Changes in Water Dynamics and Vigor of Recalcitrant Phoebe chekiangensis Seeds during Desiccation by Nuclear Magnetic Resonance and Transmission Electron Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatments
2.2. Seed Standard Germination Test and Seed Size Calculation
2.3. Observations on the Ultrastructure of Seeds
2.4. Changes in Moisture Distribution during Seed Desiccation
2.4.1. Changes in Moisture Distribution
2.4.2. Calculation of Signal-to-Noise Ratio for Measurement Region of Interest of Seeds
2.5. Changes in Seed Moisture Status and Content during Desiccation
2.5.1. Changes in Seed Moisture Status and Content
2.5.2. Establishment of NMR Detection Method for Seed Moisture
2.6. Statistical Analysis
3. Results
3.1. Changes in Germination Percentage and Seed Size during Desiccation
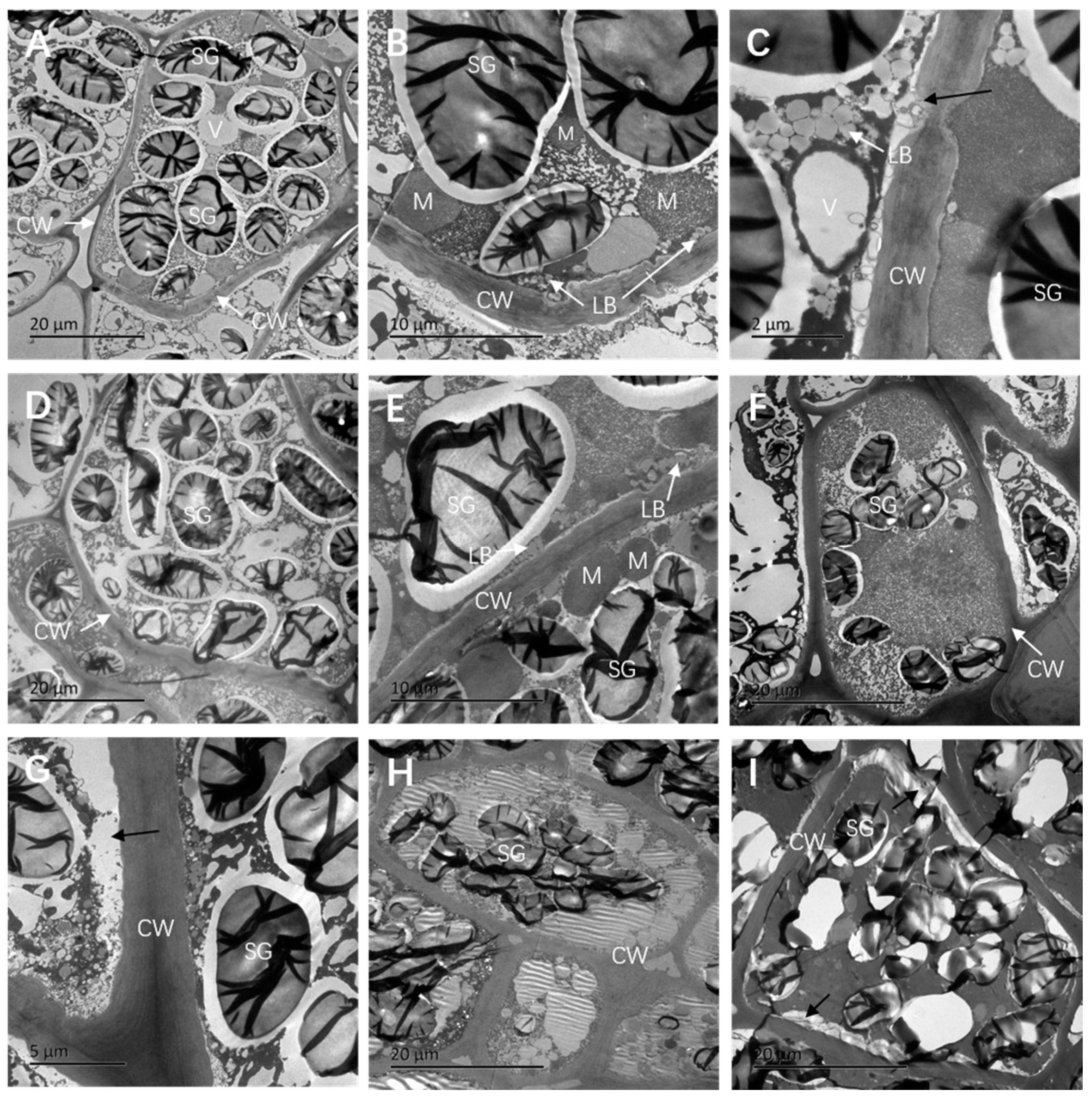
3.2. Changes in the Ultrastructure of Seed Embryos
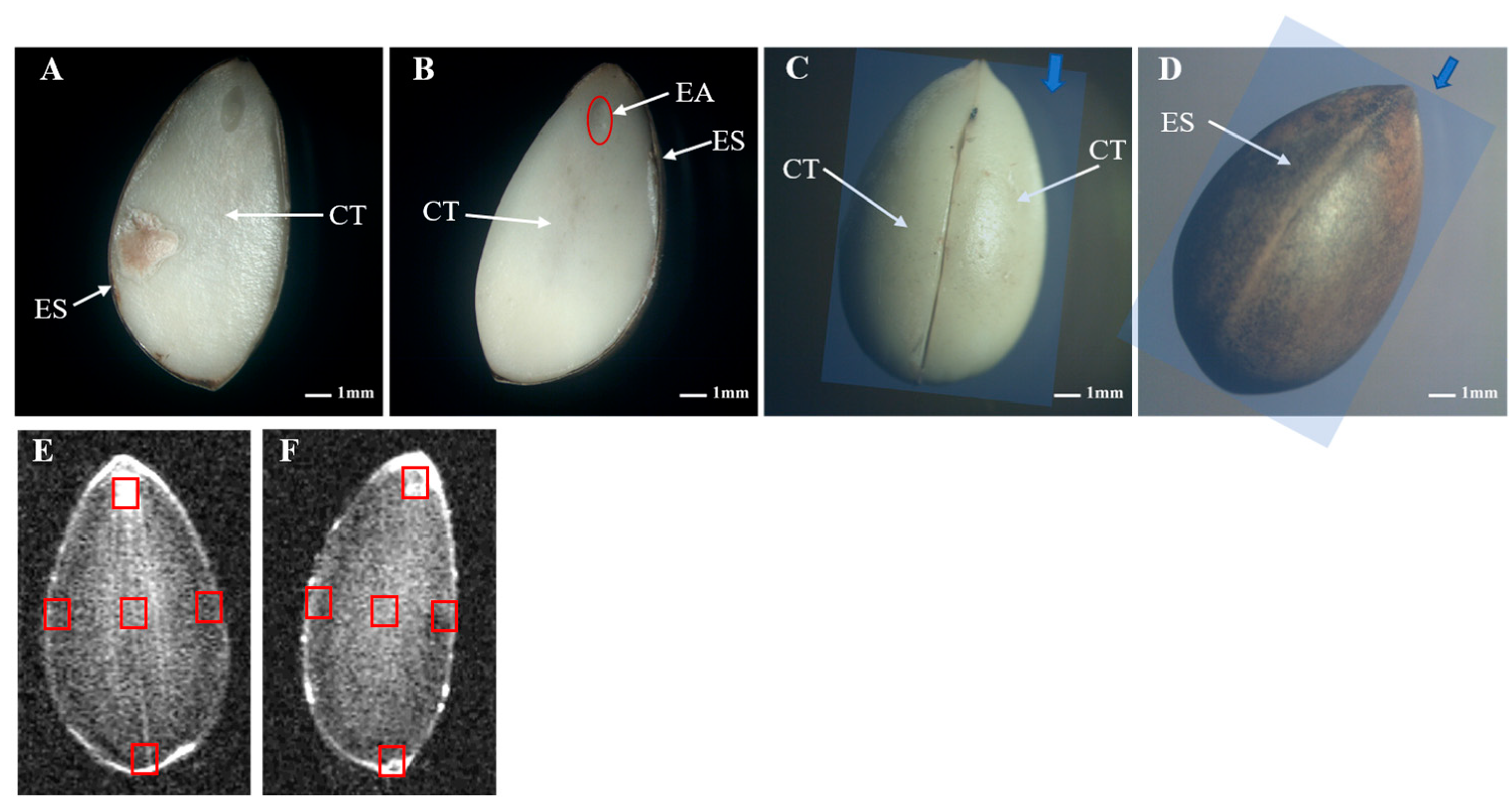
3.3. MRI Analysis
3.4. Changes in T2 Inversion Spectrum during Seed Desiccation
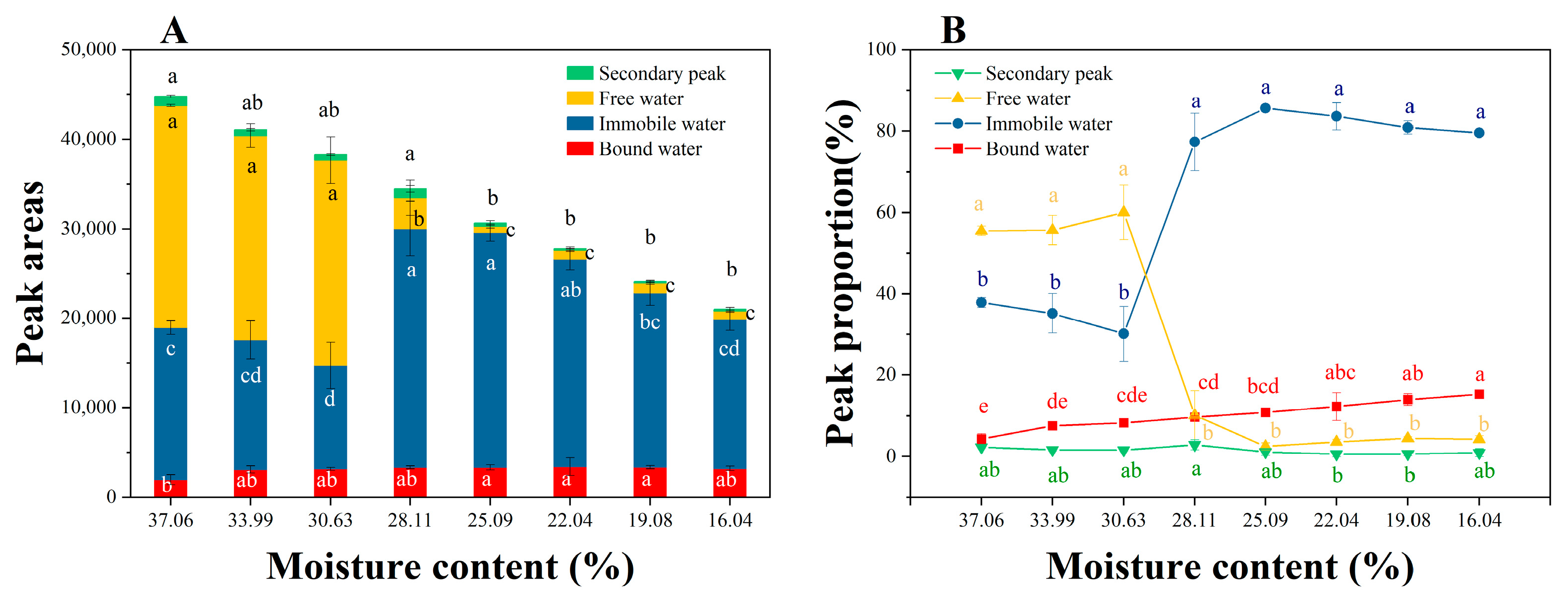
3.5. Dynamic Changes in Peak Area and Its Proportion
3.6. Correlation Analysis
4. Discussion
4.1. Effect of Desiccation on Seed Germination of P. chekiangensis
4.2. Changes in Ultrastructure of Cotyledons and Embryonic Axis of the Seeds
4.3. Water Distribution of P. chekiangensis Seeds during Desiccation
4.4. Changes in Water Status and Water Content of Each State during Desiccation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pennisi, E. Tending the global garden. Science 2010, 329, 1274–1277. [Google Scholar] [CrossRef]
- Walters, C.; Volk, G.M.; Richards, C.M. Genebanks in the post-genomic age: Emerging roles and anticipated uses. Biodiversity 2008, 9, 68–71. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guan, Y. (Eds.) Seed Biology, 2nd ed.; Higher Education Press: Beijing, China, 2022; pp. 284–290. [Google Scholar]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Singh, R.; Verma, A.; Joshi, D.; Singh, S. Changes in seed water status as characterized by NMR in developing soybean seed grown under moisture stress conditions. Biochem. Biophys. Res. Commun. 2014, 444, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Vertucci, C.W.; Leopold, A.C. The relationship between water binding and desiccation tolerance in tissues. Plant Physiol. 1987, 85, 232–238. [Google Scholar] [CrossRef]
- Farrant, J.M.; Pammenter, N.W.; Berjak, P. Recalcitrance—A current assessment. Seed Sci. Technol. 1988, 16, 155–166. [Google Scholar]
- Pritchard, H. Water potential and embryonic axis viability in recalcitrant seeds of Quercus rubra. Ann. Bot. 1991, 67, 43–49. [Google Scholar] [CrossRef]
- Sakurai, M.; Kawai, H.; Inoue, Y.; Hino, A.; Kobayashi, S. Effects of trehalose on the water structure in yeast cells as studied by in vivo 1H NMR spectroscopy. Bull. Chem. Soc. Jpn. 1995, 68, 3621–3627. [Google Scholar] [CrossRef]
- El-Saidi, M.; Gabr, A.; El-Kadi, M.; El-Zeiny, H. The effect of certain pre-sowing seed treatments and early phosphorus supplement on cell sap concentration and water fractions in leaves of maize (Zea mays L.) plants grown under soil moisture stress conditions. Biol. Plant. 1975, 17, 281–291. [Google Scholar] [CrossRef]
- Rascio, A.; Russo, M.; Platani, C.; Di Fonzo, N. Drought intensity effects on genotypic differences in tissue affinity for strongly bound water. Plant Sci. 1998, 132, 121–126. [Google Scholar] [CrossRef]
- Misik, S. Bound water in vine cane studied by microwave method. Acta Hortic. 2000, 526, 177–182. [Google Scholar] [CrossRef]
- Joardder, M.U.; Karim, A.; Kumar, C.; Brown, R.J. Porosity: Establishing the Relationship between Drying Parameters and Dried Food Quality; Springer: Berlin/Heidelberg, Germany, 2015; pp. 15–19. [Google Scholar]
- Sun, W.Q. Dielectric Relaxation of Water and Water-Plasticized Biomolecules in Relation to Cellular Water Organization, Cytoplasmic Viscosity, and Desiccation Tolerance in Recalcitrant Seed Tissues. Plant Physiol. 2000, 124, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, D.; Wang, L. Drying damage on physiological properties of rice seed associated with ultrastructure changes. Int. J. Food Eng. 2017, 13, 20170096. [Google Scholar] [CrossRef]
- Donadon, J.R.; Resende, O.; Teixeira, S.d.P.; Santos, J.M.d.; Moro, F.V. Effect of hot air drying on ultrastructure of crambe seeds. Dry. Technol. 2013, 31, 269–276. [Google Scholar] [CrossRef]
- Seyedin, N.; Burris, J.S.; Flynn, T.E. Physiological studies on the effects of drying temperatures on corn seed quality. Can. J. Plant Sci. 1984, 64, 497–504. [Google Scholar] [CrossRef]
- Siddique, A.B.; Wright, D. Effects of Different Drying Time and Temperature on Moisture Percentage and Seed Quality (Viability and Vigour) of Pea Seeds (Pisum sativum L.). Asian J. Plant Sci. 2003, 2, 978–982. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Y. Investigation of Water Distribution and Mobility Dynamics in Recalcitrant Quercus acutissima Seeds during Desiccation Using Magnetic Resonance Methods. Forests 2023, 14, 738. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, M.; Yang, P. Combination of LF-NMR and BP-ANN to monitor water states of typical fruits and vegetables during microwave vacuum drying. LWT 2019, 116, 108548. [Google Scholar] [CrossRef]
- Winisdorffer, G.; Musse, M.; Quellec, S.; Barbacci, A.; Le Gall, S.; Mariette, F.; Lahaye, M. Analysis of the dynamic mechanical properties of apple tissue and relationships with the intracellular water status, gas distribution, histological properties and chemical composition. Postharvest Biol. Technol. 2015, 104, 1–16. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Effect of trehalose and ultrasound-assisted osmotic dehydration on the state of water and glass transition temperature of broccoli (Brassica oleracea L. var. botrytis L.). J. Food Eng. 2013, 119, 640–647. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Black heart characterization and detection in pomegranate using NMR relaxometry and MR imaging. Postharvest Biol. Technol. 2012, 67, 96–101. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Yu, H. Effect of far-infrared drying on the water state and glass transition temperature in carrots. J. Food Eng. 2014, 136, 42–47. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cheng, D.; Li, W.; Guo, S.; Chen, C.; Liu, C.; Chen, S. An accurate and effective single-seed moisture selection method based on nuclear magnetic resonance (NMR) in maize. Comput. Electron. Agric. 2022, 193, 106649. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, Y.; Yue, X.; Xiong, P.; Pan, D.; Song, P.; Luo, B. LF-NMR/MRI Determination of Different 6-Benzylaminopurine Concentrations and Their Effects on Soybean Moisture. Front. Plant Sci. 2022, 13, 885804. [Google Scholar] [CrossRef]
- Krishnan, P.; Joshi, D.; Nagarajan, S.; Moharir, A. Characterization of germinating and non-viable soybean seeds by nuclear magnetic resonance (NMR) spectroscopy. Seed Sci. Res. 2004, 14, 355–362. [Google Scholar] [CrossRef]
- Song, P.; Kim, G.; Song, P.; Yang, T.; Yue, X.; Gu, Y. Rapid and non-destructive detection method for water status and water distribution of rice seeds with different vigor. Int. J. Agric. Biol. Eng. 2021, 14, 231–238. [Google Scholar] [CrossRef]
- Garnczarska, M.; Zalewski, T.; Kempka, M. Water uptake and distribution in germinating lupine seeds studied by magnetic resonance imaging and NMR spectroscopy. Physiol. Plant. 2007, 130, 23–32. [Google Scholar] [CrossRef]
- Garnczarska, M.; Zalewski, T.; Kempka, M. Changes in water status and water distribution in maturing lupin seeds studied by MR imaging and NMR spectroscopy. J. Exp. Bot. 2007, 58, 3961–3969. [Google Scholar] [CrossRef]
- Hu, X.; Wu, P.; Zhang, S.; Chen, S.; Wang, L. Moisture conversion and migration in single-wheat kernel during isothermal drying process by LF-NMR. Dry. Technol. 2019, 37, 803–812. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Yu, J. Determining wood moisture content by free induction decay of nuclear magnetic resonance. J. Beijing For. Univ. 2012, 34, 142–145. [Google Scholar] [CrossRef]
- Xu, F.; Jin, X.; Zhang, L.; Chen, X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017, 96, 191–197. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). International Rules for Seed Testing. Chapter 9: Determination of Moisture Content; International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2004. [Google Scholar]
- Feng, J.; Shen, Y.; Shi, F.; Li, C. Changes in seed germination ability, lipid peroxidation and antioxidant enzyme activities of Ginkgo biloba seed during desiccation. Forests 2017, 8, 286. [Google Scholar] [CrossRef]
- Shi, X.; Shi, Z. Dormancy of Phoebe chekiangensis Seed. J. Zhejiang For. Coll. 1990, 7, 377–382. [Google Scholar]
- Shi, X.; Shi, Z. Study on Seed and Dormancy of Phoebe chekiangensis and Its Releasing Ways. SEED 1990, 76. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Benites-Alfaro, O.E.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R”. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Kim, W.-S.; Oehrle, N.W.; Smith, J.R.; Gillman, J.D. Effect of heat stress on seed protein composition and ultrastructure of protein storage vacuoles in the cotyledonary parenchyma cells of soybean genotypes that are either tolerant or sensitive to elevated temperatures. Int. J. Mol. Sci. 2020, 21, 4775. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.B. The structural and chemical characteristics of the pericarp are important in Tilia miqueliana seed dormancy. New For. 2021, 52, 875–888. [Google Scholar] [CrossRef]
- Li, Y.; Obadi, M.; Shi, J.; Sun, J.; Chen, Z.; Xu, B. Determination of moisture, total lipid, and bound lipid contents in oats using low-field nuclear magnetic resonance. J. Food Compos. Anal. 2020, 87, 103401. [Google Scholar] [CrossRef]
- Song, P.; Yue, X.; Gu, Y.; Yang, T. Assessment of maize seed vigor under saline-alkali and drought stress based on low field nuclear magnetic resonance. Biosyst. Eng. 2022, 220, 135–145. [Google Scholar] [CrossRef]
- Kamal, T.; Cheng, S.; Khan, I.A.; Nawab, K.; Zhang, T.; Song, Y.; Wang, S.; Nadeem, M.; Riaz, M.; Khan, M.A.U. Potential uses of LF-NMR and MRI in the study of water dynamics and quality measurement of fruits and vegetables. J. Food Process Preserv. 2019, 43, e14202. [Google Scholar] [CrossRef]
- He, H.; Gao, H.; Xue, X.; Ren, J.; Chen, X.; Niu, B. Variation of sugar compounds in Phoebe chekiangensis seeds during natural desiccation. PLoS ONE 2024, 19, e0299669. [Google Scholar] [CrossRef] [PubMed]
- Leon-Lobos, P.; Ellis, R.H. Comparison of seed desiccation sensitivity amongst Castanea sativa, Quercus ilex and Q. cerris. Seed Sci. Technol. 2018, 46, 233–237. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsakaldimi, M. A comparative study of desiccation responses of seeds of three drought-resistant Mediterranean oaks. For. Ecol. Manag. 2013, 305, 189–194. [Google Scholar] [CrossRef]
- Panza, V.; Láinez, V.; Maldonado, S.; Maroder, H.L. Effects of desiccation on Euterpe edulis Martius seeds. Biocell 2007, 31, 383–390. [Google Scholar] [CrossRef]
- Perán, R.; Pammenter, N.; Naicker, J.; Berjak, P. The influence of rehydration technique on the response of recalcitrant seed embryos to desiccation. Seed Sci. Res. 2004, 14, 179–184. [Google Scholar] [CrossRef]
- Yan, H.; Jia, S.; Mao, P. Melatonin priming alleviates aging-induced germination inhibition by regulating β-oxidation, protein translation, and antioxidant metabolism in oat (Avena sativa L.) seeds. Int. J. Mol. Sci. 2020, 21, 1898. [Google Scholar] [CrossRef] [PubMed]
- Wei, S. Study on Biological Characteristics of Recalcitrant Seeds and Mechanism of Desiccation Sensitivity of Taxillus chinensis (DC.) Danser. Ph.D. Thesis, Peking Union Medical College, Beijing, China, 2017. [Google Scholar]
- Tuomainen, T.V.; Himanen, K.; Helenius, P.; Kettunen, M.I.; Nissi, M.J. Quantitative magnetic resonance imaging of Scots pine seeds and the assessment of germination potential. Can. J. For. Res. 2022, 52, 685–695. [Google Scholar] [CrossRef]
- Terskikh, V.V.; Feurtado, J.A.; Ren, C.; Abrams, S.R.; Kermode, A.R. Water uptake and oil distribution during imbibition of seeds of western white pine (Pinus monticola Dougl. ex D. Don) monitored in vivo using magnetic resonance imaging. Planta 2005, 221, 17–27. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Zhang, W. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. 2018, 242, 16–21. [Google Scholar] [CrossRef]
- Duan, W.; Li, Y.; Cui, L.; Liu, F.; Yang, G.; Guo, L.; Wang, X. Analyze moisture transformation and transport rules during processing of Paeoniae Radix Alba by using low-field NMR. China J. Chin. Mater. Medica 2017, 42, 2092–2096. [Google Scholar] [CrossRef]
- Xu, J.; Xu, G.; Zhang, X.; Gu, Z.; Zhang, S.; Li, H. Moisture transport in carrot during hot air drying using magnetic resonance imaging. Trans. Chin. Soc. Agric. Eng. 2013, 29, 271–276. [Google Scholar] [CrossRef]
- Song, P.; Xu, J.; Ma, H.; Wang, C.; Yang, T.; Gao, H. Moisture phase state and distribution characteristics of seed during rice seed soaking process by low field nuclear magnetic resonance. Trans. Chin. Soc. Agric. Eng. 2016, 32, 204–210. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, H.; Feng, X.; Huang, X.; Wang, N. Studies on the Change of Moisture State of Fresh Jujube during Storage Base on LF-NMR and MRI. J. Chin. Inst. Food Sci. Technol. 2016, 16, 252–257. [Google Scholar] [CrossRef]
- Cheng, E.; Song, P.; Hou, T.; Zhang, B.; Wu, L. Determining the effects of LFHV-PEF treatment on the water distribution and vigor of aged rice seeds using LF-NMR. Int. J. Agric. Biol. Eng. 2022, 15, 190–196. [Google Scholar] [CrossRef]
- Luo, J.; Tang, M.; Qiu, Y.; Liu, J.; Wang, Q. Change of water status during olecranon peach storage by LF-NMR technique. J. Zhongkai Univ. Agric. Eng. 2019, 32, 23–27. [Google Scholar] [CrossRef]
- Levitt, J. Frost, drought, and heat resistance. Annu. Rev. Plant Physiol. 1951, 2, 245–268. [Google Scholar] [CrossRef]
- Ambastha, V.; Tiwari, B.S. Cellular water and anhydrobiosis in plants. J. Plant Growth Regul. 2015, 34, 665–671. [Google Scholar] [CrossRef]
- Moore, J.P.; Le, N.T.; Brandt, W.F.; Driouich, A.; Farrant, J.M. Towards a systems-based understanding of plant desiccation tolerance. Trends Plant Sci. 2009, 14, 110–117. [Google Scholar] [CrossRef]
- Xiaocheng, J.; Xiaoquan, Y.; Jiarui, F.; Junxian, H. Differences between the water status in orthodox seeds and that in recalcitrant seeds. Acta Sci. Nat. Univ. Norm. Hunan 1996, 19, 54–58. [Google Scholar]
- Kuroki, S.; Tsenkova, R.; Moyankova, D.; Muncan, J.; Morita, H.; Atanassova, S.; Djilianov, D. Water molecular structure underpins extreme desiccation tolerance of the resurrection plant Haberlea rhodopensis. Sci. Rep. 2019, 9, 3049. [Google Scholar] [CrossRef] [PubMed]




| Moisture Content/% | Transverse Diameter/mm | Longitudinal Diameter/mm | Thickness/mm |
|---|---|---|---|
| 37.06 | 7.82 ± 0.06 a | 12.59 ± 0.08 a | 7.08 ± 0.13 a |
| 33.99 | 7.70 ± 0.01 b | 12.50 ± 0.04 ab | 6.86 ± 0.04 b |
| 30.63 | 7.61 ± 0.03 c | 12.42 ± 0.07 abc | 6.85 ± 0.05 b |
| 28.11 | 7.59 ± 0.03 c | 12.40 ± 0.08 abcd | 6.81 ± 0.06 bc |
| 25.09 | 7.56 ± 0.02 c | 12.37 ± 0.07 bcde | 6.80 ± 0.06 bcd |
| 22.04 | 7.42 ± 0.04 d | 12.30 ± 0.05 cde | 6.68 ± 0.02 cd |
| 19.08 | 7.39 ± 0.02 d | 12.25 ± 0.07 de | 6.66 ± 0.03 d |
| 16.04 | 7.39 ± 0.02 d | 12.21 ± 0.09 e | 6.67 ± 0.01 cd |
| Moisture Content/% | T21 | T22 | T23 | T24 | ||||
|---|---|---|---|---|---|---|---|---|
| Relaxation Range/ms | Peak Time/ms | Relaxation Range/ms | Peak Time/ms | Relaxation Range/ms | Peak Time/ms | Relaxation Range/ms | Peak Time/ms | |
| 37.06 | 0.14 ± 0.02 de | 0.09 ± 0.01 b | 3.06 ± 0.28 d | 1.61 ± 0.31 a | 180.48 ± 32.46 a | 8.42 ± 0.58 c | 322.97 ± 43.03 a | 312.32 ± 38.76 a |
| 33.99 | 0.13 ± 0.03 e | 0.07 ± 0.01 b | 2.71 ± 1.00 d | 1.75 ± 0.88 a | 149.34 ± 21.75 a | 6.99 ± 0.28 c | 308.40 ± 11.69 a | 284.36 ± 29.61 ab |
| 30.63 | 0.11 ± 0.00 e | 0.07 ± 0.00 b | 1.41 ± 0.45 d | 1.20 ± 0.00 a | 118.59 ± 9.09 a | 3.85 ± 0.57 c | 239.95 ± 64.49 a | 209.82 ± 8.51 ab |
| 28.11 | 0.18 ± 0.01 cd | 0.08 ± 0.01 b | 21.40 ± 6.61 c | 2.04 ± 0.21 a | 55.96 ± 22.30 a | 28.19 ± 16.30 bc | 306.84 ± 153.93 a | 140.12 ± 51.04 b |
| 25.09 | 0.20 ± 0.01 bc | 0.08 ± 0.00 b | 52.72 ± 1.85 a | 2.17 ± 0.08 a | 143.06 ± 88.15 a | 80.53 ± 21.81 a | 201.58 ± 29.22 a | 286.96 ± 95.79 ab |
| 22.04 | 0.20 ± 0.03 bc | 0.09 ± 0.02 b | 40.31 ± 1.65 b | 2.06 ± 0.21 a | 222.41 ± 136.67 a | 84.79 ± 20.04 a | 186.83 ± 60.26 a | 267.26 ± 46.08 ab |
| 19.08 | 0.34 ± 0.01 a | 0.15 ± 0.04 a | 29.84 ± 4.20 c | 1.52 ± 0.06 a | 172.21 ± 64.10 a | 64.99 ± 10.85 ab | 168.19 ± 20.52 a | 266.28 ± 69.40 ab |
| 16.04 | 0.25 ± 0.01 b | 0.09 ± 0.01 b | 26.66 ± 3.99 c | 1.39 ± 0.10 a | 142.34 ± 70.76 a | 61.65 ± 16.56 ab | 187.72 ± 112.27 a | 235.81 ± 83.96 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Gao, H.; Gu, W.; Huang, Y. Changes in Water Dynamics and Vigor of Recalcitrant Phoebe chekiangensis Seeds during Desiccation by Nuclear Magnetic Resonance and Transmission Electron Microscopy. Forests 2024, 15, 1508. https://doi.org/10.3390/f15091508
He H, Gao H, Gu W, Huang Y. Changes in Water Dynamics and Vigor of Recalcitrant Phoebe chekiangensis Seeds during Desiccation by Nuclear Magnetic Resonance and Transmission Electron Microscopy. Forests. 2024; 15(9):1508. https://doi.org/10.3390/f15091508
Chicago/Turabian StyleHe, Huangpan, Handong Gao, Wen Gu, and Ying Huang. 2024. "Changes in Water Dynamics and Vigor of Recalcitrant Phoebe chekiangensis Seeds during Desiccation by Nuclear Magnetic Resonance and Transmission Electron Microscopy" Forests 15, no. 9: 1508. https://doi.org/10.3390/f15091508






