Using Headspace Gas Chromatography–Mass Spectrometry to Investigate the Volatile Terpenoids Released from the Liquidambar formosana Leaf and Its Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Hydrodistillation of Leaf Essential Oil
2.3. Chemical Analysis of Leaf Essential Oil via the GC-MS Method
2.4. VOC Analyses of Leaves and Leaf Essential Oils via the HS-GC-MS Method
2.5. Statistical Analysis
3. Results and Discussion
3.1. Constituent Analysis of L. formosana Leaf Essential Oils
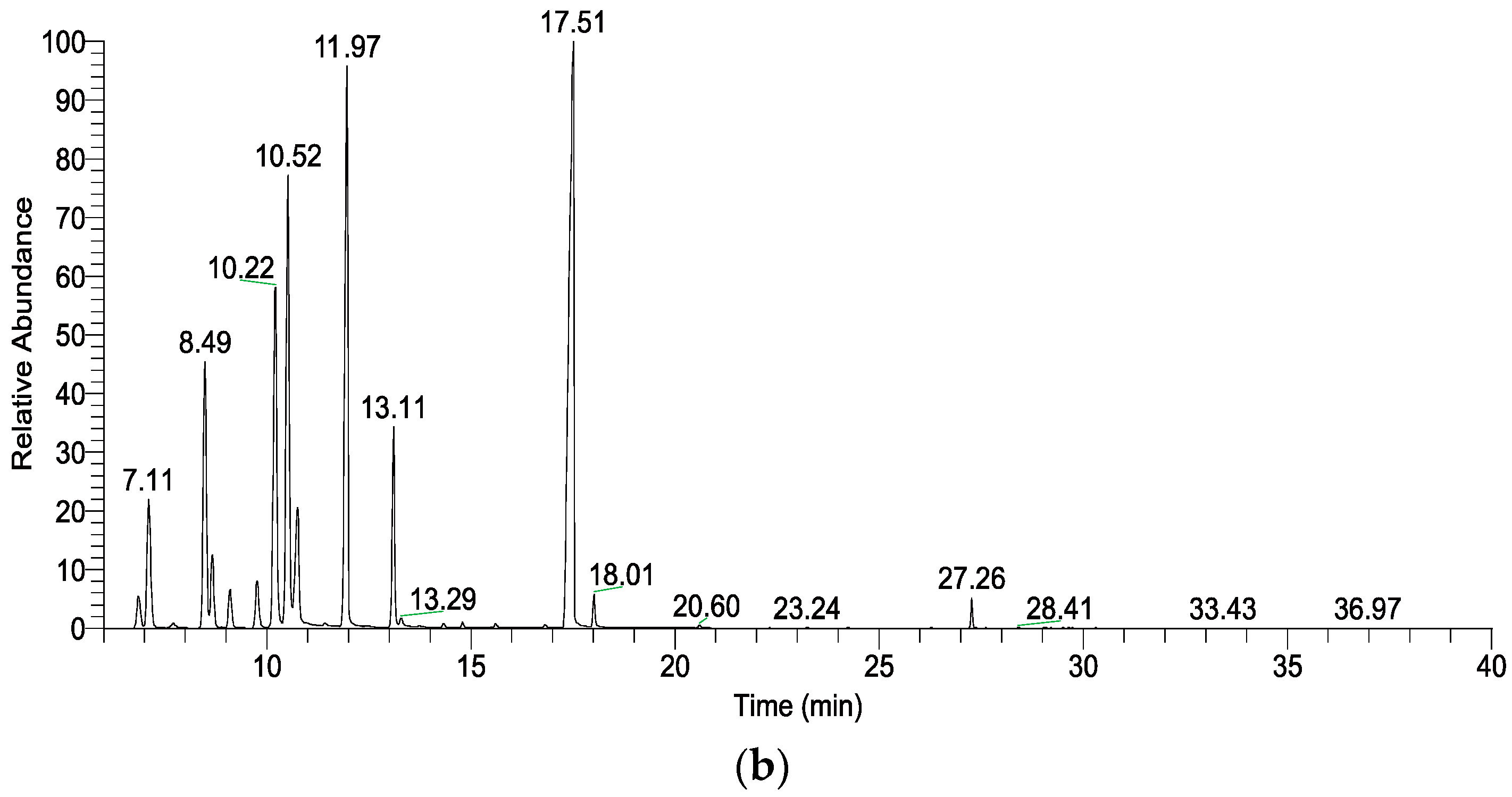
3.2. VOC Analyses of L. formosana Leaves at Different Temperatures
3.3. VOC Analyses of Leaf Essential Oils at Different Temperatures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 40 CFR 51.100. Available online: https://www.law.cornell.edu/cfr/text/40/51.100 (accessed on 17 July 2024).
- Hayward, S.; Tani, A.; Owen, S.M.; Hewitt, C.N. Online analysis of volatile organic compound emissions from Sitka spruce (Picea sitchensis). Tree Physiol. 2004, 24, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Dicke, M.; Schnitzler, J.P.; Turlings, T.C. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, Q.; Xu, H.; Zuo, Z. Dynamic physiological responses of Cinnamomum camphora with monoterpene protection under high temperature shock. Forests 2023, 14, 2005. [Google Scholar] [CrossRef]
- Walker, H.; Jena, A.; McEwan, K.; Evans, G.; Campbell, S. Natural volatile organic compounds (NVOCs) are greater and more diverse in UK forests compared with a public garden. Forests 2023, 14, 92. [Google Scholar] [CrossRef]
- Dehimeche, N.; Buatois, B.; Bertin, N.; Staudt, M. Insights into the intraspecific variability of the above and belowground emissions of volatile organic compounds in tomato. Molecules 2021, 26, 237. [Google Scholar] [CrossRef]
- Isidorov, V.; Jdanova, M. Volatile organic compounds from leaves litter. Chemosphere 2002, 48, 975–979. [Google Scholar] [CrossRef]
- Lemaitre-Guillier, C.; Dufresne, C.; Chartier, A.; Cluzet, S.; Valls, J.; Jacquens, L.; Douillet, A.; Aveline, N.; Adrian, M.; Daire, X. VOCs are relevant biomarkers of elicitor-induced defences in grapevine. Molecules 2021, 26, 4258. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre-Guillier, C.; Chartier, A.; Dufresne, C.; Douillet, A.; Cluzet, S.; Valls, J.; Aveline, N.; Daire, X.; Adrian, M. Elicitor-induced VOC emission by grapevine leaves: Characterisation in the vineyard. Molecules 2022, 27, 6028. [Google Scholar] [CrossRef]
- MacDougall, S.; Bayansal, F.; Ahmadi, A. Emerging methods of monitoring volatile organic compounds for detection of plant pests and disease. Biosensors 2022, 12, 239. [Google Scholar] [CrossRef]
- Althoff, E.R.; O’Loughlin, T.J.; Wakarchuk, D.A.; Aukema, K.G.; Aukema, B.H. Monoterpene composition of phloem of Eastern Larch (Larix laricina (Du Roi) K. Koch) in the great lakes region: With what must the eastern larch beetle (Dendroctonus simplex LeConte) contend? Forests 2023, 14, 566. [Google Scholar] [CrossRef]
- Stříbrská, B.; Moliterno, A.A.C.; Hüttnerová, T.; Leiner, M.; Surový, P.; Jirošová, A. Pilot study of 3D spatial distribution of α-pinene emitted by Norway spruce (L.) Karst recently infested by Ips typographus (L. 1758) (Coleoptera: Scolytinae). Forests 2024, 15, 10. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest volatile organic compounds and their effects on human health: A state-of-the-art review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar]
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. Int. J. Mol. Sci. 2020, 21, 8956. [Google Scholar] [CrossRef] [PubMed]
- García-Nicolás, M.; Arroyo-Manzanares, N.; Arce, L.; Hernández-Córdoba, M.; Viñas, P. Headspace gas chromatography coupled to mass spectrometry and ion mobility spectrometry: Classification of virgin olive oils as a study case. Foods 2020, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Jakubska-Busse, A.; Dziadas, M.; Gruss, I.; Kobyłka, M.J. Floral volatile organic compounds and a list of pollinators of Fallopia baldschuanica (Polygonaceae). Insects 2022, 13, 904. [Google Scholar] [CrossRef]
- Caser, M.; Scariot, V. The contribution of volatile organic compounds (VOCs) emitted by petals and pollen to the scent of garden roses. Horticulturae 2022, 8, 1049. [Google Scholar] [CrossRef]
- Karami, A.; Niazi, A.; Kavoosi, G.; Khosh-Khui, M.; Salehi, H. Temporal characterization of 2-phenylethanol in strongly and weakly scented genotypes of damask rose. Physiol. Mol. Biol. Plants 2015, 21, 43–49. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.M. Comparison of static headspace, headspace solid phase microextraction, headspace sorptive extraction, and direct thermal desorption techniques on chemical composition of French olive oils. J. Agric. Food Chem. 2003, 51, 7709–7716. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Okereke, C.N.; Jiang, Y.; Talts, E.; Kaurilind, E.; Niinemets, Ü. Wounding-Induced VOC emissions in five tropical agricultural species. Molecules 2021, 26, 2602. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Riikonen, J.; Valolahti, H.; Elina, H.; Holopainen, J.K.; Holopainen, T. Effects of elevated ozone and warming on terpenoid emissions and concentrations of Norway spruce depend on needle phenology and age. Tree Physiol. 2022, 42, 1570–1586. [Google Scholar] [CrossRef]
- Mariano, A.P.X.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; García, Y.M.; de Paula, A.C.C.F.F.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Optimization of extraction conditions and characterization of volatile organic compounds of Eugenia klotzschiana O. berg fruit pulp. Molecules 2022, 27, 935. [Google Scholar] [CrossRef] [PubMed]
- Lingbeck, J.M.; O’Bryan, C.A.; Martin, E.M.; Adams, J.P.; Crandall, P.G. Sweetgum: An ancient source of beneficial compounds with modern benefits. Pharmacogn. Rev. 2015, 9, 1–11. [Google Scholar] [PubMed]
- Lai, Y.J.; Li, S.J.; Wang, W.M. Evolutionary trends in leaf morphology and biogeography of Altingiaceae based on fossil evidence. Palaeoworld 2018, 27, 415–422. [Google Scholar] [CrossRef]
- Mancarz, G.F.F.; Laba, L.C.; da Silva, E.C.P.; Prado, M.R.M.; de Souza, L.M.; de Souza, D.; Nakashima, T.; Mello, R.G. Liquidambar styraciflua L.: A new potential source for therapeutic uses. J. Pharm. Biomed. Anal. 2019, 174, 422–431. [Google Scholar] [CrossRef]
- Chien, S.C.; Xiao, J.H.; Tseng, Y.H.; Kuo, Y.H.; Wang, S.Y. Composition and antifungal activity of balsam from Liquidambar formosana Hance. Holzforschung 2013, 67, 345–351. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.F.; Tu, Z.C.; Zhao, Y.; Wang, H.; Li, G.J.; Wang, H.; Sha, X.M. α-Glucosidase inhibition, anti-glycation and antioxidant activities of Liquidambar formosana Hance leaf, and identification of phytochemical profile. S. Afr. J. Bot. 2017, 113, 239–247. [Google Scholar] [CrossRef]
- Chuang, L.; Wen, C.H.; Lee, Y.R.; Lin, Y.L.; Hsu, L.R.; Wang, S.Y.; Chu, F.H. Identification, functional characterization, and seasonal expression patterns of five sesquiterpene synthases in Liquidambar formosana. J. Nat. Prod. 2018, 81, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, A.; Zeng, T.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The essential oil composition and antimicrobial activity of Liquidambar formosana oleoresin. Plants 2020, 9, 822. [Google Scholar] [CrossRef]
- Zhu, Y.; Guan, Y.J.; Chen, Q.Z.; Yuan, L.H.; Xu, Q.Q.; Zhou, M.L.; Liu, H.; Lin, W.; Zhang, Z.D.; Zhou, Z.L.; et al. Pentacyclic triterpenes from the resin of Liquidambar formosana have anti-angiogenic properties. Phytochemistry 2021, 184, 112676. [Google Scholar] [CrossRef]
- Chen, S.; Dong, M.; Zhang, Y.; Qi, S.; Liu, X.; Zhang, J.; Zhao, J. Development and characterization of simple sequence repeat markers for, and genetic diversity analysis of Liquidambar formosana. Forests 2020, 11, 203. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lee, C.J. Sleep-enhancing effects of phytoncide via behavioral, electrophysiological, and molecular modeling approaches. Exp. Neurobiol. 2020, 29, 120–129. [Google Scholar] [CrossRef]
- Wen, Y.; Yan, Q.; Pan, Y.; Gu, X.; Liu, Y. Medical empirical research on forest bathing (Shinrin-yoku): A systematic review. Environ. Health Prev. Med. 2019, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential oils, phytoncides, aromachology, and aromatherapy—A review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Morita, E.; Fukuda, S.; Nagano, J.; Hamajima, N.; Yamamoto, H.; Iwai, Y.; Nakashima, T.; Ohira, H.; Shirakawa, T. Psychological effects of forest environments on healthy adults: Shinrin-yoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health 2007, 121, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Mardani, A.; Basirinezhad, M.H.; Hamidzadeh, A.; Eskandari, F. The effects of Lavender and Chamomile essential oil inhalation aromatherapy on depression, anxiety and stress in older community-dwelling people: A randomized controlled trial. Explore 2022, 18, 272–278. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, S.L.; Ko, C.H.; Chang, H.T. Antifungal sesquiterpenoids from Michelia formosana leaf essential oil against wood-rotting fungi. Molecules 2022, 27, 2136. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, Y.Y.; Chang, S.T.; Chang, H.T. Xanthine oxidase inhibitory activity and chemical composition of Pistacia chinensis leaf essential oil. Pharmaceutics 2022, 14, 1982. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 6–611. ISBN 978-1932633214. [Google Scholar]
- Chang, H.T.; Chang, M.L.; Chen, Y.T.; Chang, S.T.; Hsu, F.L.; Wu, C.C.; Ho, C.K. Evaluation of motor coordination and antidepressant activities of Cinnamomum osmophloeum ct. linalool leaf oil in rodent model. Molecules 2021, 26, 3037. [Google Scholar] [CrossRef]
- Chen, G.R.; Chang, M.L.; Chang, S.T.; Ho, Y.T.; Chang, H.T. Cytotoxicity and apoptosis induction of 6,7-dehydroroyleanone from Taiwania cryptomerioides bark essential oil in hepatocellular carcinoma cells. Pharmaceutics 2022, 14, 351. [Google Scholar] [CrossRef]
- Ho, Y.T.; Liu, I.H.; Chang, S.T.; Wang, S.Y.; Chang, H.T. In vitro and in vivo antimelanogenesis effects of leaf essential oil from Agathis dammara. Pharmaceutics 2023, 15, 2269. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.F.; Yang, T.J.; Chiu, H.W.; Ho, C.L. Essential oil from leaves of Liquidambar formosana ameliorates inflammatory response in lipopolysaccharide-activated mouse macrophages. Nat. Prod. Commun. 2014, 9, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Liu, H.; Liu, W.; Zhang, R.; Xian, M.; Liu, H. Biosynthesis and production of sabinene: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Phillips, M.A. Structural diversity and biosynthesis of plant derived p-menthane monoterpenes. Phytochem. Rev. 2021, 20, 433–459. [Google Scholar] [CrossRef]
- Filella, I.; Wilkinson, M.J.; Llusià, J.; Hewitt, C.N.; Peñuelas, J. Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol. Plant 2007, 130, 58–66. [Google Scholar] [CrossRef]
- Yuan, J.S.; Himanen, S.J.; Holopainen, J.K.; Chen, F.; Stewart, C.N., Jr. Smelling global climate change: Mitigation of function for plant volatile organic compounds. Trends Ecol. Evol. 2009, 24, 323–331. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef] [PubMed]
- Yatagai, M.; Ohira, M.; Ohira, T.; Nagai, S. Seasonal variations of terpene emission from trees and influence of temperature, light, and contact stimulation on terpene emission. Chemosphere 1995, 30, 1137–1149. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene prevents skeletal muscle atrophy by inhibiting the MAPK–MuRF-1 pathway in rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
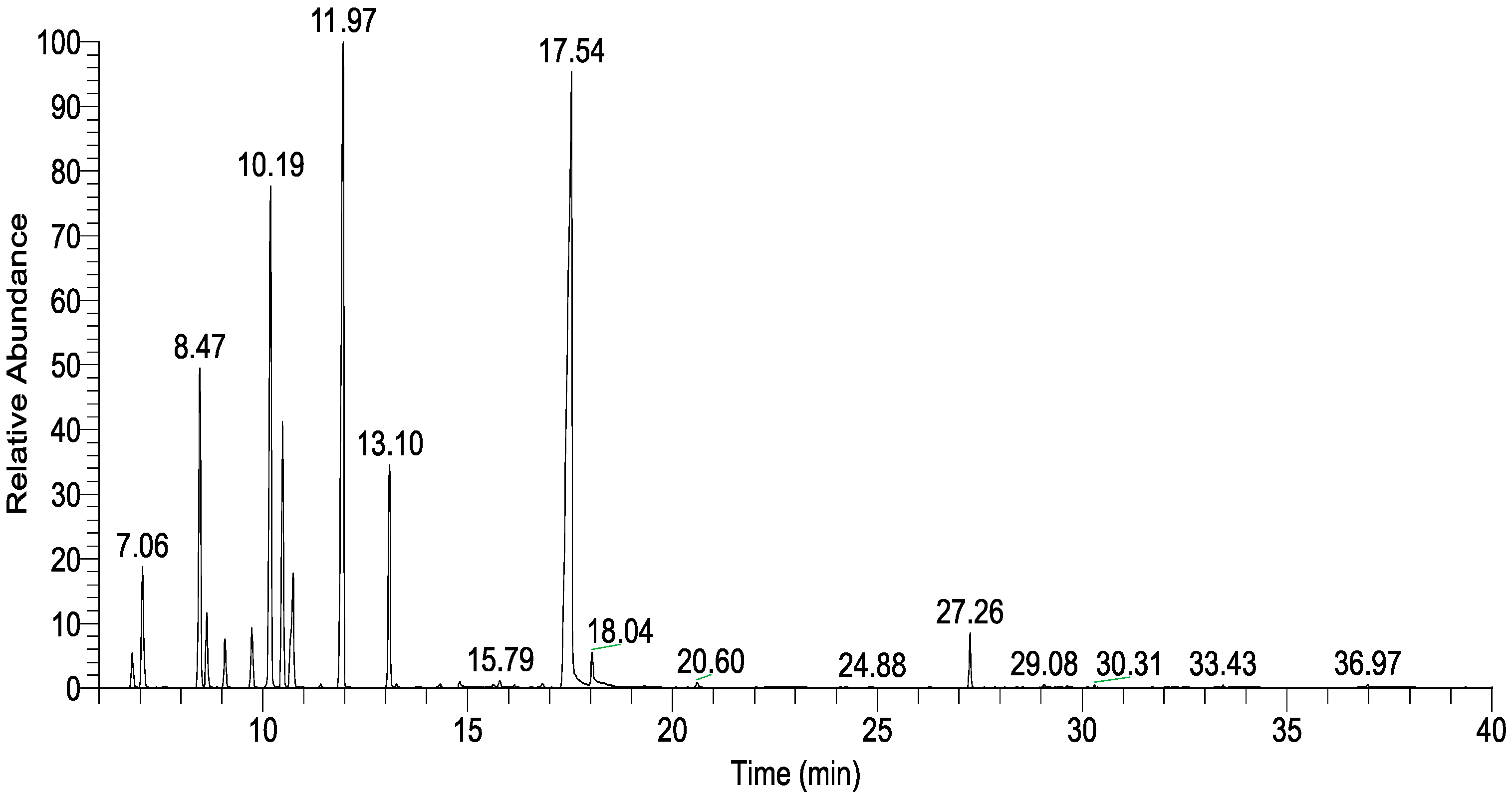

| RT (min) | Compound | Formula | KI | rKI | Relative Content (%) |
|---|---|---|---|---|---|
| 6.81 | α-Thujene | C10H16 | 929 | 930 | 0.86 ± 0.00 |
| 7.06 | α-Pinene | C10H16 | 936 | 939 | 3.11 ± 0.02 |
| 7.62 | Camphene | C10H16 | 953 | 954 | 0.03 ± 0.01 |
| 8.47 | Sabinene | C10H16 | 975 | 975 | 7.89 ± 0.08 |
| 8.64 | β-Pinene | C10H16 | 979 | 979 | 1.70 ± 0.01 |
| 9.08 | Myrcene | C10H16 | 990 | 990 | 1.10 ± 0.01 |
| 9.73 | α-Phellandrene | C10H16 | 1006 | 1002 | 1.43 ± 0.03 |
| 10.19 | α-Terpinene | C10H16 | 1018 | 1017 | 12.79 ± 0.08 |
| 10.49 | p-Cymene | C10H14 | 1026 | 1024 | 5.91 ± 0.06 |
| 10.70 | Limonene | C10H16 | 1032 | 1029 | 1.12 ± 0.14 |
| 10.74 | β-Phellandrene | C10H16 | 1033 | 1029 | 2.23 ± 0.16 |
| 11.41 | trans-β-Ocimene | C10H16 | 1049 | 1050 | 0.08 ± 0.00 |
| 11.97 | γ-Terpinene | C10H16 | 1062 | 1059 | 19.32 ± 0.02 |
| 13.10 | Terpinolene | C10H16 | 1086 | 1088 | 4.87 ± 0.03 |
| 13.26 | p-Cymenene | C10H12 | 1089 | 1091 | 0.07 ± 0.00 |
| 16.82 | Umbellulone | C10H14O | 1169 | 1171 | 0.12 ± 0.00 |
| 17.54 | Terpinen-4-ol | C10H18O | 1183 | 1177 | 34.14 ± 0.08 |
| 18.04 | α-Terpineol | C10H18O | 1193 | 1188 | 0.97 ± 0.06 |
| 18.33 | γ-Terpineol | C10H18O | 1199 | 1199 | 0.07 ± 0.00 |
| 24.25 | δ-Elemene | C15H24 | 1336 | 1338 | 0.02 ± 0.00 |
| 26.27 | β-Elemene | C15H24 | 1387 | 1390 | 0.03 ± 0.00 |
| 27.26 | trans-Caryophyllene | C15H24 | 1416 | 1419 | 1.10 ± 0.01 |
| 28.41 | α-Caryophyllene | C15H24 | 1453 | 1454 | 0.02 ± 0.00 |
| 29.20 | Germacrene D | C15H24 | 1478 | 1481 | 0.03 ± 0.01 |
| 29.64 | Viridiflorene | C15H24 | 1492 | 1496 | 0.06 ± 0.01 |
| 30.31 | γ-Cadinene | C15H24 | 1516 | 1513 | 0.04 ± 0.00 |
| 34.04 | α-Cadinol | C15H26O | 1660 | 1654 | 0.05 ± 0.00 |
| Monoterpenes | 62.47 ± 0.13 | ||||
| Oxygenated monoterpenes | 35.30 ± 0.14 | ||||
| Sesquiterpenes | 1.29 ± 0.01 | ||||
| Oxygenated sesquiterpenes | 0.05 ± 0.00 | ||||
| Total identified | 99.11 ± 0.03 | ||||
| RT (min) | Compound | Formula | KI | rKI | Relative Content (%) | |
|---|---|---|---|---|---|---|
| 25 °C | 50 °C | |||||
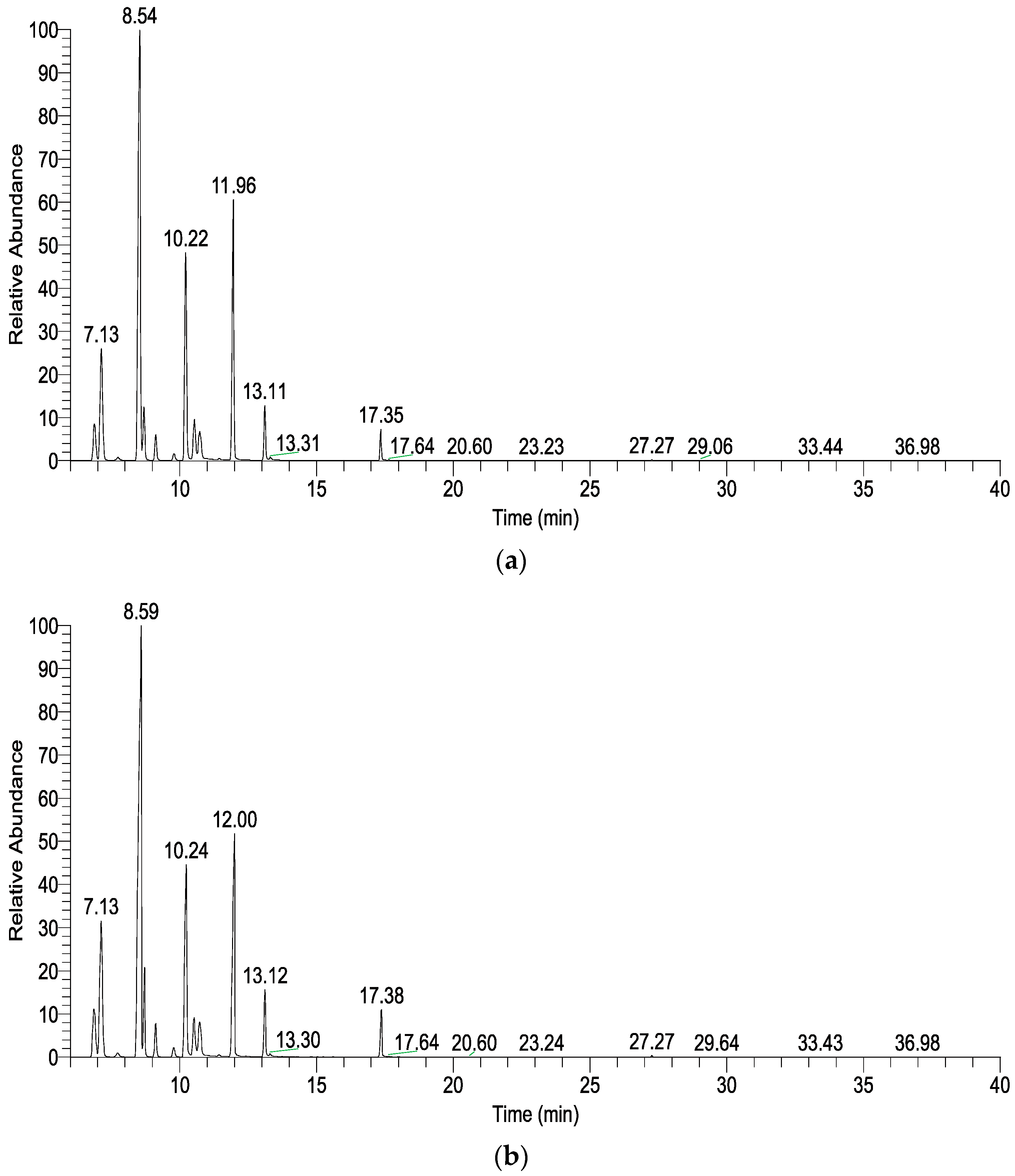
| 6.87 | α-Thujene | C10H16 | 930 | 930 | 3.50 ± 0.04 | 4.36 ± 0.31 |
| 7.13 | α-Pinene | C10H16 | 938 | 939 | 10.96 ± 0.80 | 10.80 ± 0.49 |
| 7.74 | Camphene | C10H16 | 956 | 954 | 0.36 ± 0.03 | 0.34 ± 0.04 |
| 8.54 | Sabinene | C10H16 | 977 | 975 | 34.67 ± 2.55 | 41.05 ± 2.11 |
| 8.68 | β-Pinene | C10H16 | 980 | 979 | 3.74 ± 0.32 | 3.45 ± 0.30 |
| 9.12 | Myrcene | C10H16 | 991 | 990 | 1.88 ± 0.08 | 1.88 ± 0.03 |
| 9.80 | α-Phellandrene | C10H16 | 1008 | 1002 | 0.59 ± 0.00 | 0.65 ± 0.03 |
| 10.22 | α-Terpinene | C10H16 | 1019 | 1017 | 15.07 ± 0.36 | 12.03 ± 0.95 |
| 10.54 | p-Cymene | C10H14 | 1027 | 1024 | 3.39 ± 0.09 | 2.83 ± 0.40 |
| 10.73 | Limonene | C10H16 | 1032 | 1029 | 2.76 ± 0.07 | 2.72 ± 0.07 |
| 11.45 | trans-β-Ocimene | C10H16 | 1050 | 1050 | 0.09 ± 0.00 | 0.09 ± 0.00 |
| 11.96 | γ-Terpinene | C10H16 | 1061 | 1059 | 17.16 ± 0.66 | 13.93 ± 0.70 * |
| 12.42 | cis-Sabinene hydrate | C10H18O | 1071 | 1070 | - | 0.04 ± 0.01 |
| 13.11 | Terpinolene | C10H16 | 1086 | 1088 | 3.29 ± 0.03 | 3.20 ± 0.14 |
| 13.31 | p-Cymenene | C10H12 | 1090 | 1091 | 0.23 ± 0.01 | 0.15 ± 0.02 * |
| 17.35 | Terpinen-4-ol | C10H18O | 1179 | 1177 | 1.62 ± 0.03 | 2.11 ± 0.13 * |
| 27.27 | trans-Caryophyllene | C15H24 | 1416 | 1419 | 0.06 ± 0.02 | 0.05 ± 0.00 |
| Monoterpenes | 97.67 ± 0.08 | 97.47 ± 0.10 | ||||
| Oxygenated monoterpenes | 1.62 ± 0.03 | 2.15 ± 0.13 | ||||
| Sesquiterpenes | 0.06 ± 0.02 | 0.05 ± 0.00 | ||||
| Total identified | 99.35 ± 0.08 | 99.65 ± 0.06 | ||||
| Specimen | Intensity of Total VOCs (1 × 108) | |
|---|---|---|
| 25 °C | 50 °C | |
| VOCs of leaves | 8.05 ± 0.97 | 23.41 ± 2.18 * |
| VOCs of leaf essential oils | 11.34 ± 0.63 | 12.70 ± 2.14 |
| RT (min) | Compound | Formula | KI | rKI | Relative Content (%) | |
|---|---|---|---|---|---|---|
| 25 °C | 50 °C | |||||
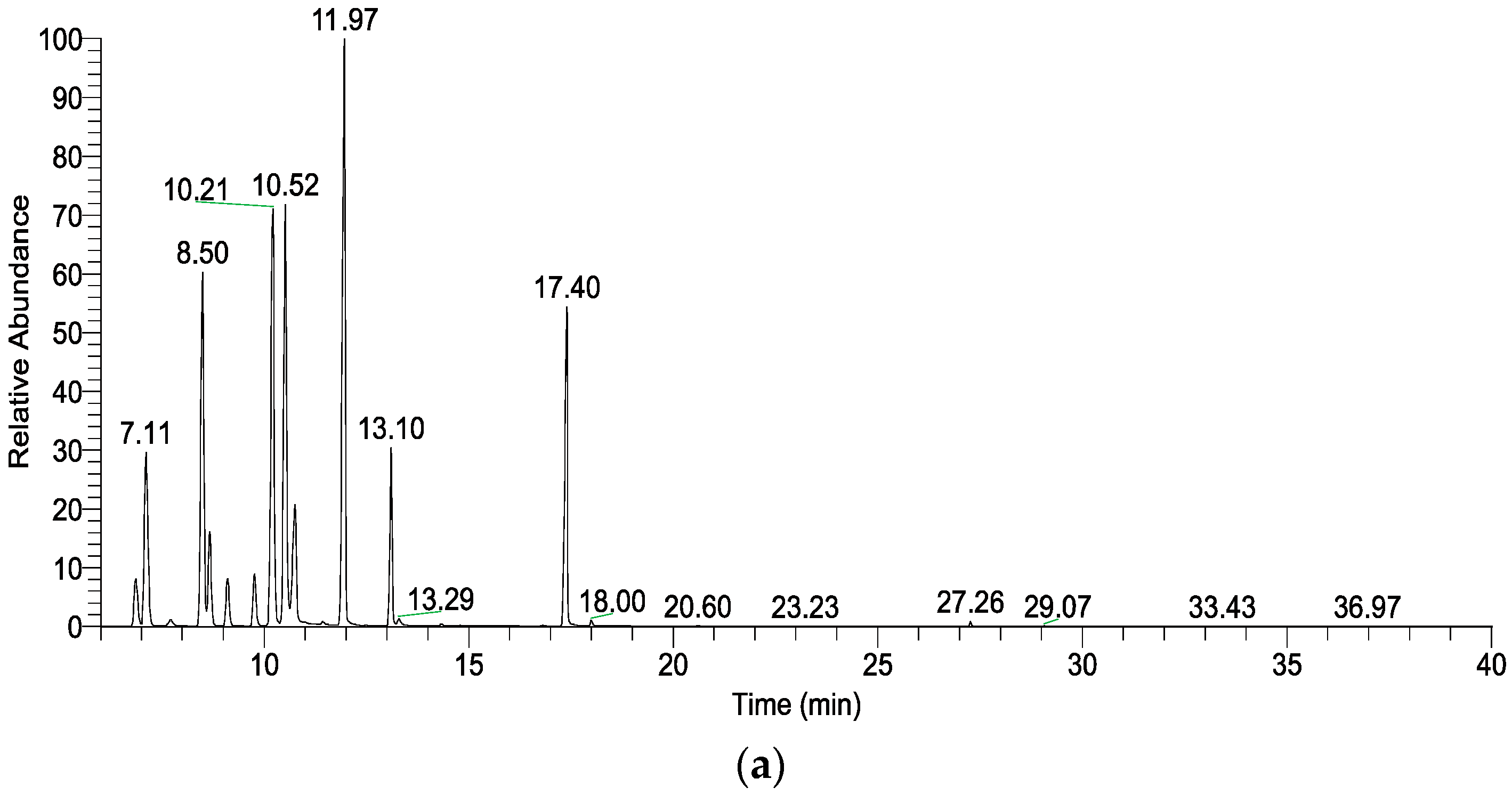
| 6.86 | α-Thujene | C10H16 | 930 | 930 | 2.05 ± 0.03 | 1.00 ± 0.24 * |
| 7.11 | α-Pinene | C10H16 | 937 | 939 | 7.60 ± 0.04 | 4.28 ± 0.77 * |
| 7.73 | Camphene | C10H16 | 955 | 954 | 0.29 ± 0.04 | 0.17 ± 0.04 |
| 8.50 | Sabinene | C10H16 | 976 | 975 | 13.14 ± 0.12 | 7.53 ± 1.65 * |
| 8.67 | β-Pinene | C10H16 | 980 | 979 | 3.22 ± 0.01 | 1.94 ± 0.34 * |
| 9.10 | Myrcene | C10H16 | 990 | 990 | 1.66 ± 0.06 | 1.02 ± 0.21 |
| 9.77 | α-Phellandrene | C10H16 | 1007 | 1002 | 1.92 ± 0.03 | 1.55 ± 0.11 * |
| 10.21 | α-Terpinene | C10H16 | 1019 | 1017 | 15.22 ± 0.22 | 10.13 ± 1.44 * |
| 10.52 | p-Cymene | C10H14 | 1027 | 1024 | 13.99 ± 0.93 | 12.91 ± 1.53 |
| 10.75 | β-Phellandrene | C10H16 | 1033 | 1029 | 4.93 ± 0.10 | 3.84 ± 0.46 |
| 11.43 | trans-β-Ocimene | C10H16 | 1049 | 1050 | 0.09 ± 0.00 | 0.08 ± 0.01 |
| 11.97 | γ-Terpinene | C10H16 | 1061 | 1059 | 19.15 ± 0.02 | 15.01 ± 1.20 * |
| 13.10 | Terpinolene | C10H16 | 1086 | 1088 | 4.81 ± 0.04 | 4.43 ± 0.32 |
| 13.29 | p-Cymenene | C10H12 | 1090 | 1091 | 0.23 ± 0.03 | 0.27 ± 0.02 |
| 13.74 | trans-Sabinene hydrate | C10H18O | 1098 | 1098 | - | 0.05 ± 0.01 |
| 16.81 | Umbellulone | C10H14O | 1168 | 1171 | 0.04 ± 0.00 | 0.12 ± 0.02 * |
| 17.40 | Terpinen-4-ol | C10H18O | 1180 | 1177 | 10.85 ± 1.47 | 33.10 ± 7.55 ** |
| 18.00 | α-Terpineol | C10H18O | 1192 | 1188 | 0.17 ± 0.02 | 0.85 ± 0.25 |
| 27.26 | trans-Caryophyllene | C15H24 | 1416 | 1419 | 0.13 ± 0.02 | 0.70 ± 0.25 |
| 29.74 | Viridiflorene | C15H24 | 1495 | 1496 | - | 0.01 ± 0.00 |
| 30.31 | γ-Cadinene | C15H24 | 1516 | 1513 | - | 0.01 ± 0.00 |
| Monoterpenes | 88.31 ± 1.47 | 64.16 ± 8.31 ** | ||||
| Oxygenated monoterpenes | 11.05 ± 1.50 | 34.12 ± 7.84 ** | ||||
| Sesquiterpenes | 0.13 ± 0.02 | 0.72 ± 0.26 | ||||
| Total identified | 99.49 ± 0.06 | 99.00 ± 0.21 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-Y.; Huang, Y.-M.; Chang, H.-T. Using Headspace Gas Chromatography–Mass Spectrometry to Investigate the Volatile Terpenoids Released from the Liquidambar formosana Leaf and Its Essential Oil. Forests 2024, 15, 1495. https://doi.org/10.3390/f15091495
Chang Y-Y, Huang Y-M, Chang H-T. Using Headspace Gas Chromatography–Mass Spectrometry to Investigate the Volatile Terpenoids Released from the Liquidambar formosana Leaf and Its Essential Oil. Forests. 2024; 15(9):1495. https://doi.org/10.3390/f15091495
Chicago/Turabian StyleChang, Yu-Yi, Yu-Mei Huang, and Hui-Ting Chang. 2024. "Using Headspace Gas Chromatography–Mass Spectrometry to Investigate the Volatile Terpenoids Released from the Liquidambar formosana Leaf and Its Essential Oil" Forests 15, no. 9: 1495. https://doi.org/10.3390/f15091495





