Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling
2.3. DNA Extraction and Metagenomic Analysis
2.4. Soil Amino Sugars and MRC
2.5. Soil Aggregates
2.6. Mycorrhizal Hyphal Length Density
2.7. Glomalin-Related Soil Protein
2.8. Statistical Analysis
3. Results
3.1. Soil MRC
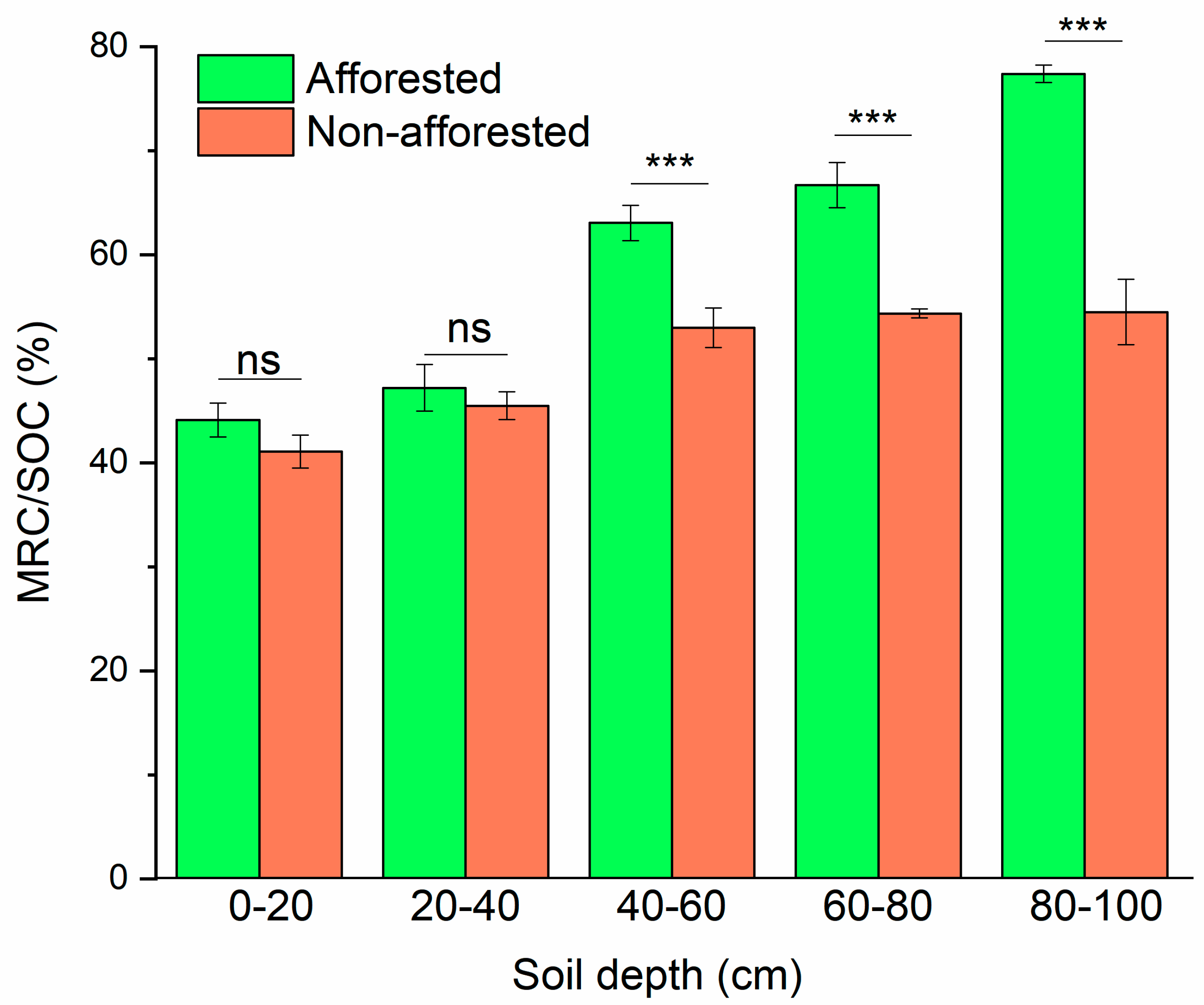
3.2. Relative Contributions of MRC to SOC
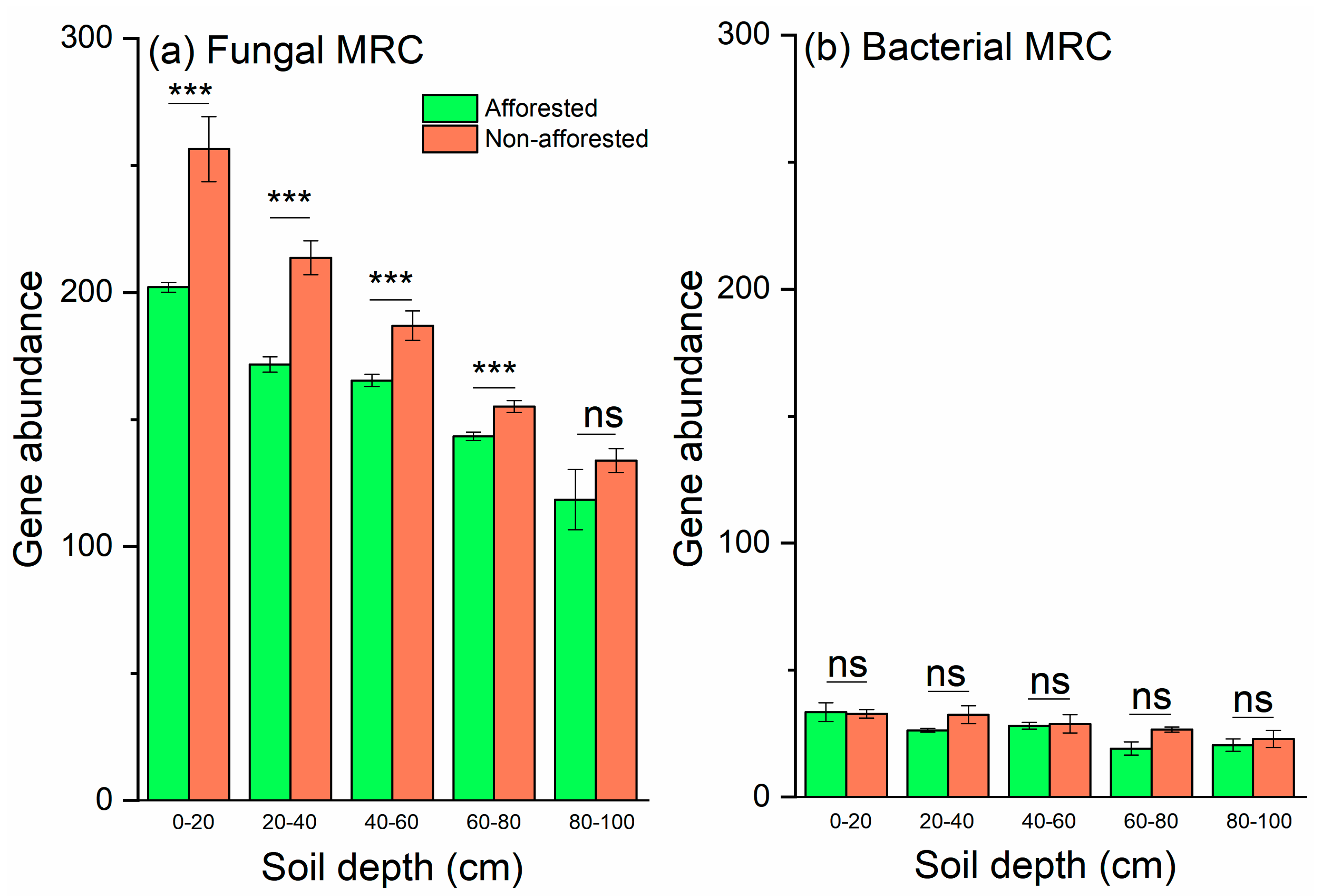
3.3. Ratio of Fungal to Bacterial MRC
3.4. CAZy Gene Families
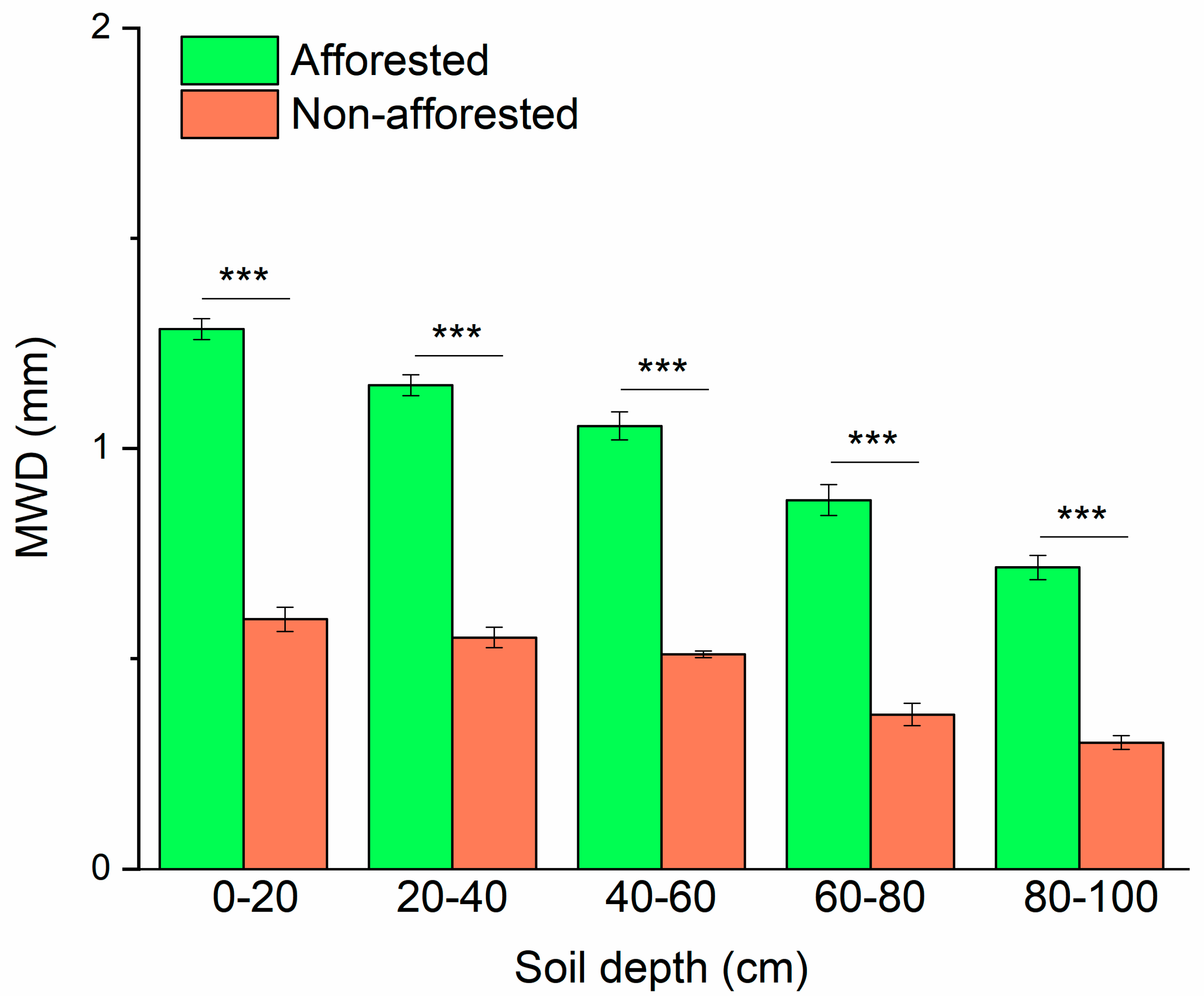
3.5. Variations of Soil MWD
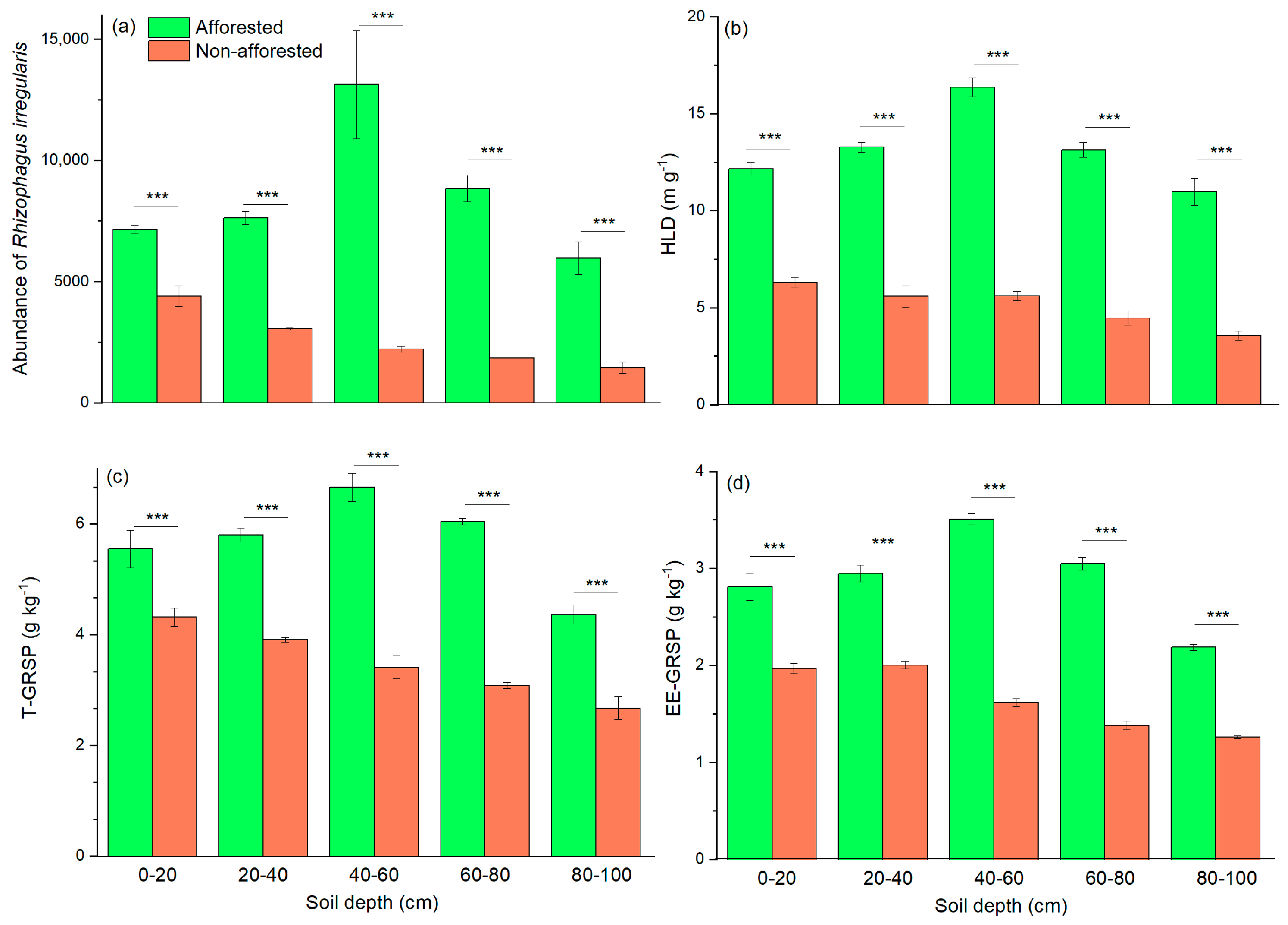
3.6. AMF-Related Characteristics
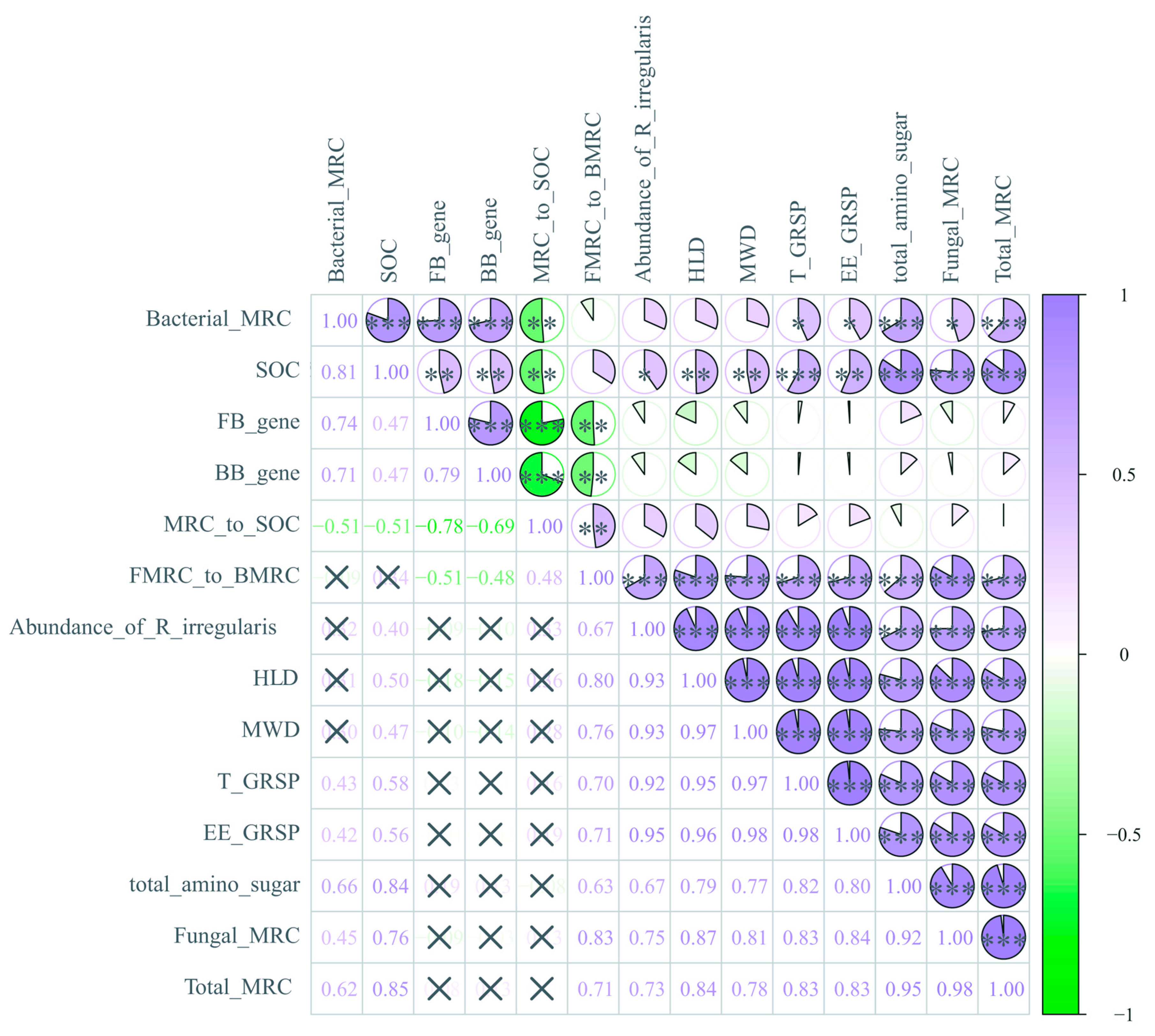
3.7. Pearson Correlations
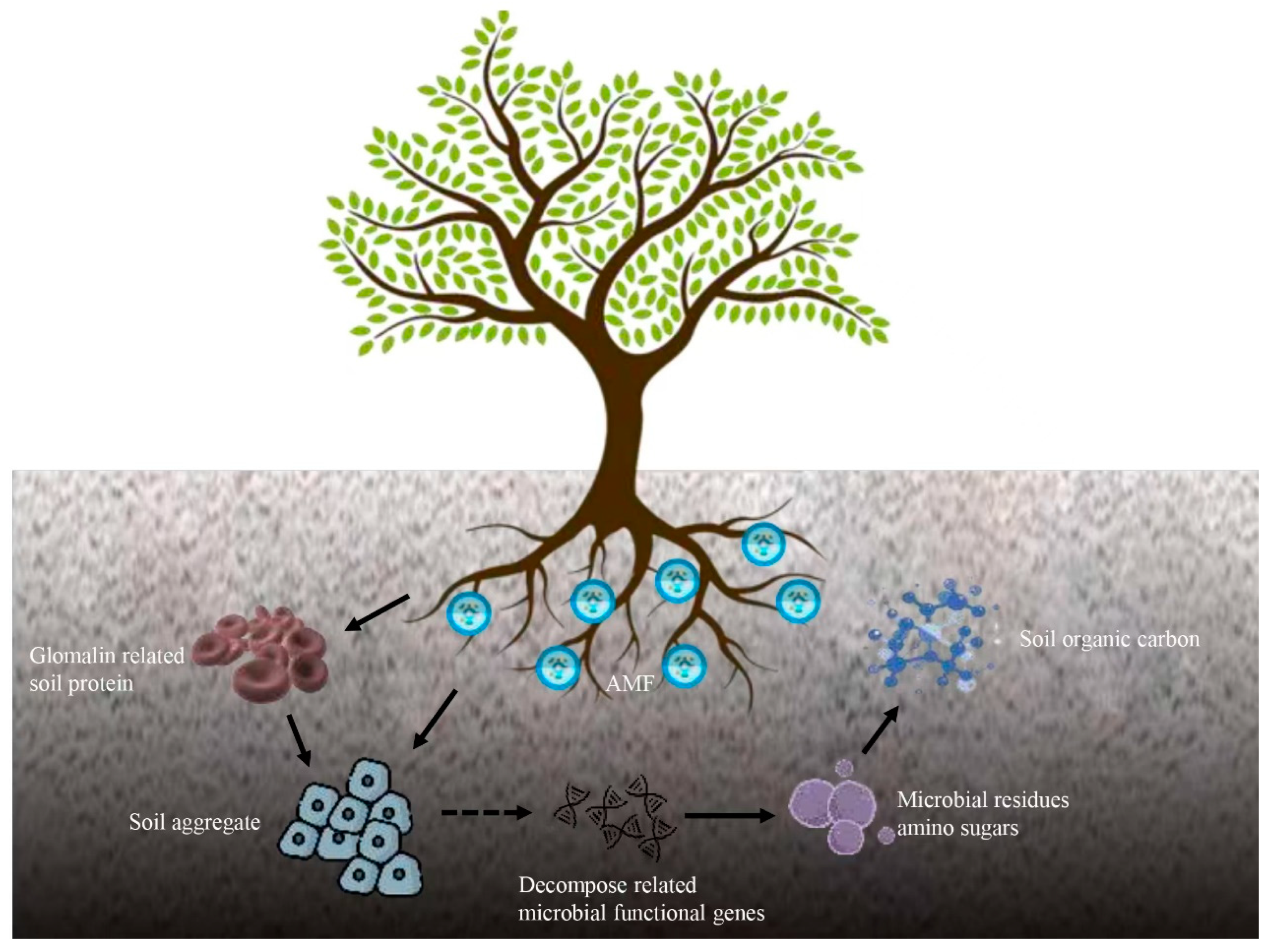
4. Discussion
4.1. Afforestation Promotes MRC Accrual in Deep Soil
4.2. Afforestation Promotes AMF and Soil Aggregation
4.3. Afforestation Decreased Microbial Functionality on MRC
4.4. Research Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Chen, S.; Yang, Y.; Wang, Q.; Wu, Y.; Zhou, Z.; Wang, H.; Wang, W. Shelterbelt farmland-afforestation induced SOC accrual with higher temperature stability: Cross-sites 1 m soil profiles analysis in NE China. Sci. Total Environ. 2022, 814, 151942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, B.; Zhang, Y.; Ao, D.; Feng, C.; Wang, P.; Bai, X.; An, S. Afforestation increased the microbial necromass carbon accumulation in deep soil on the Loess Plateau. J. Environ. Manag. 2024, 349, 119508. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Lal, R. The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv. Agron. 2005, 88, 35–66. [Google Scholar]
- Harrison, R.B.; Footen, P.W.; Strahm, B.D. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. For. Sci. 2011, 57, 67–76. [Google Scholar] [CrossRef]
- Johnson, D.W.; Murphy, J.D.; Rau, B.M.; Miller, W.W. Subsurface carbon contents: Some case studies in forest soils. For. Sci. 2011, 57, 3–10. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Tláskal, V.; Větrovský, T.; Štursová, M.; Toscan, R.; da Rocha, U.N.; Baldrian, P. Metagenomics and stable isotope probing reveal the complementary contribution of fungal and bacterial communities in the recycling of dead biomass in forest soil. Soil Biol. Biochem. 2020, 148, 107875. [Google Scholar] [CrossRef]
- Huff, M.; Seaman, J.; Wu, D.; Zhebentyayeva, T.; Kelly, L.J.; Faridi, N.; Nelson, C.D.; Cooper, E.; Best, T.; Steiner, K.; et al. A high-quality reference genome for Fraxinus pennsylvanica for ash species restoration and research. Mol. Ecol. Resour. 2022, 22, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, X.; Luo, Y.; Liu, W.; Liu, G. Identifying carbon-degrading enzyme activities in association with soil organic carbon accumulation under land-use changes. Ecosystems 2022, 25, 1219–1233. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, X.; Zhang, S.; Wang, J.; Xu, M.; Guo, Y.; Wang, J.; Han, X.; Zhao, F.; Yang, G.; et al. Altered microbial CAZyme families indicated dead biomass decomposition following afforestation. Soil Biol. Biochem. 2021, 160, 108362. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol. Lett. 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ramsey, P.W.; Morris, S.; Paul, E.A. Glomalin, an arbuscular-mycorrhizal fungal soil protein, responds to land-use change. Plant Soil 2003, 253, 293–299. [Google Scholar] [CrossRef]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.; Shew, H.D.; Rufty, T.W.; Hu, S. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef]
- Carrillo, Y.; Dijkstra, F.A.; LeCain, D.; Pendall, E. Mediation of soil C decomposition by arbuscular mycorrizhal fungi in grass rhizospheres under elevated CO2. Biogeochemistry 2016, 127, 45–55. [Google Scholar] [CrossRef]
- Shahzad, T.; Chenu, C.; Genet, P.; Barot, S.; Perveen, N.; Mougin, C.; Fontaine, S. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol. Biochem. 2015, 80, 146–155. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.; Vogt, S.; Gleber, S.; Bigalke, M.; Wilcke, W.; Rillig, M. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef]
- Jiang, T.-t.; Pan, J.-f.; Pu, X.-M.; Wang, B.; Pan, J.-J. Current status of coastal wetlands in China: Degradation, restoration, and future management. Estuar. Coast. Shelf Sci. 2015, 164, 265–275. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Sun, Q.-X.; Yang, Y.-F. Research on sustainable use of the middle and lower beach land of the Yangtze River. Resour. Environ. Yangtze Basin 2010, 19, 878–883. [Google Scholar]
- Gao Shenghua, C.; Zhang Xudong, C.; Yuxi, T. Short-term effects of clear-cutting of Populus deltoides plantation on methane flux on the beach land of Yangtze River. Sci. Silvae Sin. 2013, 49, 7–13. [Google Scholar]
- Zhang, Q.; Tang, J.; Angel, R.; Wang, D.; Hu, X.; Gao, S.; Zhang, L.; Tang, Y.; Zhang, X.; Koide, R.T.; et al. Soil properties interacting with microbial metagenome in decreasing CH4 emission from seasonally flooded marshland following different stages of afforestation. Front. Microbiol. 2022, 13, 830019. [Google Scholar] [CrossRef]
- Yang, H.; Koide, R.T.; Zhang, Q. Short-term waterlogging increases arbuscular mycorrhizal fungal species richness and shifts community composition. Plant Soil 2016, 404, 373–384. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; LoCascio, P.F.; Hauser, L.J.; Uberbacher, E.C. Gene and translation initiation site prediction in metagenomic sequences. Bioinformatics 2012, 28, 2223–2230. [Google Scholar] [CrossRef]
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/taxonomyhome.html/index.cgi?chapter=tgencodes#SG1 (accessed on 1 June 2023).
- Tool. Cd-hit. Available online: http://www.bioinformatics.org/cd-hit/ (accessed on 15 August 2023).
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Steinegger, M.; Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Indorf, C.; Dyckmans, J.; Khan, K.S.; Joergensen, R.G. Optimisation of amino sugar quantification by HPLC in soil and plant hydrolysates. Biol. Fertil. Soils 2011, 47, 387–396. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Peñuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob. Chang. Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, Y.; Zhang, W.; Hu, G.; Xie, H.; Yan, J.; Han, S.; He, H.; Zhang, X. Linkage of microbial residue dynamics with soil organic carbon accumulation during subtropical forest succession. Soil Biol. Biochem. 2017, 114, 114–120. [Google Scholar] [CrossRef]
- Appuhn, A.; Joergensen, R.G. Microbial colonisation of roots as a function of plant species. Soil Biol. Biochem. 2006, 38, 1040–1051. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 425–442. [Google Scholar]
- Jakobsen, I.; Abbott, L.; Robson, A. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Rillig, M.C.; Field, C.B.; Allen, M.F. Soil biota responses to long-term atmospheric CO2 enrichment in two California annual grasslands. Oecologia 1999, 119, 572–577. [Google Scholar] [CrossRef]
- Emran, M.; Gispert, M.; Pardini, G. Patterns of soil organic carbon, glomalin and structural stability in abandoned Mediterranean terraced lands. Eur. J. Soil Sci. 2012, 63, 637–649. [Google Scholar] [CrossRef]
- Han, X.; Ren, C.; Li, B.; Yan, S.; Fu, S.; Gao, D.; Zhao, F.; Deng, J.; Yang, G. Growing seasonal characteristics of soil and plants control the temporal patterns of bacterial communities following afforestation. Catena 2019, 178, 288–297. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Huang, L.; Qi, Q.; Liu, L.; Zhao, Y.; Wang, Z.; Zhou, H.; Lv, X.; Mao, Z.; et al. Shifts in soil microbial community functional gene structure across a 61-year desert revegetation chronosequence. Geoderma 2019, 347, 126–134. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, W.; Lu, X.; Gu, Y.; Wu, S.; Shen, Z.; Han, X.; Yang, G.; Ren, C. Adaptive pathways of soil microorganisms to stoichiometric imbalances regulate microbial respiration following afforestation in the Loess Plateau, China. Soil Biol. Biochem. 2020, 151, 108048. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Han, F.; Ju, W.; Ye, L.; Wang, X.; Tan, W.; Zhang, X. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Yin, R.; Deng, H.; Wang, H.-l.; Zhang, B. Vegetation type affects soil enzyme activities and microbial functional diversity following re-vegetation of a severely eroded red soil in sub-tropical China. Catena 2014, 115, 96–103. [Google Scholar] [CrossRef]
- Huang, H.; Tian, D.; Zhou, L.; Su, H.; Ma, S.; Feng, Y.; Tang, Z.; Zhu, J.; Ji, C.; Fang, J. Effects of afforestation on soil microbial diversity and enzyme activity: A meta-analysis. Geoderma 2022, 423, 115961. [Google Scholar] [CrossRef]
- Kong, W.; Wei, X.; Wu, Y.; Shao, M.; Zhang, Q.; Sadowsky, M.J.; Ishii, S.; Reich, P.B.; Wei, G.; Jiao, S.; et al. Afforestation can lower microbial diversity and functionality in deep soil layers in a semiarid region. Glob. Chang. Biol. 2022, 28, 6086–6101. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Herman, D.J.; He, Z.; Pett-Ridge, J.; Wu, L.; Zhou, J.; Firestone, M.K. Plant roots alter microbial functional genes supporting root litter decomposition. Soil Biol. Biochem. 2018, 127, 90–99. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The M icrobial E fficiency-M atrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Boyno, G.; Yerli, C.; Çakmakci, T.; Sahin, U.; Demir, S. The effect of arbuscular mycorrhizal fungi on carbon dioxide (CO2) emission from turfgrass soil under different irrigation intervals. J. Water Clim. Chang. 2024, 15, 541–553. [Google Scholar] [CrossRef]
- Hu, X.; Chen, D.; Yan, F.; Zheng, X.; Fang, X.; Bai, Y.; Zhao, J.; Ma, X.; Ma, C.; Cai, X.; et al. Global research trends on the effects of arbuscular mycorrhizal fungi on the soil carbon cycle: A bibliometric analysis. Ecol. Indic. 2024, 158, 111543. [Google Scholar] [CrossRef]
- Ni, X.; Liao, S.; Tan, S.; Peng, Y.; Wang, D.; Yue, K.; Wu, F.; Yang, Y. The vertical distribution and control of microbial necromass carbon in forest soils. Glob. Ecol. Biogeogr. 2020, 29, 1829–1839. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Liu, J.-B.; Jia, X.; Qin, S.-G. Soil Organic Carbon Accumulation in Arid and Semiarid Areas after Afforestation: A Meta-Analysis. Pol. J. Environ. Stud. 2013, 22, 611–620. [Google Scholar]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, X.; Zheng, X.; Deng, S.; Hu, Y.; Zheng, S.; He, X.; Wu, J.; Kuzyakov, Y.; Su, Y. Preferential uptake of hydrophilic and hydrophobic compounds by bacteria and fungi in upland and paddy soils. Soil Biol. Biochem. 2020, 148, 107879. [Google Scholar] [CrossRef]
- Di Lonardo, D.; De Boer, W.; Gunnewiek, P.K.; Hannula, S.; Van der Wal, A. Priming of soil organic matter: Chemical structure of added compounds is more important than the energy content. Soil Biol. Biochem. 2017, 108, 41–54. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, F.; Zhu, J.; Ni, X. Leaf and root inputs additively contribute to soil organic carbon formation in various forest types. J. Soils Sediments 2023, 23, 1135–1145. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- He, M.; Fang, K.; Chen, L.; Feng, X.; Qin, S.; Kou, D.; He, H.; Liang, C.; Yang, Y. Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands. Glob. Chang Biol. 2022, 28, 936–949. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, G.; Zhou, X.; Zhou, L.; Shao, J.; Liu, R.; Gao, J.; He, Y.; Du, Z.; Tang, J.; et al. Effects of tree mycorrhizal type on soil respiration and carbon stock via fine root biomass and litter dynamic in tropical plantations. J. Plant Ecol. 2023, 16, rtac056. [Google Scholar] [CrossRef]
- Cissé, G.; Essi, M.; Kedi, B.; Nicolas, M.; Staunton, S. Accumulation and vertical distribution of glomalin-related soil protein in French temperate forest soils as a function of tree type, climate and soil properties. Catena 2023, 220, 106635. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, L.; Cao, Y.; Yang, Y.; Wang, P.; Li, Z.; Lin, Y. Effects of land-use type on soil organic carbon and carbon pool management index through arbuscular mycorrhizal fungi pathways. Glob. Ecol. Conserv. 2023, 43, e02432. [Google Scholar] [CrossRef]
- Wright, S.F.; a Nichols, K. Glomalin: Hiding place for a third of the world’s stored soil carbon. Agric. Res. 2002, 50, 4. [Google Scholar]
- Gispert, M.; Pardini, G.; Emran, M.; Doni, S.; Masciandaro, G. Seasonal evolution of soil organic matter, glomalin and enzymes and potential for C storage after land abandonment and renaturalization processes in soils of NE Spain. Catena 2018, 162, 402–413. [Google Scholar] [CrossRef]
- Kohler, J.; Roldán, A.; Campoy, M.; Caravaca, F. Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil 2017, 410, 273–281. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, B.; Shen, J.; Xu, F.; Li, N.; Jia, P.; Jia, Y.; An, S.; Amoah, I.D.; Huang, Y. Shifts in C-degradation genes and microbial metabolic activity with vegetation types affected the surface soil organic carbon pool. Soil Biol. Biochem. 2024, 192, 109371. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Liu, E.; Li, Y.; Tang, Y.; Tian, Y.; Du, S.; Li, H.; Wan, L.; Zhang, Q. Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland. Forests 2024, 15, 1542. https://doi.org/10.3390/f15091542
Tang J, Liu E, Li Y, Tang Y, Tian Y, Du S, Li H, Wan L, Zhang Q. Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland. Forests. 2024; 15(9):1542. https://doi.org/10.3390/f15091542
Chicago/Turabian StyleTang, Jie, En Liu, Yongjin Li, Yuxi Tang, Ye Tian, Shuhui Du, Haoyang Li, Long Wan, and Qian Zhang. 2024. "Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland" Forests 15, no. 9: 1542. https://doi.org/10.3390/f15091542
APA StyleTang, J., Liu, E., Li, Y., Tang, Y., Tian, Y., Du, S., Li, H., Wan, L., & Zhang, Q. (2024). Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland. Forests, 15(9), 1542. https://doi.org/10.3390/f15091542





