Temperate Himalayan Regime. Biocatal. Agric. Biotechnol. 2019, 21, 101311. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, C.; Shi, Z.; Yin, X.; Liu, C. Spatial Distribution of Glomalin-Related Soil Proteins in Coniferous and Broadleaf Mixed Temperate Forest. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1087–1093. [Google Scholar] [CrossRef]
- Ji, L.; Tan, W.; Chen, X. Arbuscular Mycorrhizal Mycelial Networks and Glomalin-Related Soil Protein Increase Soil Aggregation in Calcaric Regosol under Well-Watered and Drought Stress Conditions. Soil Tillage Res. 2019, 185, 1–8. [Google Scholar] [CrossRef]
- Liu, G.; Duan, X.; Yan, G.; Sun, X.; Jiang, S.; Xing, Y.; Wang, Q. Changes in Soil Aggregates and Glomalin-Related Soil Protein Stability During the Successional Process of Boreal Forests. J. Soil Sci. Plant Nutr. 2024, 24, 1335–1348. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Cao, M.-Q.; Zou, Y.-N.; He, X. Direct and Indirect Effects of Glomalin, Mycorrhizal Hyphae and Roots on Aggregate Stability in Rhizosphere of Trifoliate Orange. Sci. Rep. 2014, 4, 5823. [Google Scholar] [CrossRef] [PubMed]
- Voets, L.; Dupré de Boulois, H.; Renard, L.; Strullu, D.-G.; Declerck, S. Development of an Autotrophic Culture System for the in Vitro Mycorrhization of Potato Plantlets. FEMS Microbiol. Lett. 2005, 248, 111–118. [Google Scholar] [CrossRef] [PubMed]
| Parameter | EE-GRSP | T-GRSP | EE-GRSP/ T-GRSP | EE-GRSP-C/SOC | T-GRSP-C/SOC | EE-GRSP-N/TN | T-GRSP-N/TN | |
|---|---|---|---|---|---|---|---|---|
| Tree | F | 2.867 | 4.976 | 7.049 | 7.478 | 15.375 | 2.522 | 11.305 |
| p | 0.048 | 0.005 | <0.001 | <0.001 | <0.001 | 0.071 | <0.001 | |
| Depth | F | 66.830 | 49.071 | 0.928 | 4.988 | 1.178 | 3.927 | 1.825 |
| p | <0.001 | <0.001 | 0.458 | 0.002 | 0.335 | 0.009 | 0.143 | |
| Tree × Depth | F | 3.961 | 2.615 | 1.968 | 2.052 | 3.197 | 2.900 | 2.677 |
| p | <0.001 | 0.011 | 0.055 | 0.044 | 0.003 | 0.006 | 0.010 | |
| Plantation | Y | Depth | Parameter | Unstandardized Coefficient, B | Standardized Coefficient, β | Sig. | R2 |
|---|---|---|---|---|---|---|---|
| AM | EE-GESP | Top soil, 0–40 cm | SOC | 0.039 | 0.995 | <0.001 | 0.990 |
| Deep soil, 40–100 cm | CP | 0.010 | 0.984 | <0.001 | 0.968 | ||
| T-GRSP | Top soil, 0–40 cm | TN | 0.789 | 0.993 | <0.001 | 0.985 | |
| Deep soil, 40–100 cm | CP | 0.028 | 0.987 | <0.001 | 0.974 | ||
| PC | EE-GESP | Top soil, 0–40 cm | SWC | 0.017 | 0.531 | 0.002 | 0.995 |
| TN | 0.163 | 0.473 | 0.004 | ||||
| Deep soil, 40–100 cm | SOC | 0.057 | 0.998 | <0.001 | 0.996 | ||
| T-GRSP | Top soil, 0–40 cm | SOC | 0.070 | 0.559 | 0.016 | 0.992 | |
| TP | 1.072 | 0.442 | 0.041 | ||||
| Deep soil, 40–100 cm | SOC | 0.154 | 0.980 | <0.001 | 0.961 | ||
| EU | EE-GESP | Top soil, 0–40 cm | SOC | 0.049 | 0.995 | <0.001 | 0.991 |
| Deep soil, 40–100 cm | CP | 0.015 | 0.989 | <0.001 | 0.978 | ||
| T-GRSP | Top soil, 0–40 cm | TP | 2.521 | 0.993 | <0.001 | 0.987 | |
| Deep soil, 40–100 cm | SOC | 0.182 | 0.990 | <0.001 | 0.979 | ||
| MG | EE-GESP | Top soil, 0–40 cm | SWC | 0.018 | 0.630 | 0.002 | 0.992 |
| TN | 0.122 | 0.373 | 0.039 | ||||
| Deep soil, 40–100 cm | SOC | 0.046 | 0.995 | <0.001 | 0.990 | ||
| T-GRSP | Top soil, 0–40 cm | SWC | 0.096 | 0.983 | <0.001 | 0.966 | |
| Deep soil, 40–100 cm | CP | 0.025 | 0.983 | <0.001 | 0.965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Zhang, S.; Gu, X.; He, Z.; Liu, Y.; Mo, Q. Accumulation of Glomalin-Related Soil Protein Regulated by Plantation Types and Vertical Distribution of Soil Characteristics in Southern China. Forests 2024, 15, 1479. https://doi.org/10.3390/f15081479
Wu M, Zhang S, Gu X, He Z, Liu Y, Mo Q. Accumulation of Glomalin-Related Soil Protein Regulated by Plantation Types and Vertical Distribution of Soil Characteristics in Southern China. Forests. 2024; 15(8):1479. https://doi.org/10.3390/f15081479
Chicago/Turabian StyleWu, Miaolan, Shaochun Zhang, Xiaojuan Gu, Zhihang He, Yue Liu, and Qifeng Mo. 2024. "Accumulation of Glomalin-Related Soil Protein Regulated by Plantation Types and Vertical Distribution of Soil Characteristics in Southern China" Forests 15, no. 8: 1479. https://doi.org/10.3390/f15081479





