Environmental Driving Mechanism and Response of Soil’s Fungal Functional Structure to Near-Naturalization in a Warm Temperate Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Research Area and Setup of Quadrats
2.2. Analysis of Plant Community Structure and Diversity
2.2.1. Calculation of Importance Values
2.2.2. Biomass Calculation
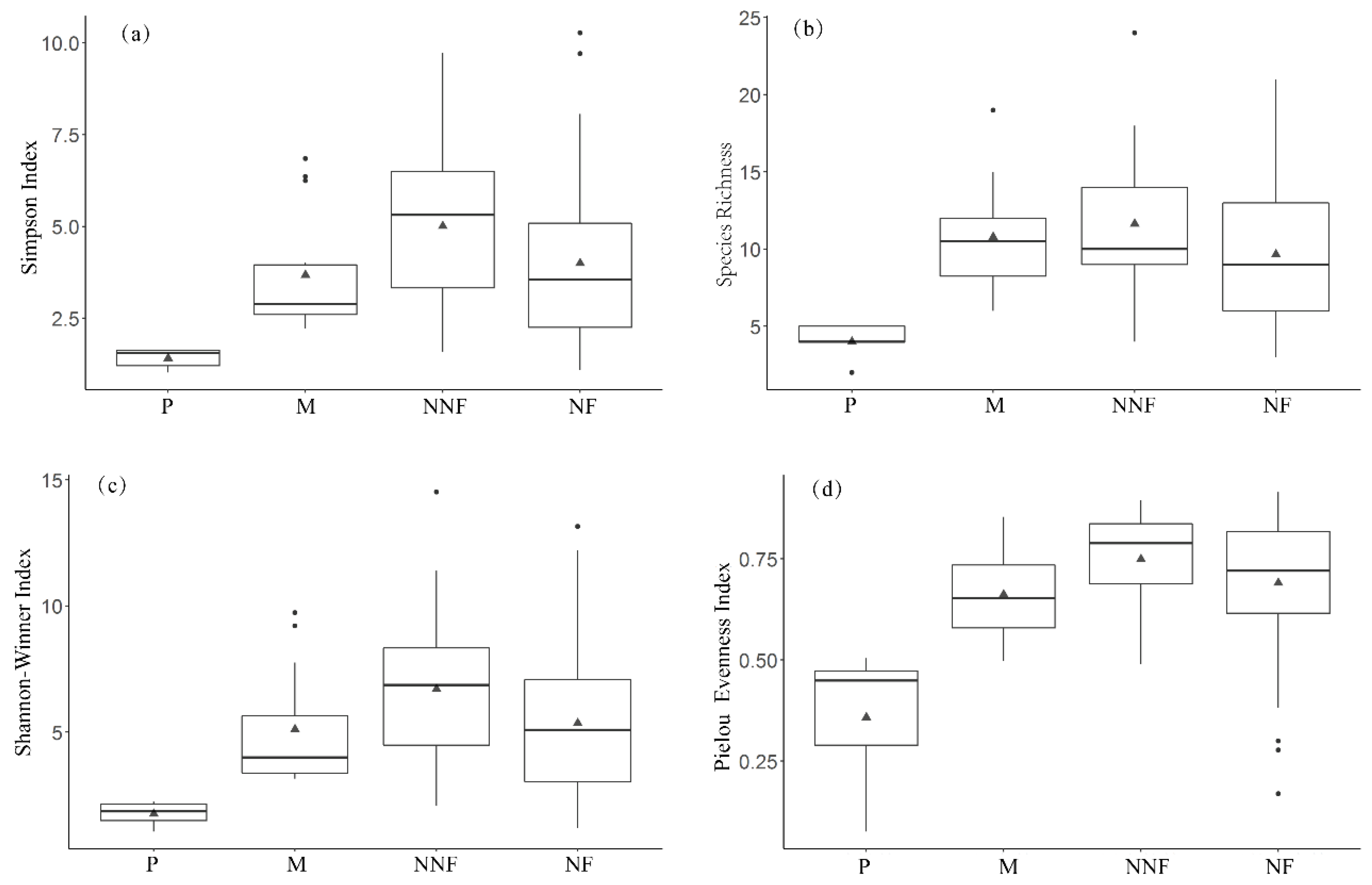
2.2.3. α-Diversity Calculation
2.3. Determination of Soil Physicochemical Properties
2.3.1. Soil Sampling
2.3.2. Determination of Soil Indices
2.4. Soil Microbial Sequencing and Annotation
2.5. Prediction of Fungal Function
2.6. Data Processing and Analysis
3. Results and Analysis
3.1. Changes in Plant Community Structure and Soil Properties
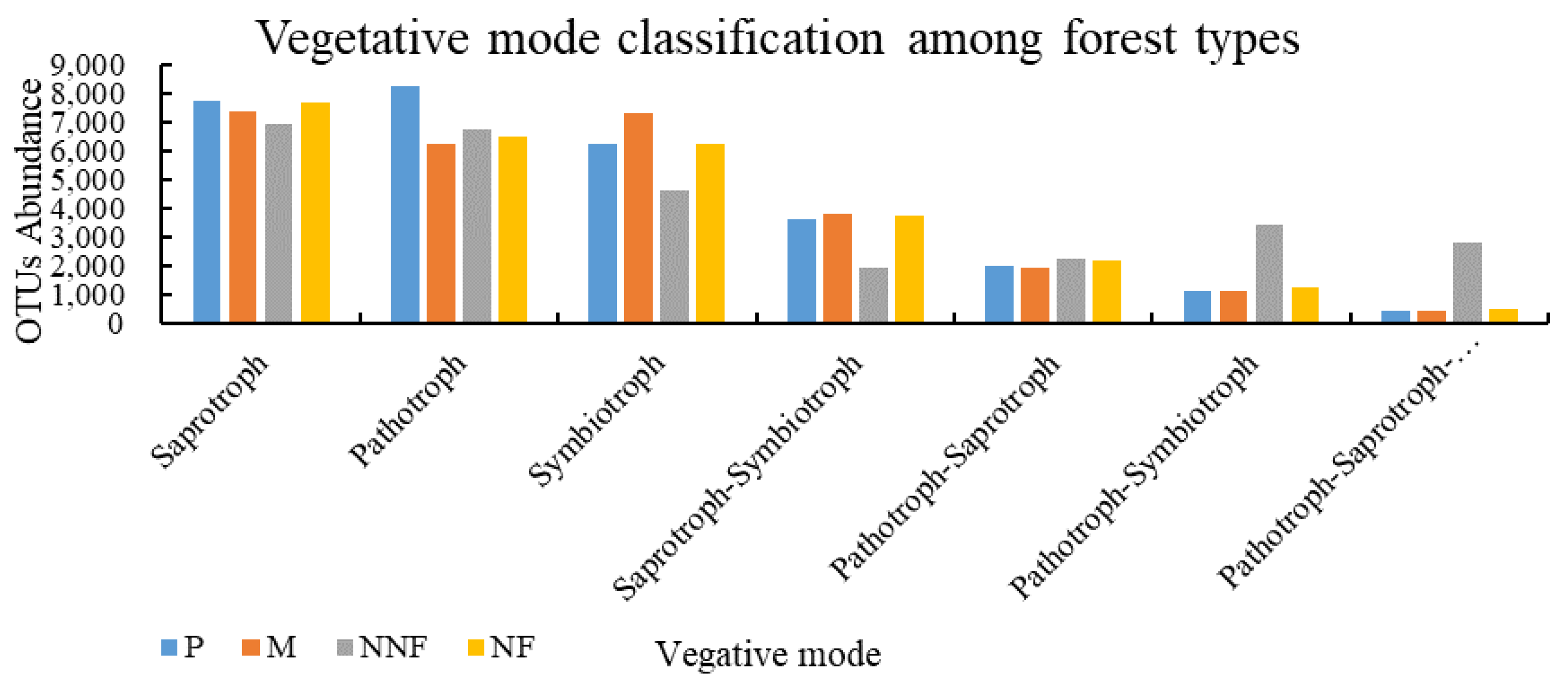
3.2. Influence of Near-Naturalization on the Abundance of Fungal Functional Groups
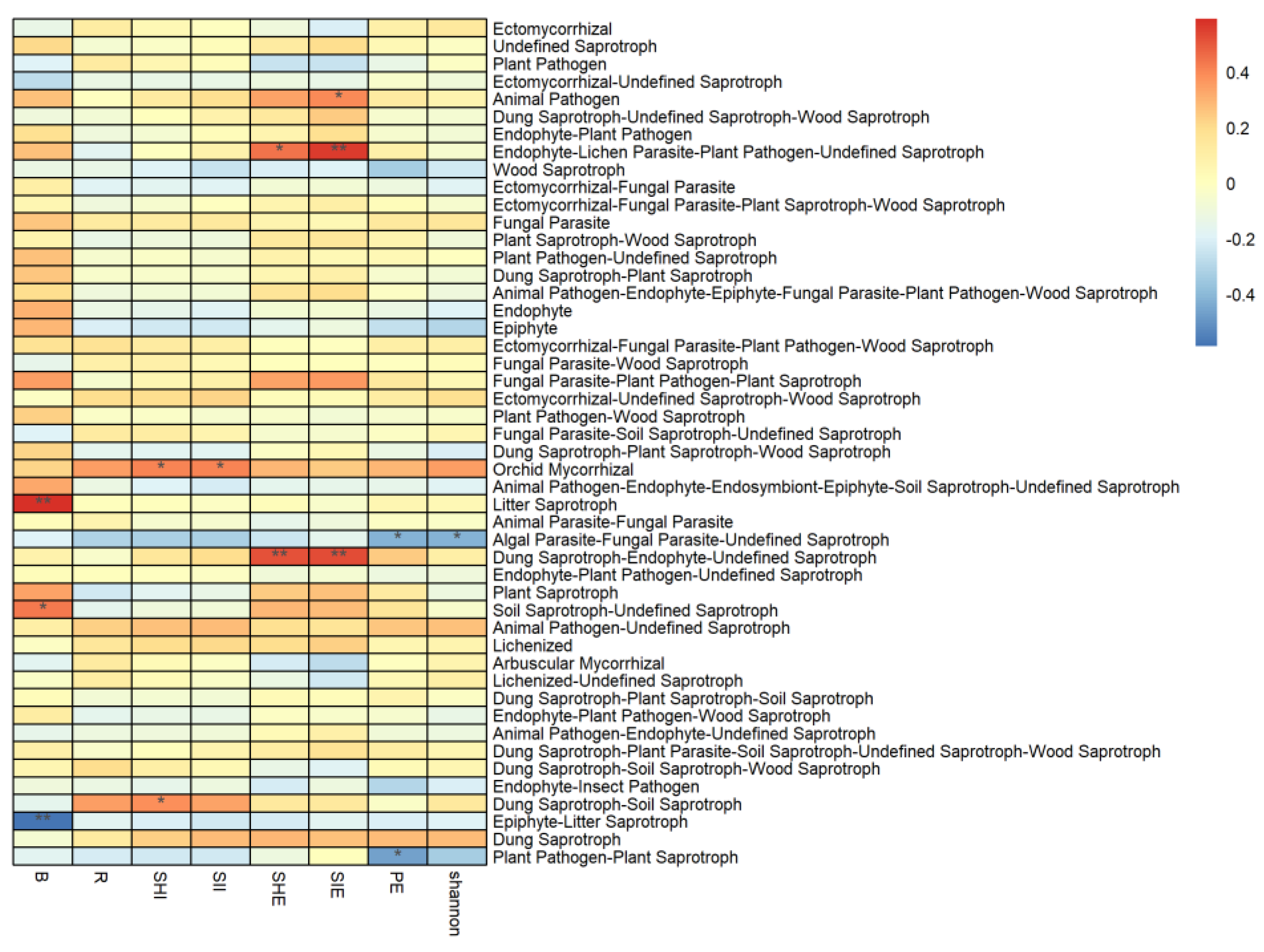
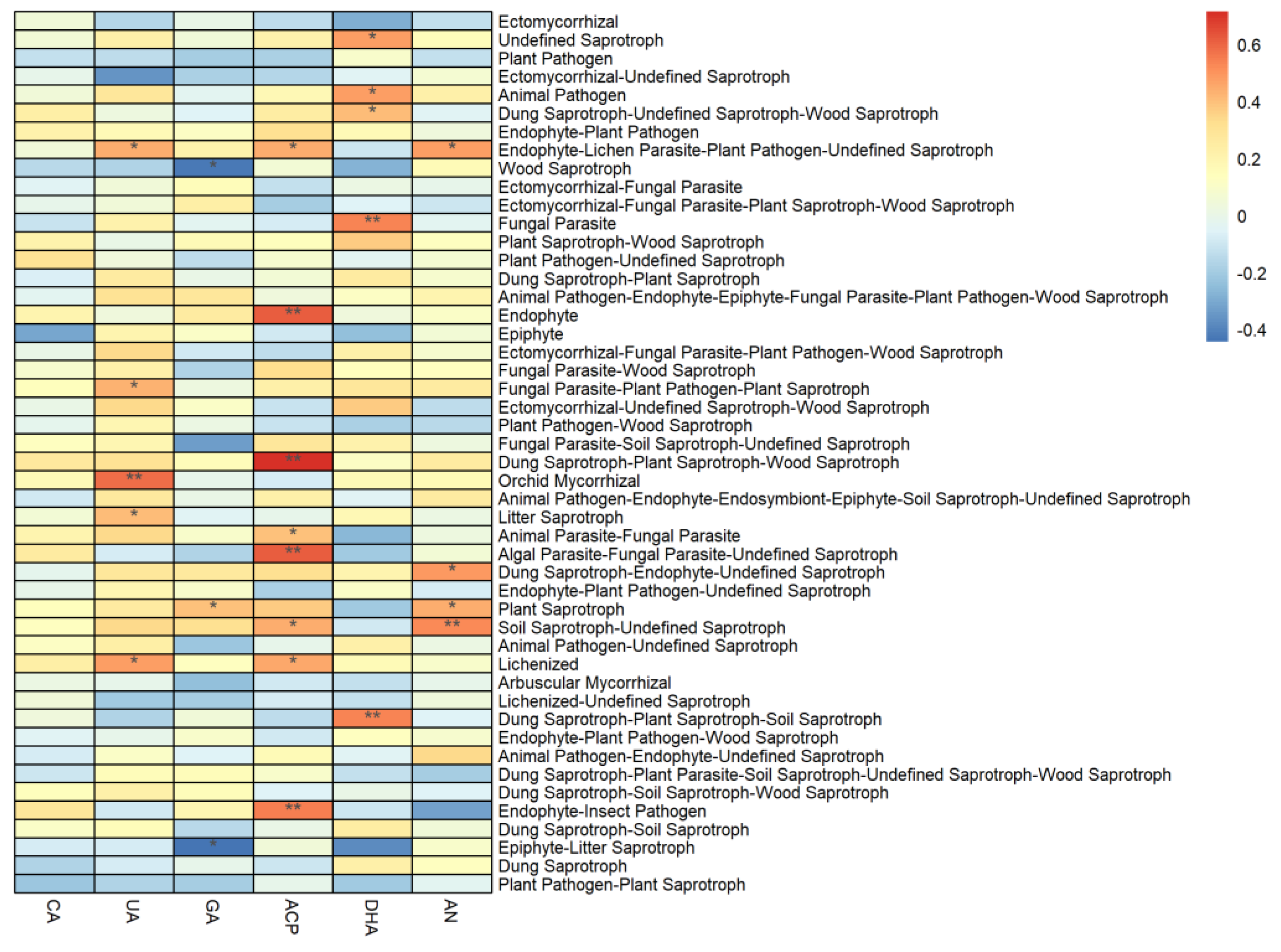
3.3. Correlation between Vegetation and Soil Properties, and the Abundance of Soil Fungal Functional Groups
3.4. Effects of Near-Naturalization on the Functional Structure of Fungal Community of Soil Fungi
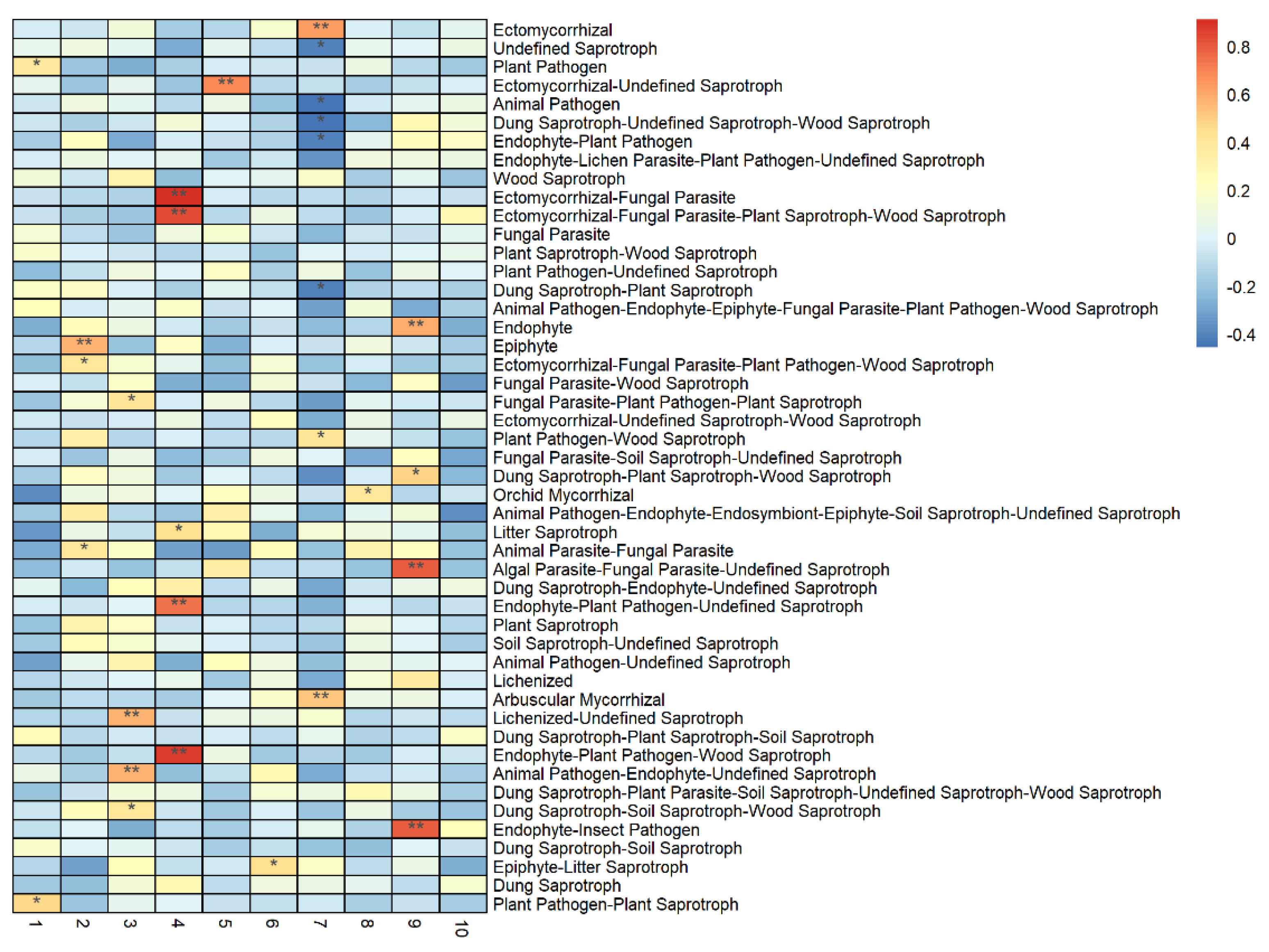
3.5. Factors Influencing the Near-Naturalization of the Soil Fungi Community’s Functional Structure
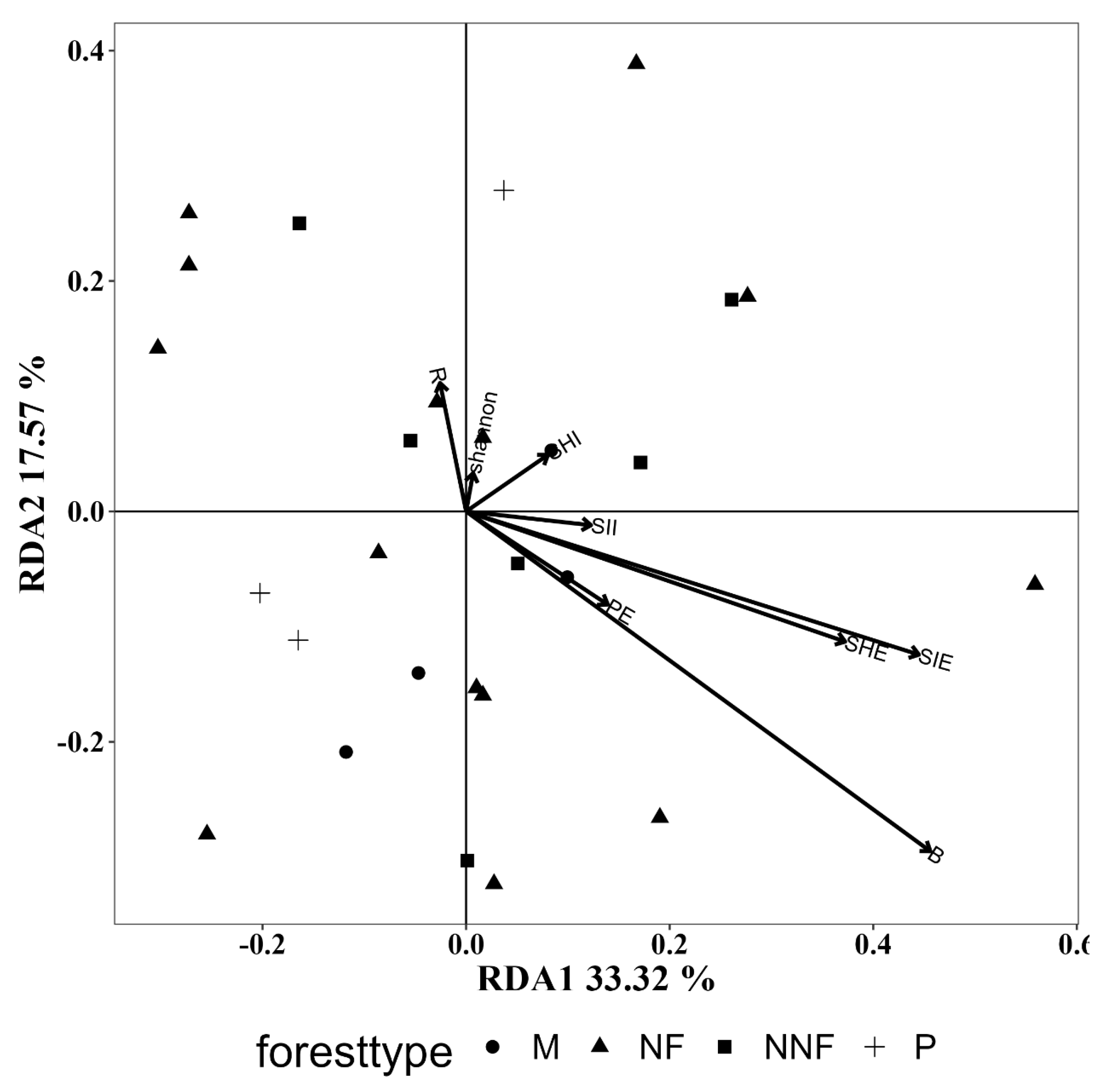
3.5.1. Influence of Tree Layer Diversity
3.5.2. Influence of Vegetation Composition
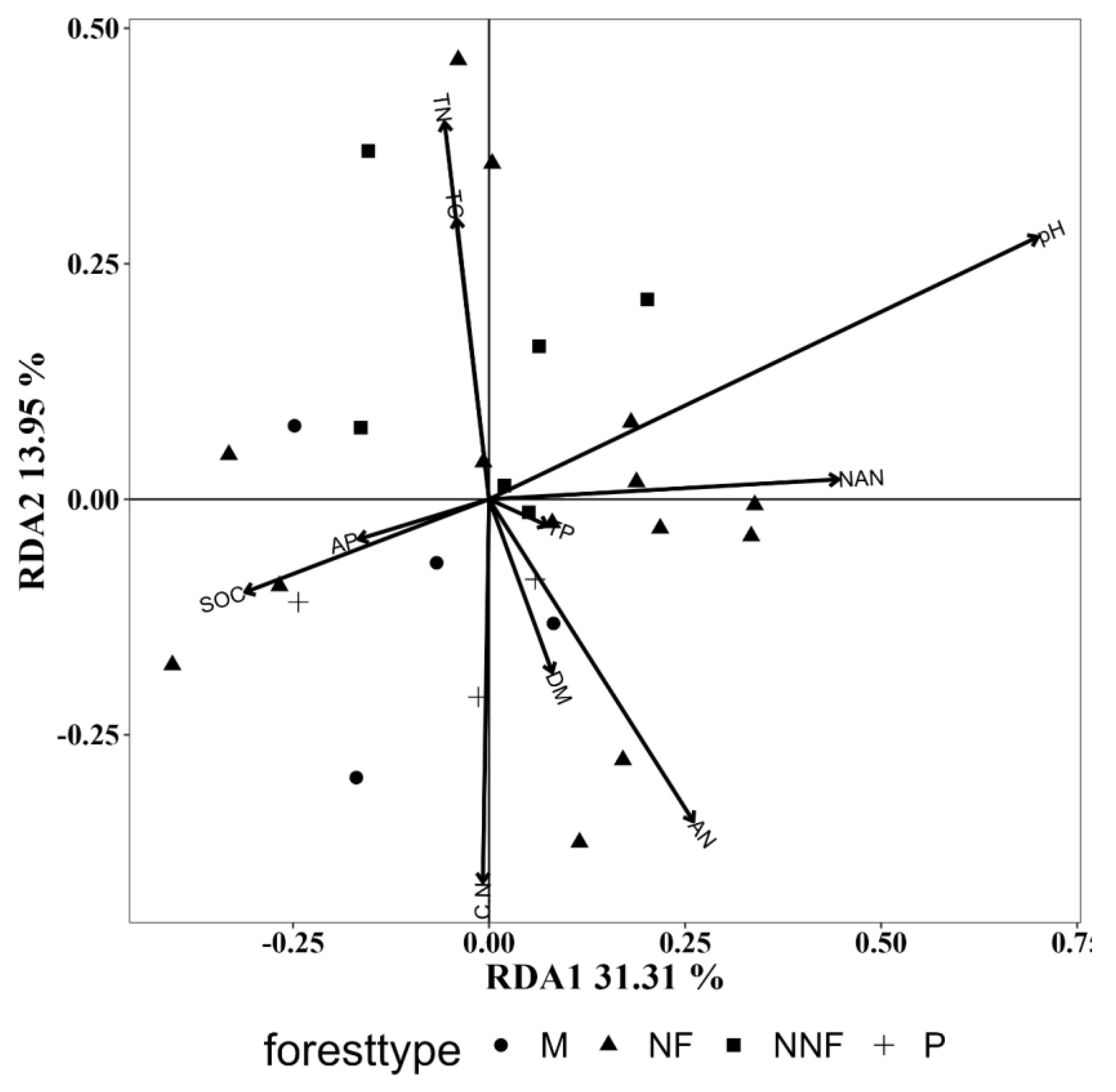
3.5.3. Influence of Soil Physicochemical Properties
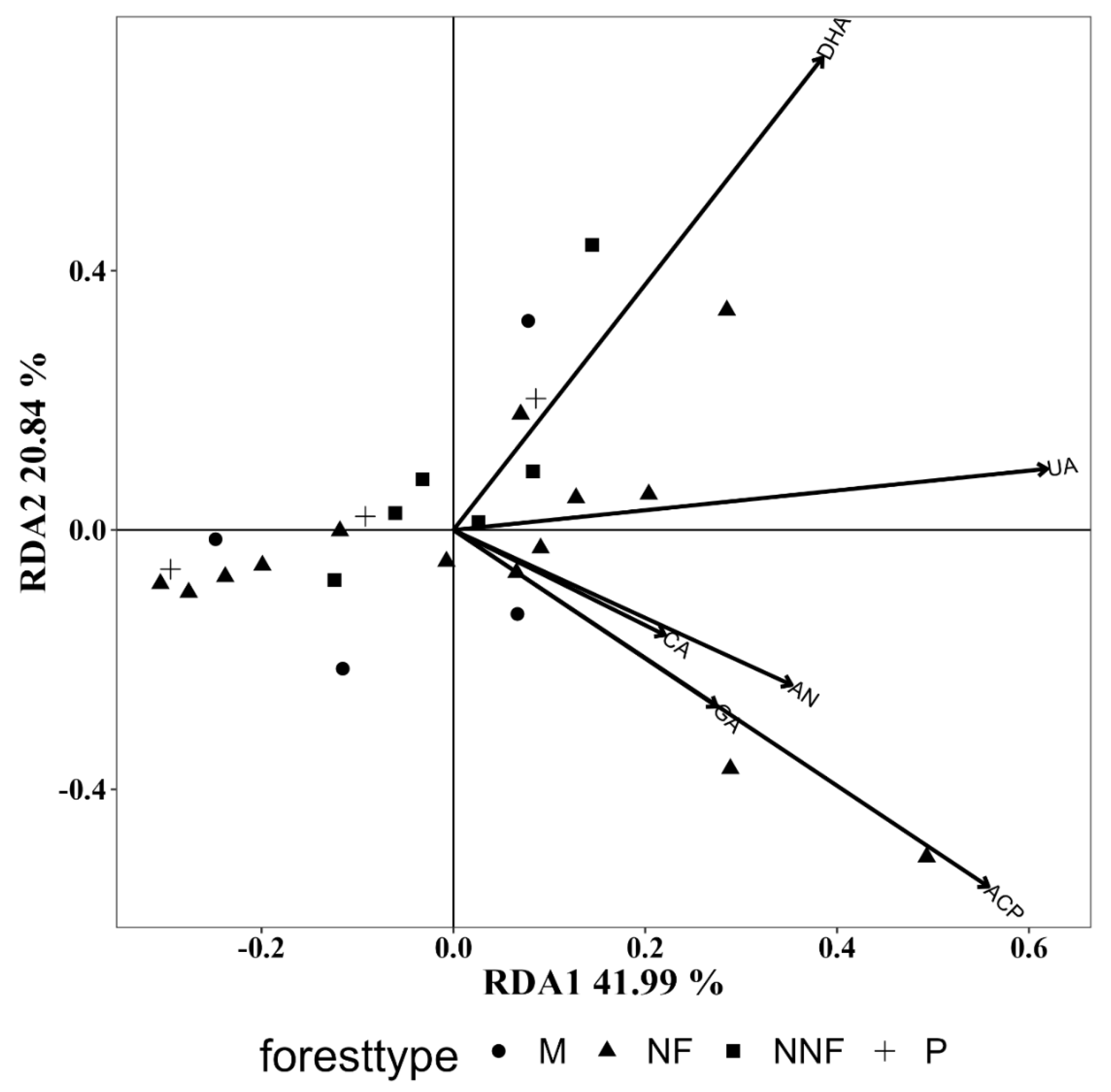
3.5.4. Influence of Soil Enzyme Activity
4. Discussion
4.1. Effects of Near-Naturalization on the Abundance of Fungal Functional Groups
4.2. Effects of Environmental Factors on the Abundance of Functional Groups of Fungi
4.3. Effects of Near-Naturalization on the Functional Structure of the Fungal Community
4.4. Effects of Environmental Factors on the Functional Structure of the Soil Fungal Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sasmito, S.-D.; Kuzyakov, Y.; Lubis, A.-A.; Murdiyarso, D.; Hutley, L.-B.; BAchri, S.; Friess, D.-A.; Martius, C.; Borchard, N. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Li, X.-J.; Li, X.-R.; Wang, X.-P.; Yang, H.-T. Changes in soil organic carbon fractions after afforestation with xerophytic shrubs in the Tengger Desert, northern China. Eur. J. Soil. Sci. 2016, 67, 184–195. [Google Scholar] [CrossRef]
- Zhou, Q.-Q.; Li, F.; Cai, X.-A.; Rao, X.-Q.; Zhou, L.-X.; Liu, Z.-F.; Lin, Y.-B.; Fu, S.-L. Survivorship of plant species from soil seedbank after translocation from subtropical natural forests to plantation forests. For. Ecol. Manag. 2019, 432, 741–747. [Google Scholar] [CrossRef]
- Chen, G.-P.; Gao, Z.-Y.; Zu, L.-H.; Tang, L.-L.; Yang, T.; Feng, X.-M.; Zhao, T.-J.; Shi, F.-C. Soil aggregate characteristics and stability of soil carbon stocks in a Pinus tabulaeformis plantation. New For. 2017, 48, 837–853. [Google Scholar] [CrossRef]
- Peay, K.-G.; Baraloto, C.; Fine, P.-V.-A. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef]
- Bahram, M.; Plme, S.; Kljalg, U.; Zarre, S.; Tedersoo, L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol. 2015, 193, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, R.; van der Wal, A.; Kardol, P.; van der Putten, W.-H.; de Ruiter, P.-C.; Dekker, S.-C. Modelling C and N mineralisation in soil food webs during secondary succession on ex-arable land. Soil Biol. Biochem. 2021, 43, 251–260. [Google Scholar] [CrossRef]
- Kardol, P.; Todd, D.-E.; Hanson, P.-J.; Mulholland, P.-J. Long-term successional forest dynamics: Species and community responses to climatic variability. J. Veg. Sci. 2021, 21, 627–642. [Google Scholar] [CrossRef]
- Sun, C.; Liu, G.; Xue, S. Natural succession of grassland on the Loess Plateau of China affects multifractal characteristics of soil particle-size distribution and soil nutrients. Ecol. Res. 2016, 31, 891–902. [Google Scholar] [CrossRef]
- Jaroslav, Š.; Petra, D.; Michaela, U.; Mirka, P.; Tomáš, C.; Jan, F.; Petr, B. Dominant trees affect microbial community composition and activity in post-mining afforested soils. Soil Biol. Biochem. 2013, 56, 105–115. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.-M.; Peng, M.; Sui, X.-H.; Li, W.; Sun, G.-Y. Variations in soil functional fungal community structure associated with pure and mixed plantations in Typical Temperate forests of China. Front. Microbiol. 2019, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-L.; Shi, C.; Zhang, M.; Shi, F.-C. Effects of close-to-nature management of plantation on the structure and ecological functions of soil microorganisms with different habitat specialization. Plant Soil 2022, 482, 347–367. [Google Scholar] [CrossRef]
- Flores-Rentería, D.; Rincón, A.; Valladares, F.; Yuste, J. Agricultural matrix affects differently the alpha and beta structural and functional diversity of soil microbial communities in a fragmented mediterranean holm oak forest. Soil Biol. Biochem. 2016, 92, 79–90. [Google Scholar] [CrossRef]
- Žifčáková, L.; Vetrovsky, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Frᶏc, M.; Hannula, S.-E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Tomer, A.; Singh, R.; Singh, S.-K.; Dwivedi, S.-A.; Reddy, C.-U.; Ram, M.; Keloth, A.; Rachel, R. Role of fungi in bioremediation and environmental sustainability. In Fungal Biology; Springer: Cham, Switzerland, 2021; pp. 187–200. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Liu, Q.; Yin, H. Plant nitrogen acquisition from inorganic and organic sources via root and mycelia pathways in ecto mycorrhizal alpine forests. Soil Biol. Biochem. 2019, 136, 107517. [Google Scholar] [CrossRef]
- Teste, F.-P.; Jones, M.-D.; Dickie, I.-A. Dual-mycorrhizal plants: Their ecology and relevance. New Phytol. 2020, 225, 1835–1851. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Li, X.-L.; Qu, Z.-L.; Zhang, Y.-M.; Ge, Y.; Sun, H. Soil fungal community and potential function in different forest ecosystems. Diversity 2022, 14, 520. [Google Scholar] [CrossRef]
- Frey, S.-D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.-H.; Wu, H.-L.; Ouyang, S.; Lei, P.-F.; Hu, Y.-J.; Ge, T.-D.; Ye, J.; Kuzyakov, Y. Contrasting patterns and drivers of soil fungal communities in subtropical deciduous and evergreen broadleaved forests. Appl. Microbiol. Biotechnol. 2019, 103, 5421–5433. [Google Scholar] [CrossRef]
- Nguyen, N.-H.; Song, Z.-W.; Bates, S.-T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.-S.; Kennedy, P.-G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Bello, A.; Wang, B.; Zhao, Y.; Yang, W.; Ogundeji, A.; Deng, L.; Egbeagu, U.-U.; Yu, S.; Zhao, L.-Y.; Li, D.-T.; et al. Composted biochar affects structural dynamics, function and co-occurrence network patterns of fungi community. Sci. Total Environ. 2021, 775, 145672. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Nisson, R.-H.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Qiu, Z.-L.; Zhang, M.; Wang, K.-F.; Shi, F.-C. Vegetation community dynamics during naturalized developmental restoration of Pinus tabulaeformis plantation in North warm temperate zone. J. Plant Ecol. 2023, 16, rtac102. [Google Scholar] [CrossRef]
- Feroz, S.-M.; Hagihara, Y.-A.; Hagihara, A. Stand stratification and woody species diversity of a subtropical forest in limestone habitat in the northern part of Okinawa Island. J. Plant Res. 2008, 121, 329–337. [Google Scholar] [CrossRef]
- Shannon, C.-E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Simpson, E.-H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.-C. An Introduction to mathematical ecology. BioScience 2011, 24, 7–12. [Google Scholar]
- HJ615–2011b; Soil Determination of Orgaic Carbon-Potassium Dichromate Oxidation Spectrophotometric Method. Ministry of Environmental Protection, PRC: Beijing, China, 2011. (In Chinese)
- HJ634–2012; Soil-Determination of Ammonium, Nitrite and Nitrate by Extraction with Potassium Chloride Solution-Spectrophotometric Methods. Ministry of Environmental Protection, PRC: Beijing, China, 2012. (In Chinese)
- GB/T 32737–2016; Determination of Nitrate Nitrogen in Soil-Ultraviolet Spectrophotometry Method. Standardization Administration of China: Beijing, China, 2016. (In Chinese)
- NY/T 1121.7–2014; Soil Testing-Method for Determination of Available Phosphorus in Soil. Ministry of Agriculture, PRC: Beijing, China, 2012. (In Chinese)
- HJ613–2011a; Soil-Determination of Dry Matter and Water Content-Gravimetric Method. Ministry of Environmental Protection, PRC: Beijing, China, 2011. (In Chinese)
- Lin, X.-G. Principles and Methods of Soil Microbiology Research; High Education Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- White, T.-J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Bolger, A.-M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Edgar, R.-C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.-C.; Haas, B.-J.; Clemente, J.-C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatic 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xing, Y.-J.; Liu, G.-C.; Hu, C.-Y.; Wang, X.-C.; Yan, G.-Y.; Wang, Q.-G. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Liu, S.-E.; Wang, H.; Tian, P.; Yao, X.; Sun, H.; Wang, Q.-K.; Baqueizo, M.-D. Decoupled diversity patterns in bacteria and fungi across continental forest ecosystems. Soil Biol. Biochem. 2020, 144, 107763. [Google Scholar] [CrossRef]
- Sheng, Y.-Y.; Cong, W.; Yang, L.-S.; Liu, Q.; Zhang, Y.-G. Forest soil fungal community elevational distribution pattern and their ecological assembly processes. Front. Microbiol. 2019, 10, 02226. [Google Scholar] [CrossRef] [PubMed]
- Monkai, J.; Purahong, W.; Nawaz, A.; Wubet, T.; Hyde, K.-D.; Goldberg, S.-D.; Mortimer, P.-E.; Xu, J.-C.; Harrison, R.-D. Conversion of rainforest to rubber plantations impacts the rhizosphere soil mycobiome and alters soil biological activity. Land Degrad. Dev. 2022, 33, 3411–3426. [Google Scholar] [CrossRef]
- Ceci, A.; Pinzari, F.; Russo, F.; Persiani, A.-M.; Gadd, G.-M. Roles of saprotrophic fungi in biodegradation or transformation of organic and inorganic pollutants in co-contaminated sites. Appl. Microbiol. Biotechnol. 2019, 103, 53–68. [Google Scholar] [CrossRef]
- Al-Yahya’ei, M.N.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of Southern Arabia. Mycorrhiza 2021, 21, 195–209. [Google Scholar] [CrossRef]
- Wilson, H.; Johnson, R.-R.; Bohannan, B.; Pfeifer-Meister, L.; Mueller, R.; Bridgham, S.-D. Experimental warming decreases arbuscular mycorrhizal fungal colonization in prairie plants along a Mediterranean climate gradient. PeerJ 2016, 4, e2083. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.-P.; Brzostek, E.; Midgley, M.-G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.-D.; Tunlid, A. Ectomycorrhizal fungi-potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.-W.; van den Hoogen, J.; Wan, J.; Mayes, M.-A.; Keiser, A.-D.; Mo, L.; Averill, C.; Maynard, D.-S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Nie, S.-A.; Lei, S.-M.; Zhao, L.-X.; Brookes, P.-C.; Wang, F.; Chen, C.-R.; Yang, W.-H.; Xing, S.-H. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Gilmartin, E.-C.; Jusino, M.-A.; Pyne, E.-J.; Banik, M.-T.; Lindner, D.-L.; Boddy, L. Fungal endophytes and origins of decay in beech (Fagus sylvatica) sapwood. Fungal Ecol. 2022, 59, 101161. [Google Scholar] [CrossRef]
- Averill, C. Slowed decomposition in ectomycorrhizal ecosystems is independent of plant chemistry. Soil Biol. Biochem. 2016, 102, 52–54. [Google Scholar] [CrossRef]
- Liang, M.-X.; Liu, X.-B.; Parker, I.-M.; Johnson, D.; Zheng, Y.; Luo, S.; Gilbert, G.-S.; Yu, S.-X. Soil microbes drive phylogenetic diversity-productivity relationships in a subtropical forest. Sci. Adv. 2019, 5, eaax5088. [Google Scholar] [CrossRef]
- Lang, C.; Seven, J.; Polle, A. Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed Central European forest. Mycorrhiza 2011, 21, 297–308. [Google Scholar] [CrossRef]
- Uroz, S.; Buee, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the forest microbiome: Highlights of temperate and boreal ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Shao, P.-S.; Liang, C.; Rubert-Nason, K.; Li, X.-Z.; Xie, H.-T.; Bao, X.L. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Stursova, M.; Barta, J.; Santruckova, H.; Baldrian, P. Small-scale spatial heterogeneity of ecosystem properties, microbial community composition and microbial activities in a temperate mountain forest soil. Fems Microbiol. Ecol. 2016, 92, fiw185. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; van Rijssel, S.-Q.; van Ruijven, J.; Termorshuizen, A.-J.; Cotton, T.; Dumbrell, A.-J.; Raaijmakers, J.-M.; Weigelt, A.; Mommer, L. Plant functional group drives the community structure of saprophytic fungi in a grassland biodiversity experiment. Plant Soil 2021, 461, 91–105. [Google Scholar] [CrossRef]
- Xu, J.; Liu, B.; Qu, Z.-L.; Ma, Y.; Sun, H. Age and species of Eucalyptus plantations affect soil microbial biomass and enzymatic activities. Microorgianisms 2020, 8, 811. [Google Scholar] [CrossRef]
- Bannert, A.; Kleineidam, K.; Wissing, L.; Mueller-Niggemann, C.; Vogelsang, V.; Welzl, G.; Cao, Z.; Schloter, M. Changes in diversity and functional gene abundances of microbial communities involved in nitrogen fixation, nitrification, and denitrification in a tidal wetland versus paddy soils cultivated for different time periods. Appl. Environ. Microbiol. 2011, 77, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.-E.; Grayston, S.-J. Tree species influence on microbial com munities in litter and soil: Current knowledge and research needs. Forest Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil. Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]





| Species | Biomass Allometry Equation |
|---|---|
| Pinus tabulaeformis | Y = e−1.41+6.92/T × D1.03 × H1.08 + 13.41 Y: (t), D: (m2), H: (m) |
| Quercus variabilis | Y = 0.022337662(D2H)0.993056421 + 0.006221667(D2H)1.008154429 + 0.001179057(D2H)1.298105392 + 0.018493229(D2H)0.671232912 + 0.014665102(D2H)0.950577264 Y: (kg), D: (cm2), H: (m) |
| Robinia pseudoacacia | Y = 0.020(D2H) + 1.974 Y: (kg), D: (cm2), H: (m) |
| Juglans mandshurica | lnY = −2.471 + 2.667 × ln(D) |
| Quercus mongolica | lnY = −3.453 + 1.004 × ln(D2 + H) |
| Tilia amurensis | lnY = −3.771 + 1.013 × ln(D2 + H) |
| Other species | lnY = −2.560 + 2.308 × ln(DBH) + 0.341 × lnH Y: (g), D: (cm2), H: (m) |
| Soil Indicator | P | M | NNF | NF |
|---|---|---|---|---|
| SOC (mg·kg−1) | 79.40 ± 14.02 | 95.88 ± 13.18 | 79.94 ± 17.85 | 105.41 ± 21.03 |
| AP (mg·kg−1) | 38.58 ± 32.35 | 33.89 ± 43.06 | 45.40 ± 10.99 | 40.28 ± 33.67 |
| TP (mg·kg−1) | 545.80 ± 159.6 | 472.02 ± 88.10 | 563.10 ± 193.3 | 532.09 ± 159.4 |
| NO3—N (mg·kg−1) | 13.32 ± 2.26 | 9.97 ± 3.55 | 15.42 ± 3.73 | 21.17 ± 8.00 |
| NH4+-N (mg·kg−1) | 8.10 ± 0.72 | 8.72 ± 7.46 | 4.84 ± 3.05 | 10.50 ± 3.98 |
| pH | 4.79 ± 0.19 | 4.64 ± 0.34 | 5.85 ± 0.38 | 5.74 ± 0.88 |
| DW (%) | 95.71 ± 1.19 | 97.11 ± 0.97 | 96.73 ± 3.27 | 95.89 ± 1.90 |
| TC (%) | 7.18 ± 3.55 | 5.16 ± 0.37 | 7.15 ± 1.16 | 7.55 ± 2.78 |
| TN (%) | 0.52 ± 0.26 | 0.36 ± 0.04 | 0.56 ± 0.12 | 0.61 ± 0.06 |
| C/N | 13.87 ± 1.26 | 14.55 ± 0.71 | 12.97 ± 0.77 | 12.35 ± 0.86 |
| CA (μg·g−1·min−1) | 0.10 ± 0.09 | 0.18 ± 0.05 | 0.20 ± 0.10 | 0.21 ± 0.06 |
| UA (μg·g−1·h−1) | 5.23 ± 1.63 | 5.24 ± 1.81 | 9.61 ± 1.34 | 8.98 ± 2.78 |
| GA (μg·g−1·h−1) | 0.83 ± 0.78 | 1.24 ± 0.98 | 1.01 ± 0.51 | 0.59 ± 0.54 |
| DHA (μg·g−1·h−1) | 0.39 ± 0.35 | 0.61 ± 0.54 | 0.46 ± 0.37 | 0.33 ± 0.38 |
| ACP (μg·g−1·min−1) | 5.83 ± 1.10 | 5.29 ± 1.29 | 4.36 ± 1.52 | 6.85 ± 3.73 |
| Soil Physicochemical Properties | Plant Diversity | Plant Composition | Enzyme Activity | |
|---|---|---|---|---|
| Functional mode | R = 0.021 p = 0.417 | R = −0.130 p = 0.983 | R = 0.098 p = 0.229 | R = −0.099 p = 0.794 |
| Vegetative mode | R = 0.048 p = 0.302 | R = −0.128 p = 0.892 | R = 0.055 p = 0.343 | R = −0.107 p = 0.798 |
| Growth mode | R = 0.053 p = 0.333 | R = −0.151 p = 0.929 | R = 0.111 p = 0.195 | R = −0.114 p = 0.859 |
| Functional Mode | Vegetative Mode | Growth Mode | ||||
|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | |
| B | 0.197 | 0.075 | 0.088 | 0.331 | 0.021 | 0.788 |
| R | 0.010 | 0.858 | 0.039 | 0.552 | 0.013 | 0.855 |
| SHI | 0.006 | 0.936 | 0.015 | 0.809 | 0.005 | 0.934 |
| SII | 0.009 | 0.905 | 0.006 | 0.938 | 0.017 | 0.801 |
| SHE | 0.095 | 0.291 | 0.031 | 0.710 | 0.050 | 0.548 |
| SIE | 0.134 | 0.176 | 0.075 | 0.387 | 0.113 | 0.265 |
| PE | 0.017 | 0.802 | 0.015 | 0.844 | 0.063 | 0.442 |
| Shannon | 0.001 | 0.999 | 0.042 | 0.588 | 0.031 | 0.662 |
| Functional Mode | Vegetative Mode | Growth Mode | ||||
|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | |
| 1 | 0.025 | 0.696 | 0.050 | 0.473 | 0.052 | 0.487 |
| 2 | 0.046 | 0.529 | 0.011 | 0.899 | 0.110 | 0.241 |
| 3 | 0.021 | 0.739 | 0.058 | 0.414 | 0.019 | 0.781 |
| 4 | 0.832 | 0.001 | 0.867 | 0.002 | 0.754 | 0.001 |
| 5 | 0.004 | 0.887 | 0.116 | 0.134 | 0.034 | 0.530 |
| 6 | 0.019 | 0.688 | 0.050 | 0.378 | 0.026 | 0.676 |
| 7 | 0.349 | 0.043 | 0.410 | 0.028 | 0.303 | 0.050 |
| 8 | 0.001 | 0.984 | 0.015 | 0.839 | 0.000 | 0.998 |
| 9 | 0.145 | 0.125 | 0.000 | 0.984 | 0.020 | 0.574 |
| 10 | 0.018 | 0.761 | 0.016 | 0.834 | 0.087 | 0.316 |
| Functional Mode | Vegetative Mode | Growth Mode | ||||
|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | |
| SOC | 0.073 | 0.408 | 0.258 | 0.037 | 0.231 | 0.037 |
| AP | 0.020 | 0.815 | 0.128 | 0.203 | 0.100 | 0.291 |
| TP | 0.004 | 0.949 | 0.014 | 0.845 | 0.081 | 0.350 |
| NAN | 0.130 | 0.207 | 0.185 | 0.080 | 0.089 | 0.315 |
| pH | 0.394 | 0.002 | 0.232 | 0.043 | 0.180 | 0.092 |
| DM | 0.032 | 0.679 | 0.242 | 0.031 | 0.036 | 0.648 |
| TC | 0.074 | 0.390 | 0.027 | 0.739 | 0.138 | 0.161 |
| CN | 0.138 | 0.165 | 0.068 | 0.411 | 0.270 | 0.019 |
| TN | 0.135 | 0.171 | 0.042 | 0.599 | 0.249 | 0.028 |
| AN | 0.137 | 0.166 | 0.086 | 0.314 | 0.187 | 0.085 |
| Functional Mode | Vegetative Mode | Growth Mode | ||||
|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | |
| CA | 0.062 | 0.427 | 0.022 | 0.768 | 0.044 | 0.610 |
| UA | 0.243 | 0.027 | 0.221 | 0.053 | 0.206 | 0.047 |
| GA | 0.127 | 0.185 | 0.107 | 0.269 | 0.371 | 0.005 |
| ACP | 0.520 | 0.012 | 0.154 | 0.149 | 0.123 | 0.183 |
| DHA | 0.438 | 0.001 | 0.159 | 0.126 | 0.163 | 0.111 |
| AN | 0.148 | 0.157 | 0.042 | 0.621 | 0.201 | 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Liu, H.; Chen, C.; Liu, C.; Shu, J. Environmental Driving Mechanism and Response of Soil’s Fungal Functional Structure to Near-Naturalization in a Warm Temperate Plantation. Forests 2024, 15, 1540. https://doi.org/10.3390/f15091540
Qiu Z, Liu H, Chen C, Liu C, Shu J. Environmental Driving Mechanism and Response of Soil’s Fungal Functional Structure to Near-Naturalization in a Warm Temperate Plantation. Forests. 2024; 15(9):1540. https://doi.org/10.3390/f15091540
Chicago/Turabian StyleQiu, Zhenlu, Huan Liu, Chunli Chen, Congcong Liu, and Jing Shu. 2024. "Environmental Driving Mechanism and Response of Soil’s Fungal Functional Structure to Near-Naturalization in a Warm Temperate Plantation" Forests 15, no. 9: 1540. https://doi.org/10.3390/f15091540





