Impacts of Climate Change on the Potential Distribution of Three Cytospora Species in Xinjiang, China
Abstract
:1. Introduction
2. Materials and Methods
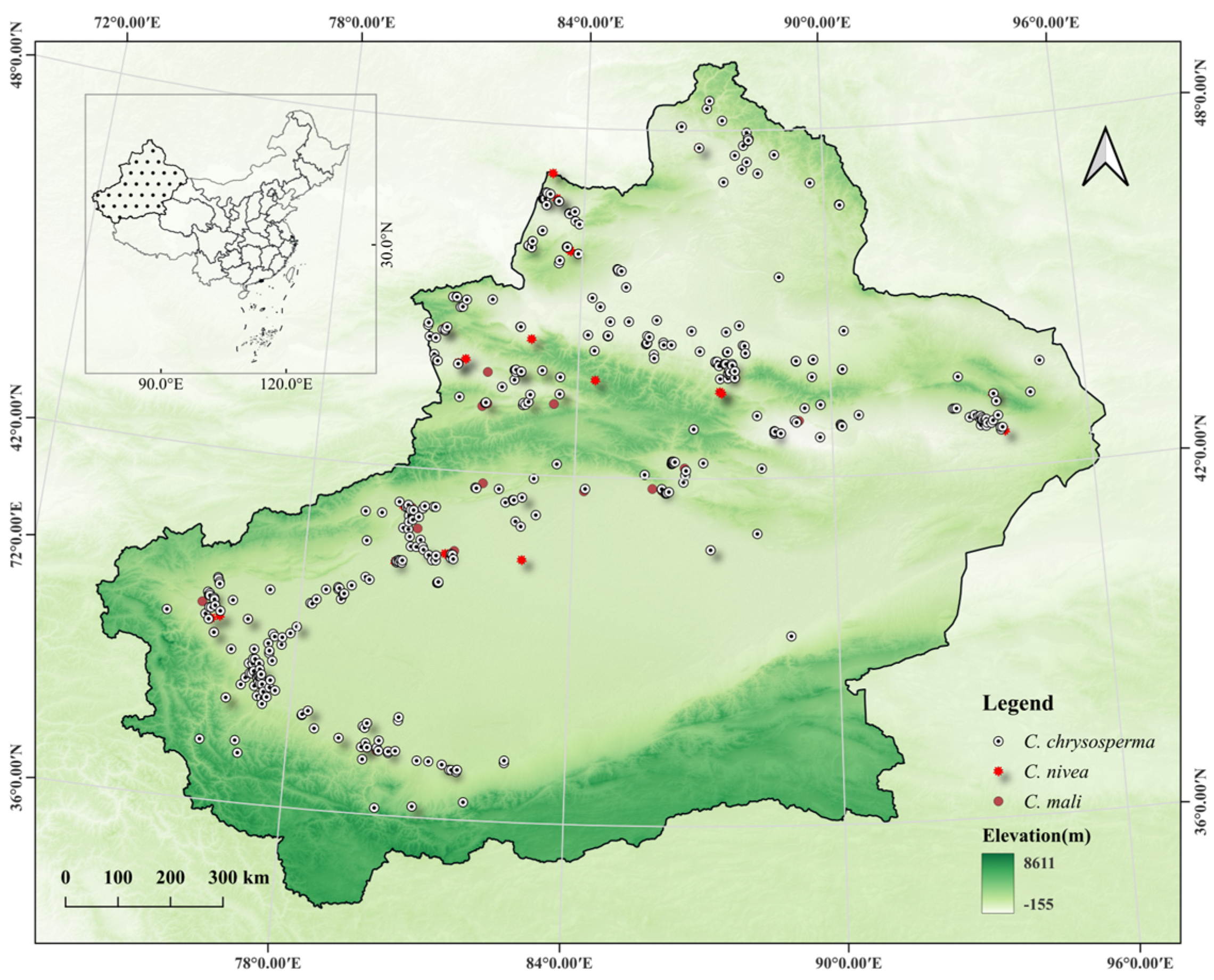
2.1. Species Geographical Distribution Data
2.2. Environmental Variables
2.3. Construction of SDM and Evaluation of Model Accuracy
2.4. Species Distribution Maps
3. Results
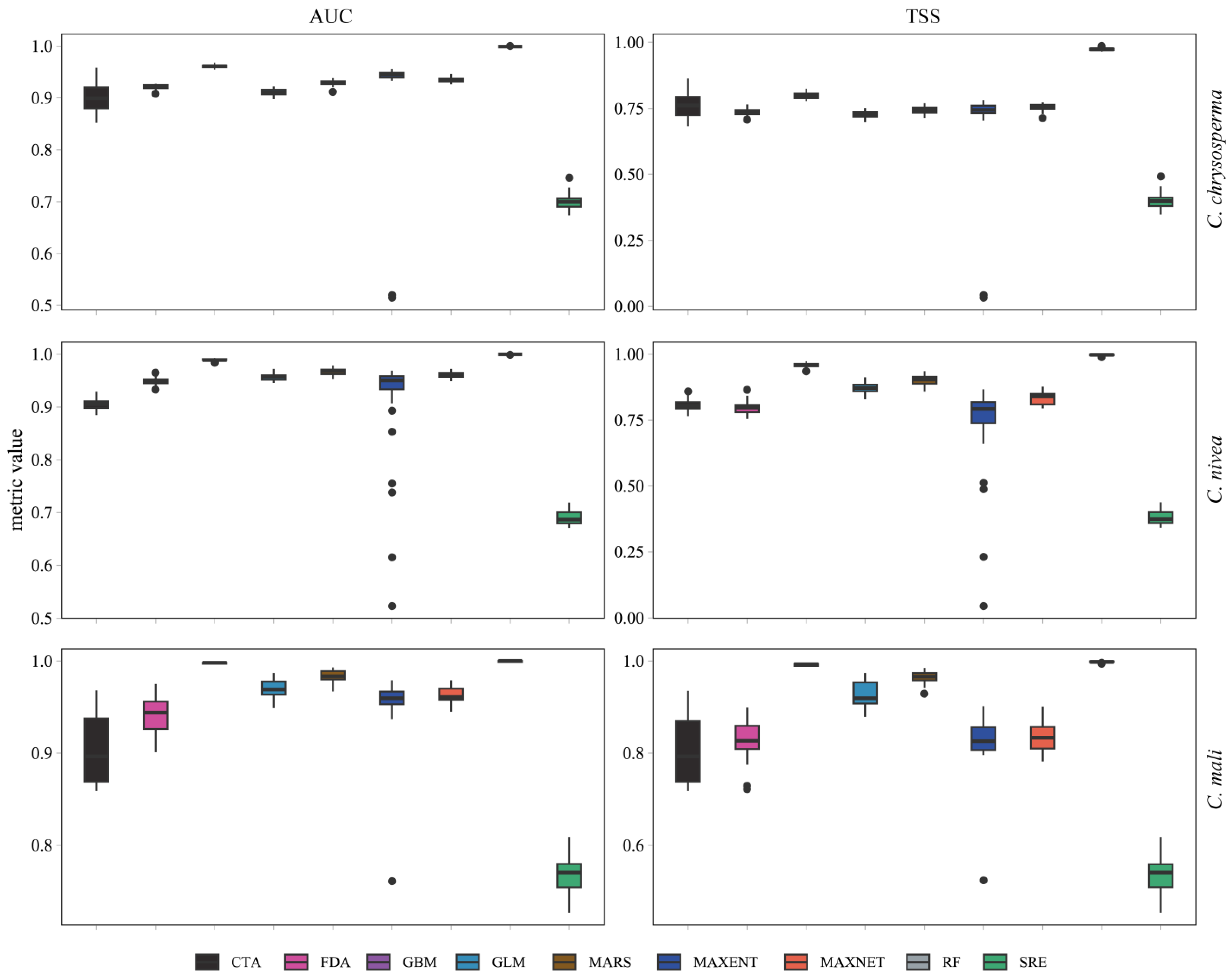
3.1. Model Accuracy Evaluation
3.2. Response Curves of Probability of Presence to Environmental Variables
3.3. Current Potential Geographical Distributions of the Three Cytospora Species
3.4. Future Potential Geographical Distributions of the Three Cytospora Species
3.5. Overlapping Geographical Distribution Areas of the Three Cytospora Species under Different Climate Scenarios
3.6. Spatial Variation in the Three Cytospora Species and Transfer of Their Distribution Centroids
4. Discussion
4.1. Evaluation of Modeling Performance
4.2. Response of Habitat Suitability to Environmental Variables
4.3. Dynamic Ecological Niches of the Three Cytospora Species
4.4. Practical Implications and Management Recommendations
4.5. Study Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.-T.; Li, J.-R.; Jiang, N. Identification of Cytospora Species Isolated from Branch Canker Diseases of Woody Plants in Tibet, China. Forests 2024, 15, 121. [Google Scholar] [CrossRef]
- Pan, M.; Zhu, H.; Bonthond, G.; Tian, C.; Fan, X. High Diversity of Cytospora Associated With Canker and Dieback of Rosaceae in China, With 10 New Species Described. Front. Plant Sci. 2020, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, K. Diseases of Trees and Shrubs. Forestry 2006, 79, 612–613. [Google Scholar] [CrossRef]
- Han, Z.; Yu, R.; Xiong, D.; Tian, C. A Sge1 Homolog in Cytospora Chrysosperma Governs Conidiation, Virulence and the Expression of Putative Effectors. Gene 2021, 778, 145474. [Google Scholar] [CrossRef]
- Yu, L.; Xiong, D.; Han, Z.; Liang, Y.; Tian, C. The Mitogen-Activated Protein Kinase Gene CcPmk1 Is Required for Fungal Growth, Cell Wall Integrity and Pathogenicity in Cytospora chrysosperma. Fungal Genet. Biol. 2019, 128, 1–13. [Google Scholar] [CrossRef]
- Christensen, C. Studies on the Biology of Valsa Sordida and Cytospora Chrysosperma [sic]. Phytopathology 1940, 30, 459–475. [Google Scholar]
- Yin, Z.; Liu, H.; Li, Z.; Ke, X.; Dou, D.; Gao, X.; Song, N.; Dai, Q.; Wu, Y.; Xu, J.; et al. Genome Sequence of Valsa Canker Pathogens Uncovers a Potential Adaptation of Colonization of Woody Bark. New Phytol. 2015, 208, 1202–1216. [Google Scholar] [CrossRef]
- Ke, X.; Huang, L.; Han, Q.; Gao, X.; Kang, Z. Histological and Cytological Investigations of the Infection and Colonization of Apple Bark by Valsa mali var. mali. Australas. Plant Pathol. 2013, 42, 85–93. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Bozorov, T.A.; Ma, R.; Ma, J.; Zhang, Y.; Yang, H.; Li, L.; Zhang, D. Characterization and Pathogenicity of Six Cytospora Strains Causing Stem Canker of Wild Apple in the Tianshan Forest, China. For. Pathol. 2020, 50, e12587. [Google Scholar] [CrossRef]
- Eken, C.; Sevindik, E. Molecular Phylogeny of Cytospora Species Associated with Canker Diseases of Apple Trees in Türkiye. Erwerbs-Obstbau 2023, 65, 2249–2257. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Pouzoulet, J.; Rolshausen, P.E.; Wilcox, W.F.; Baumgartner, K. Characterization of Cytospora Isolates from Wood Cankers of Declining Grapevine in North America, with the Descriptions of Two New Cytospora Species. Plant Pathol. 2017, 66, 713–725. [Google Scholar] [CrossRef]
- Adams, G.C.; Roux, J.; Wingfield, M.J. Cytospora Species (Ascomycota, Diaporthales, Valsaceae): Introduced and Native Pathogens of Trees in South Africa. Austral. Plant Pathol. 2006, 35, 521–548. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Y.; Chi, F.; Ji, Z.; Wu, J.; Dong, Q.; Zhou, Z. Bacillus amyloliquefaciens GB1 Can Effectively Control Apple Valsa Canker. Biol. Control 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Holland, L.A.; Nouri, M.T.; Travadon, R.; Abramians, A.; Michailides, T.J.; Trouillas, F.P. Molecular Phylogeny of Cytospora Species Associated with Canker Diseases of Fruit and Nut Crops in California, with the Descriptions of Ten New Species and One New Combination. IMA Fungus 2018, 9, 333–369. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, F.P.; Peduto, F.; Lorber, J.D.; Sosnowski, M.R.; Grant, J.; Coates, W.W.; Anderson, K.K.; Caprile, J.; Gubler, W.D. Calosphaeria Canker of Sweet Cherry Caused by Calosphaeria pulchella in California and South Australia. Plant Dis. 2012, 96, 648–658. [Google Scholar] [CrossRef]
- Adams, G.; Wingfield, M.; Common, R.; Roux, J. Phylogenetic Relationships and Morphology of Cytospora Species and Related Teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud. Mycol. 2004, 52, 1–3. [Google Scholar]
- Pan, M.; Zhu, H.-Y.; Tian, C.-M.; Alvarez, L.V.; Fan, X.-L. Cytospora piceae sp. nov. Associated with Canker Disease of Picea crassifolia in China. Phytotaxa 2018, 383, 181–196. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.-M.; Gleason, M.L.; Huang, L. Fungal Species Associated with Apple Valsa Canker in East Asia. Phytopathol. Res. 2020, 2, 35. [Google Scholar] [CrossRef]
- Azizi, R.; Ghosta, Y.; Ahmadpour, A. Apple Crown and Collar Canker and Necrosis Caused by Cytospora balanejica sp. nov. in Iran. Sci. Rep. 2024, 14, 6629. [Google Scholar] [CrossRef]
- Fan, X.-L.; Liang, Y.-M.; Ma, R.; Tian, C.-M. Morphological and Phylogenetic Studies of Cytospora (Valsaceae, Diaporthales) Isolates from Chinese Scholar Tree, with Description of a New Species. Mycoscience 2014, 55, 252–259. [Google Scholar] [CrossRef]
- Yan, C.; Hao, H.; Sha, S.; Wang, Z.; Huang, L.; Kang, Z.; Wang, L.; Feng, H. Comparative Assessment of Habitat Suitability and Niche Overlap of Three Cytospora Species in China. JoF 2024, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lu, Q.; Decock, C.; Li, Y.-X.; Zhang, X.-Y. Cytospora Species from Populus and Salix in China with C. davidiana sp. nov. Fungal Biol. 2015, 119, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-L.; Tian, C.-M.; Yang, Q.; Liang, Y.-M.; You, C.-J.; Zhang, Y.-B. Cytospora from Salix in Northern China. Mycotaxon 2015, 129, 303–315. [Google Scholar] [CrossRef]
- Wang, X.; Wei, J.; Huang, L.; Kang, Z. Re-Evaluation of Pathogens Causing Valsa Canker on Apple in China. Mycologia 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, R.; Gleason, M.L.; Sun, G. Sustainable Apple Disease Management in China: Challenges and Future Directions for a Transforming Industry. Plant Dis. 2022, 106, 786–799. [Google Scholar] [CrossRef]
- Lin, L.; Pan, M.; Bezerra, J.D.P.; Tian, C.; Fan, X. Re-Evaluation of the Fungal Diversity and Pathogenicity of Cytospora Species from Populus in China. Plant Dis. 2023, 107, 83–96. [Google Scholar] [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European Ash (Fraxinus Excelsior) Dieback—A Conservation Biology Challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Beaton, J.K.; Bellamy, P.E.; Broome, A.; Chetcuti, J.; Eaton, S.; Ellis, C.J.; Gimona, A.; Harmer, R.; Hester, A.J.; et al. Ash Dieback in the UK: A Review of the Ecological and Conservation Implications and Potential Management Options. Biol. Conserv. 2014, 175, 95–109. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Chestnut Blight: The Classical Problem of an Introduced Pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Cahill, D.M.; Rookes, J.E.; Wilson, B.A.; Gibson, L.; McDougall, K.L. Phytophthora cinnamomi and Australia’s Biodiversity: Impacts, Predictions and Progress towards Control. Aust. J. Bot. 2008, 56, 279–310. [Google Scholar] [CrossRef]
- He, T.; Cai, G.; Jia, H.; Zhai, Y.; Ma, R. Distribution Characteristics of Cytospora spp. in Xinjiang. Xinjiang Agric. Sci. 2022, 59, 2696–2706. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Yin, Y.X.; Zhang, Z.D.; Tian, C.M. Diversity of Cytospora chrysosperma from different hosts in Xinjiang. Biodivers. Sci. 2019, 27, 1122–1131. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y.; Li, Y.; Jiang, N.; Zhu, T.; Li, Z.; Song, S.; Li, J.; Luo, L. Identification of causal agent of apple, walnut and poplar Valsa canker disease in partial areas of Xinjiang. Acta Phytopathol. Sin. 2020, 50, 267–275. (In Chinese) [Google Scholar] [CrossRef]
- Kereman; Jiang, H.; Zhang, X.; Wang, C.; Jiao, S.; Wang, M.; Liu, J.; Liu, A. Records on Species of Forest Disease in Agricultural Development Area of Karamay City. Xinjiang Agric. Sci. 2006, 03, 192–194. (In Chinese) [Google Scholar]
- Wu, F.; Liu, H.; Hou, S.; Wen, J. Spatial Distribution Characteristics of Valsa Canker on Fragrant Pear. Chin. Agric. Sci. Bull. 2012, 28, 277–281. (In Chinese) [Google Scholar] [CrossRef]
- Gemingguli, M. Occurrence Regularity of Poplar Rot in Altai Region and Control Measures. J. Agric. Catastrophology 2014, 4, 13–14. (In Chinese) [Google Scholar] [CrossRef]
- Yue, C.; Kong, T.; Ayixiamu, Y.; Jiao, S.; Zhang, X. Main Factors Affecting Walnut Rot Disease. J. Northwest For. Univ. 2015, 30, 154–157. (In Chinese) [Google Scholar] [CrossRef]
- Tekauz, A. The Role of Twig Infections on the Incidence of Perennial Canker of Peach. Phytopathology 1974, 64, 683. [Google Scholar] [CrossRef]
- Biggs, A.R. Integrated Approach to Controlling Leucostoma Canker of Peach in Ontario. Plant Dis. 1989, 73, 869. [Google Scholar] [CrossRef]
- Kepley, J.B.; Jacobi, W.R. Pathogenicity of Cytospora Fungi on Six Hardwood Species. AUF 2000, 26, 326–333. [Google Scholar] [CrossRef]
- Minter, D.W. IMI Descriptions of Fungi and Bacteria. Mycopathologia 1996, 136, 147–185. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.F. Release and Dispersal of Conidia and Ascospores of Valsa Leucostoma [sic]. Phytopathology 1976, 66, 987. [Google Scholar] [CrossRef]
- Guo, Q.; Kelly, M.; Graham, C.H. Support Vector Machines for Predicting Distribution of Sudden Oak Death in California. Ecol. Modell. 2005, 182, 75–90. [Google Scholar] [CrossRef]
- Puschendorf, R.; Carnaval, A.C.; VanDerWal, J.; Zumbado-Ulate, H.; Chaves, G.; Bolanos, F.; Alford, R.A. Distribution Models for the Amphibian Chytrid Batrachochytrium Dendrobatidis in Costa Rica: Proposing Climatic Refuges as a Conservation Tool. Divers. Distrib. 2009, 15, 401–408. [Google Scholar] [CrossRef]
- Shirk, A.; Cushman, S.; Waring, K.; Wehenkel, C.; Leal-Saenz, A.; Toney, C.; Lopez-Sanchez, C. Southwestern White Pine (Pinus strobiformis) Species Distribution Models Project a Large Range Shift and Contraction Due to Regional Climatic Changes. For. Ecol. Manag. 2018, 411, 176–186. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Esmaeili, A. Future Distributions of Fusarium oxysporum f. spp. in European, Middle Eastern and North African Agricultural Regions under Climate Change. Agric. Ecosyst. Environ. 2014, 197, 96–105. [Google Scholar] [CrossRef]
- Ejaz, M.R.; Jaoua, S.; Ahmadi, M.; Shabani, F. An Examination of How Climate Change Could Affect the Future Spread of Fusarium spp. around the World, Using Correlative Models to Model the Changes. Environ. Technol. Innov. 2023, 31, 103177. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive Alien Pests Threaten the Carbon Stored in Europe’s Forests. Nat. Commun. 2018, 9, 1626. [Google Scholar] [CrossRef]
- Fuchs, A.J.; Gilbert, C.C.; Kamilar, J.M. Ecological Niche Modeling of the Genus Papio. Am. J. Phys. Anthr. 2018, 166, 812–823. [Google Scholar] [CrossRef]
- Batista, E.; Lopes, A.; Miranda, P.; Alves, A. Can Species Distribution Models Be Used for Risk Assessment Analyses of Fungal Plant Pathogens? A Case Study with Three Botryosphaeriaceae Species. Eur. J. Plant Pathol. 2023, 165, 41–56. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, B.; Zhou, X.; Zou, H.; Duan, D.; Zhang, X.; Zhang, X. Distribution and Protection of Thesium Chinense Turcz. under Climate and Land Use Change. Sci. Rep. 2024, 14, 6475. [Google Scholar] [CrossRef] [PubMed]
- Xu, S. The Occurrence, Pathogenic Structural Composition and Genetic Diversity of Walnut Canker Disease in Xingjiang. Master’s Thesis, Tarim University, Aral, China, 3 June 2022. (In Chinese) [Google Scholar] [CrossRef]
- Huete, A.; Justice, C.; Van, L.W. MODIS vegetation index (MOD13). Algorithm Theor. Basis Doc. 1999, 3, 295–309. [Google Scholar]
- Roy, D.P.; Kovalskyy, V.; Zhang, H.K.; Vermote, E.F.; Yan, L.; Kumar, S.S.; Egorov, A. Characterization of Landsat-7 to Landsat-8 Reflective Wavelength and Normalized Difference Vegetation Index Continuity. Remote Sens. Environ. 2016, 185, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Shabani, F.; Ahmadi, M.; Peters, K.J.; Haberle, S.; Champreux, A.; Saltré, F.; Bradshaw, C.J.A. Climate-driven Shifts in the Distribution of Koala-browse Species from the Last Interglacial to the near Future. Ecography 2019, 42, 1587–1599. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Shen, X.-L.; Tong, L.; Lei, F.-W.; Mu, X.-Y.; Zhang, Z.-X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera Oblata and Its Conservation Implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, B.; Ma, C.; Gao, C.; Han, D.; Chai, Y. Impacts of Climate Change on Species Distribution Patterns of Polyspora Sweet in China. Ecol. Evol. 2022, 12, e9516. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R.; Blancheteau, H. biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 4.2-6-1, 2024, 1–133. Available online: https://biomodhub.github.io/biomod2/ (accessed on 12 July 2024).
- Rather, Z.A.; Ahmad, R.; Dar, T.-U.-H.; Khuroo, A.A. Ensemble Modelling Enables Identification of Suitable Sites for Habitat Restoration of Threatened Biodiversity under Climate Change: A Case Study of Himalayan Trillium. Ecol. Eng. 2022, 176, 106534. [Google Scholar] [CrossRef]
- Resquin, F.; Duque-Lazo, J.; Acosta-Muñoz, C.; Rachid-Casnati, C.; Carrasco-Letelier, L.; Navarro-Cerrillo, R.M. Modelling Current and Future Potential Habitats for Plantations of Eucalyptus Grandis Hill Ex Maiden and E. dunnii Maiden in Uruguay. Forests 2020, 11, 948. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and Comparing the Accuracy of Species Distribution Models with Presence-Absence Data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the Distribution Pattern of Chinese Ziziphus jujuba under Climate Change Based on Optimized Biomod2 and MaxEnt Models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Xian, X.; Zhao, H.; Wang, R.; Huang, H.; Chen, B.; Zhang, G.; Liu, W.; Wan, F. Climate Change Has Increased the Global Threats Posed by Three Ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2023, 859, 160252. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, J.; Liang, T.; Feng, Q. Grassland Vegetation Phenological Variations and Responses to Climate Change in the Xinjiang Region, China. Quat. Int. 2019, 513, 56–65. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Jim, C.-Y.; Chan, N.W.; Johnson, V.C.; Liu, C.; Duan, P.; Bahtebay, J. Spatio-Temporal Variations and Drivers of Ecological Carrying Capacity in a Typical Mountain-Oasis-Desert Area, Xinjiang, China. Ecol. Eng. 2022, 180, 106672. [Google Scholar] [CrossRef]
- Du, J.; Shu, J.; Yin, J.; Yuan, X.; Jiaerheng, A.; Xiong, S.; He, P.; Liu, W. Analysis on Spatio-Temporal Trends and Drivers in Vegetation Growth during Recent Decades in Xinjiang, China. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 216–228. [Google Scholar] [CrossRef]
- van Leeuwen, W.J.D.; Orr, B.J.; Marsh, S.E.; Herrmann, S.M. Multi-Sensor NDVI Data Continuity: Uncertainties and Implications for Vegetation Monitoring Applications. Remote Sens. Environ. 2006, 100, 67–81. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Wang, G.; Wu, X.; Li, J. Spatiotemporal Characteristic and Forecast of Drought in Northern Xinjiang, China. Ecol. Indic. 2021, 127, 107712. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Zhao, Y.; Guan, X.; Mao, W.; Yang, L. Climatic and Associated Atmospheric Water Cycle Changes over the Xinjiang, China. J. Hydrol. 2020, 585, 124823. [Google Scholar] [CrossRef]
- Lin, L.; Pan, M.; Tian, C.; Fan, X. Fungal Richness of Cytospora Species Associated with Willow Canker Disease in China. J. Fungi 2022, 8, 377. [Google Scholar] [CrossRef]
- Bertrand, P.F.; English, H.; Carlson, R.M. Relation of soil physical and fertility properties to the occurrence of Cytospora canker in French prune orchards. Phytopathology 1976, 66, 1321–1324. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Juroszek, P.; von Tiedemann, A.; Duveiller, E.; Singh, R.P.; Nicol, J.M. Climate Change and Potential Future Risks through Wheat Diseases: A Review. Eur. J. Plant Pathol. 2013, 136, 21–33. [Google Scholar] [CrossRef]
- Dudley, M.M.; Tisserat, N.A.; Jacobi, W.R.; Negrón, J.; Stewart, J.E. Pathogenicity and Distribution of Two Species of Cytospora on Populus Tremuloides in Portions of the Rocky Mountains and Midwest in the United States. For. Ecol. Manag. 2020, 468, 118168. [Google Scholar] [CrossRef]
- Han, Z.; Xiong, D.; Xu, Z.; Liu, T.; Tian, C. The Cytospora chrysosperma Virulence Effector CcCAP1 Mainly Localizes to the Plant Nucleus To Suppress Plant Immune Responses. mSphere 2021, 6, e00883-20. [Google Scholar] [CrossRef]
- Duveiller, E.; Singh, R.P.; Nicol, J.M. The Challenges of Maintaining Wheat Productivity: Pests, Diseases, and Potential Epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Wollan, A.K.; Bakkestuen, V.; Kauserud, H.; Gulden, G.; Halvorsen, R. Modelling and Predicting Fungal Distribution Patterns Using Herbarium Data. J. Biogeogr. 2008, 35, 2298–2310. [Google Scholar] [CrossRef]
- Trebicki, P. Climate Change and Plant Virus Epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yan, J.; Che, M.; Zhu, Y.; Liu, Z.; Pei, H.; Zhang, H.; Xu, G.; Lin, X. Climate Change and the Ecological Responses in Xinjiang, China: Model Simulations and Data Analyses. Quat. Int. 2013, 311, 108–116. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate Change, Plant Diseases and Food Security: An Overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the Effects of Climate Change on Natural Enemies of Agricultural Pests. Biol. Control 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; d’Oultremont, T.; Ellis, C.K. Climate Change Effects on Poikilotherm Tritrophic Interactions. Clim. Chang. 2008, 87, 167–192. [Google Scholar] [CrossRef]
- Büntgen, U.; Egli, S.; Tegel, W.; Stobbe, U.; Sproll, L.; Elburg, R.; Peter, M.; Nievergelt, D.; Cherubini, P.; Stenseth, N.C. Illuminating the Mysterious World of Truffles. Front. Ecol Env. 2012, 10, 462–463. [Google Scholar] [CrossRef]
- Li, Q.; Cao, S.; Sun, W.; Zhang, Z. Prediction of the Potential Geographical Distribution of Cytospora Chrysosperma in Xinjiang, China under Climate Change Scenarios. Front. For. Glob. Change 2024, 7, 1370365. [Google Scholar] [CrossRef]
- Xu, W.; Sun, H.; Jin, J.; Cheng, J. Predicting the Potential Distribution of Apple Canker Pathogen (Valsa Mali) in China under Climate Change. Forests 2020, 11, 1126. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate Change and Forest Diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Bosso, L.; Di Febbraro, M.; Cristinzio, G.; Zoina, A.; Russo, D. Shedding Light on the Effects of Climate Change on the Potential Distribution of Xylella Fastidiosa in the Mediterranean Basin. Biol. Invasions 2016, 18, 1759–1768. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.-L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought Effects on Damage by Forest Insects and Pathogens: A Meta-Analysis. Glob. Chang. Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s Parameter Configuration and Small Samples: Are We Paying Attention to Recommendations? A Systematic Review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Smith, M.J.; Edwards, T.C.; Guisan, A.; McMahon, S.M.; Normand, S.; Thuiller, W.; Wüest, R.O.; Zimmermann, N.E.; Elith, J. What Do We Gain from Simplicity versus Complexity in Species Distribution Models? Ecography 2014, 37, 1267–1281. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of Climate Change on Plant Diseases—Opinions and Trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Savage, D.; Barbetti, M.J.; MacLeod, W.J.; Salam, M.U.; Renton, M. Temporal Patterns of Ascospore Release in Leptosphaeria Maculans Vary Depending on Geographic Region and Time of Observation. Microb. Ecol. 2013, 65, 584–592. [Google Scholar] [CrossRef]
- Meentemeyer, R.K.; Haas, S.E.; Václavík, T. Landscape Epidemiology of Emerging Infectious Diseases in Natural and Human-Altered Ecosystems. Annu. Rev. Phytopathol. 2012, 50, 379–402. [Google Scholar] [CrossRef]

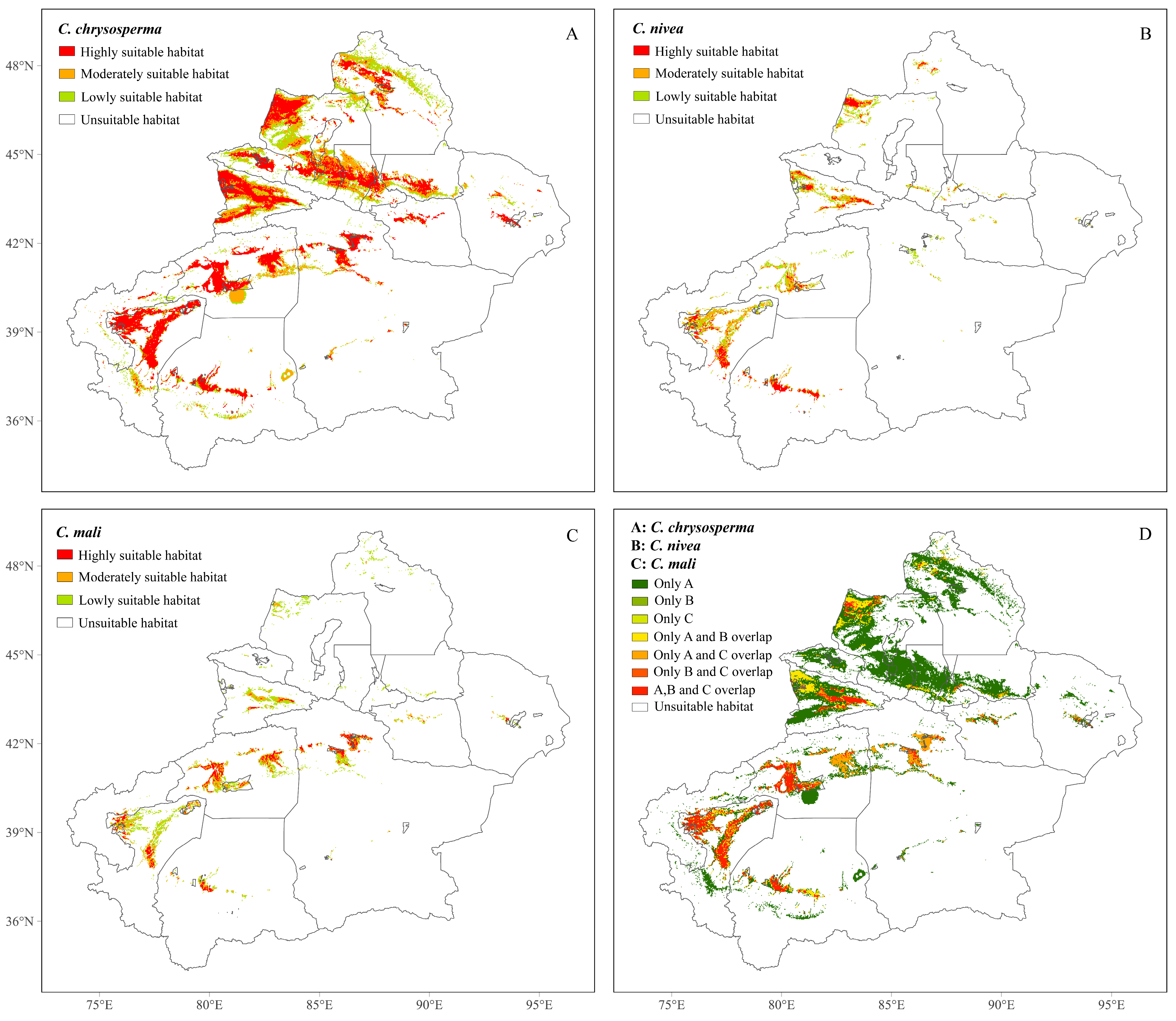
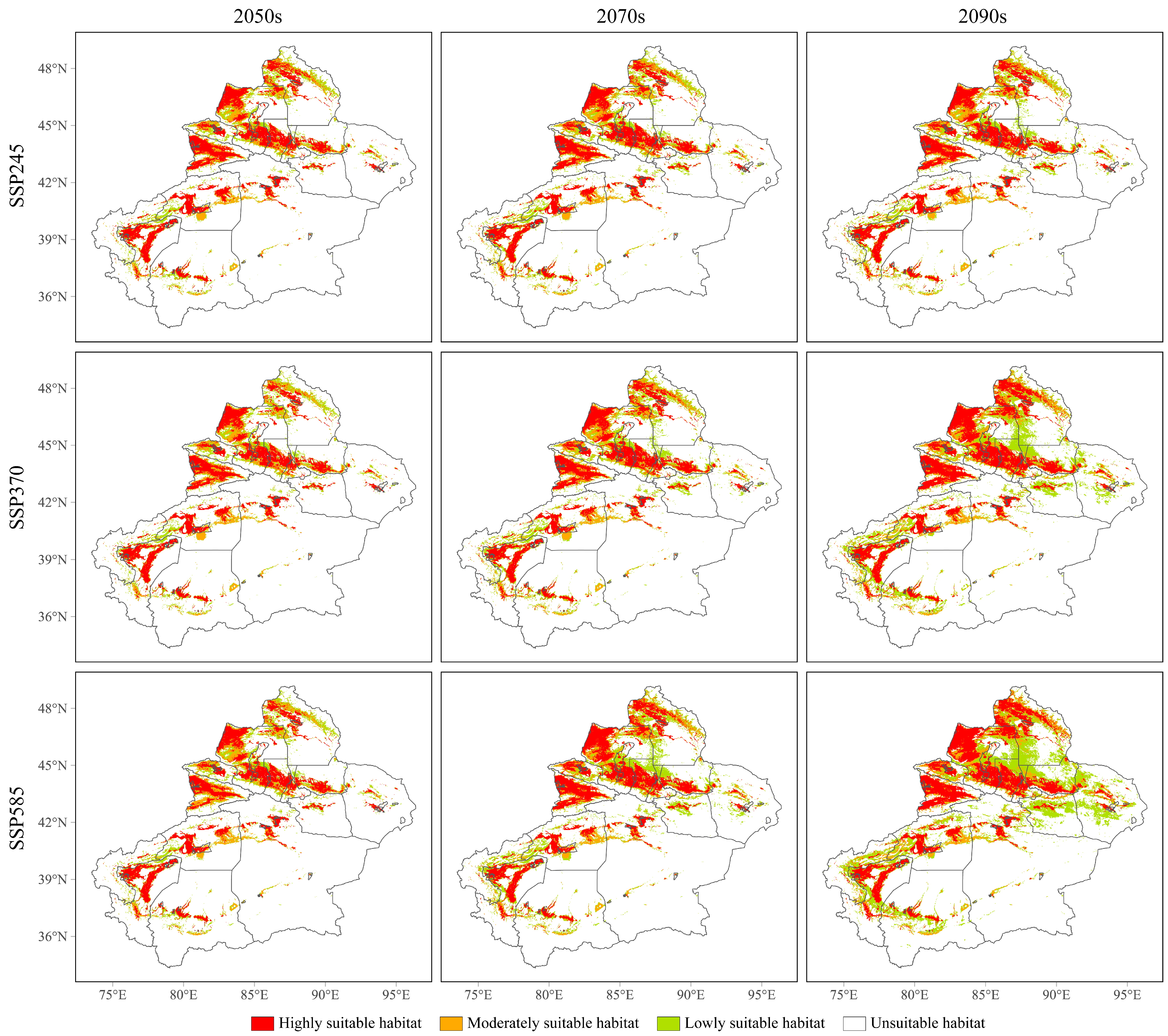
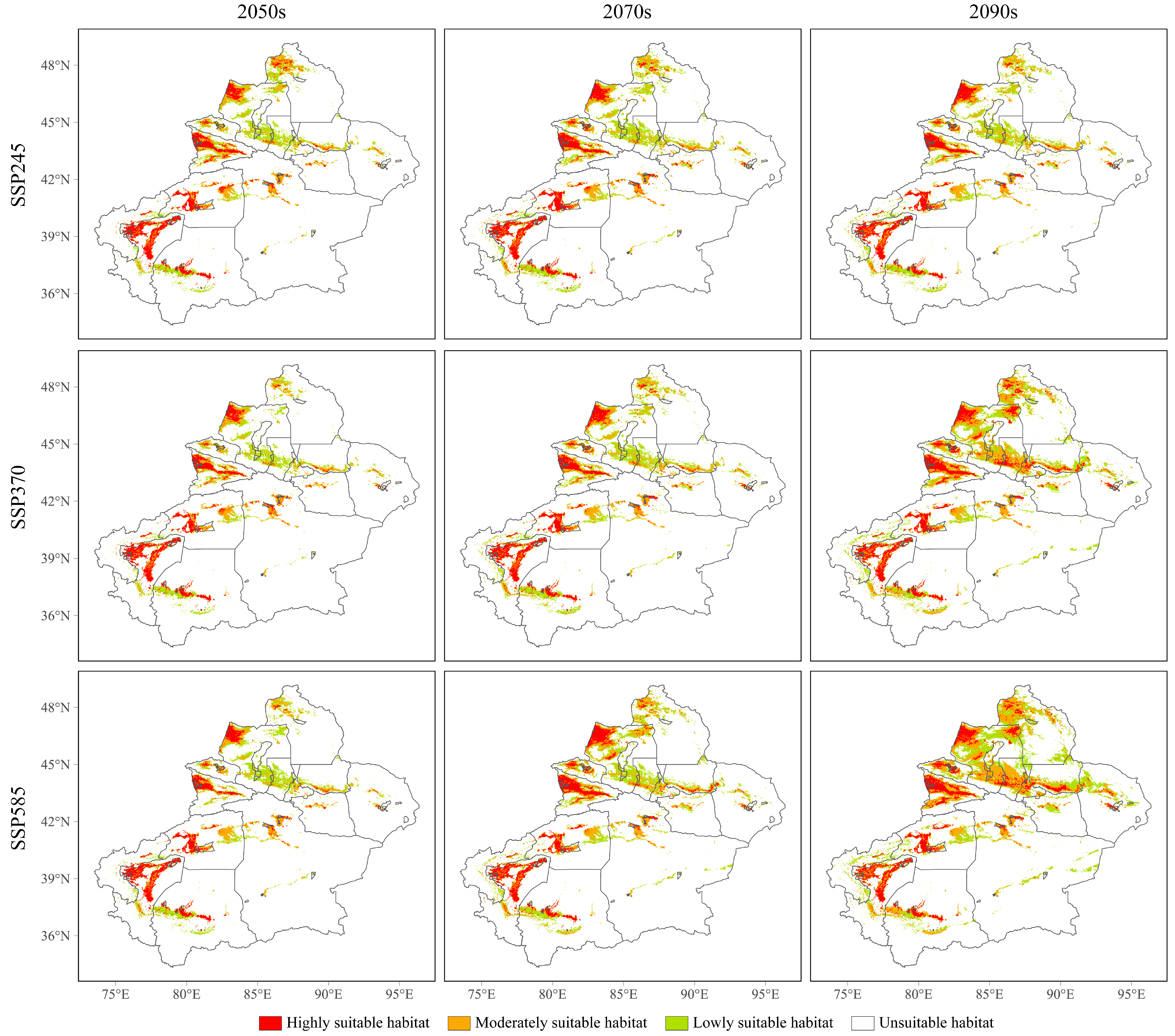
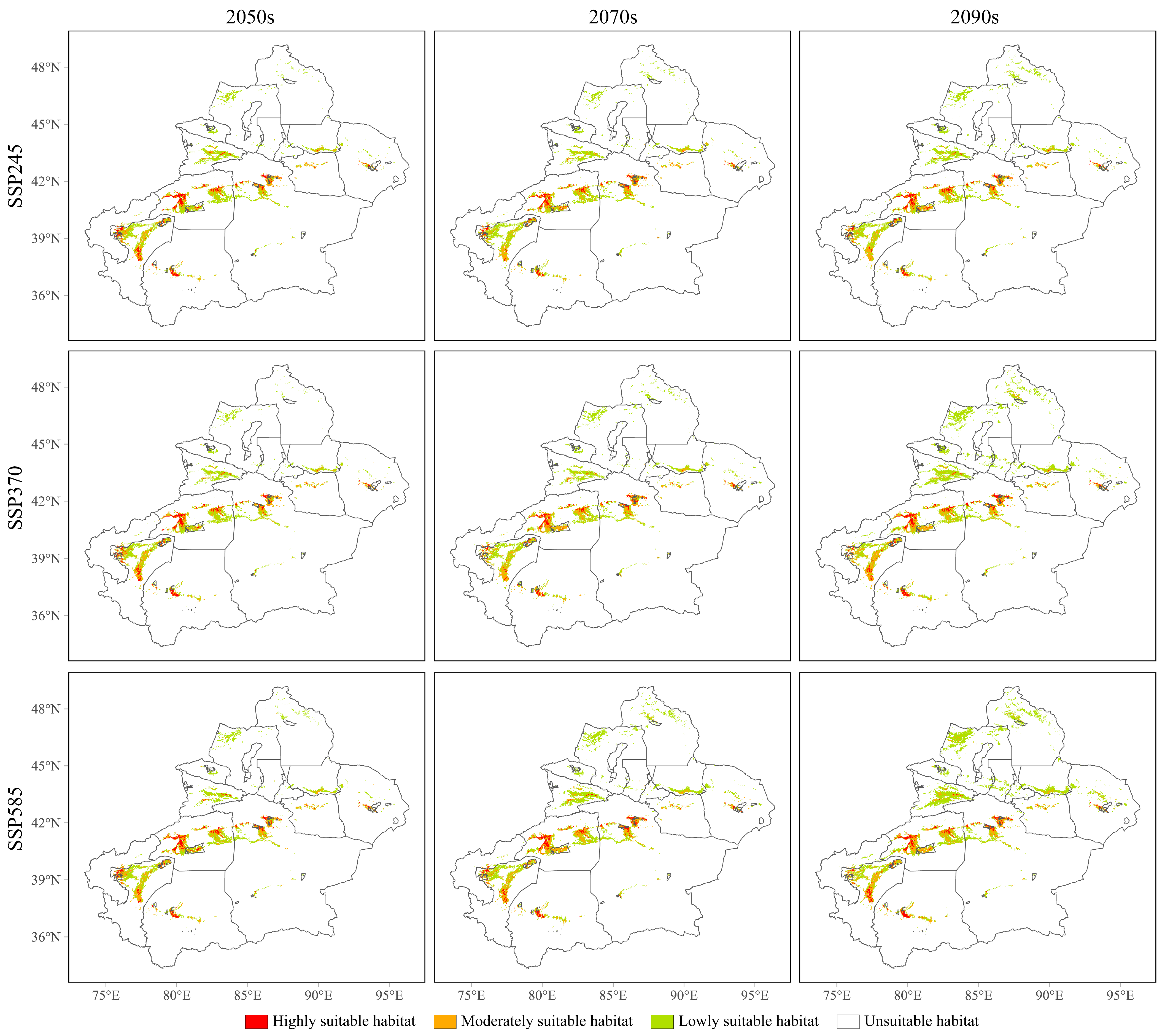
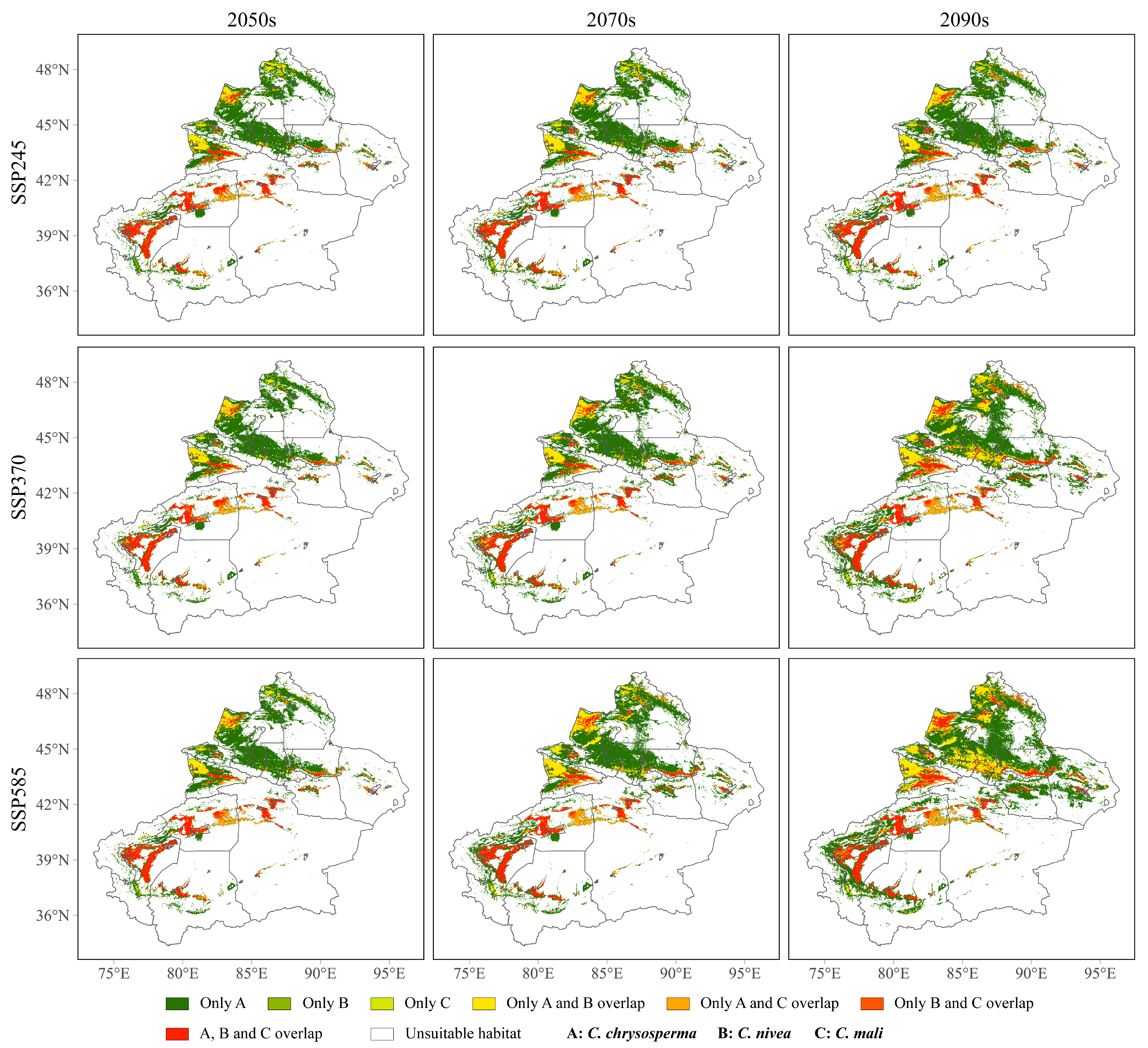
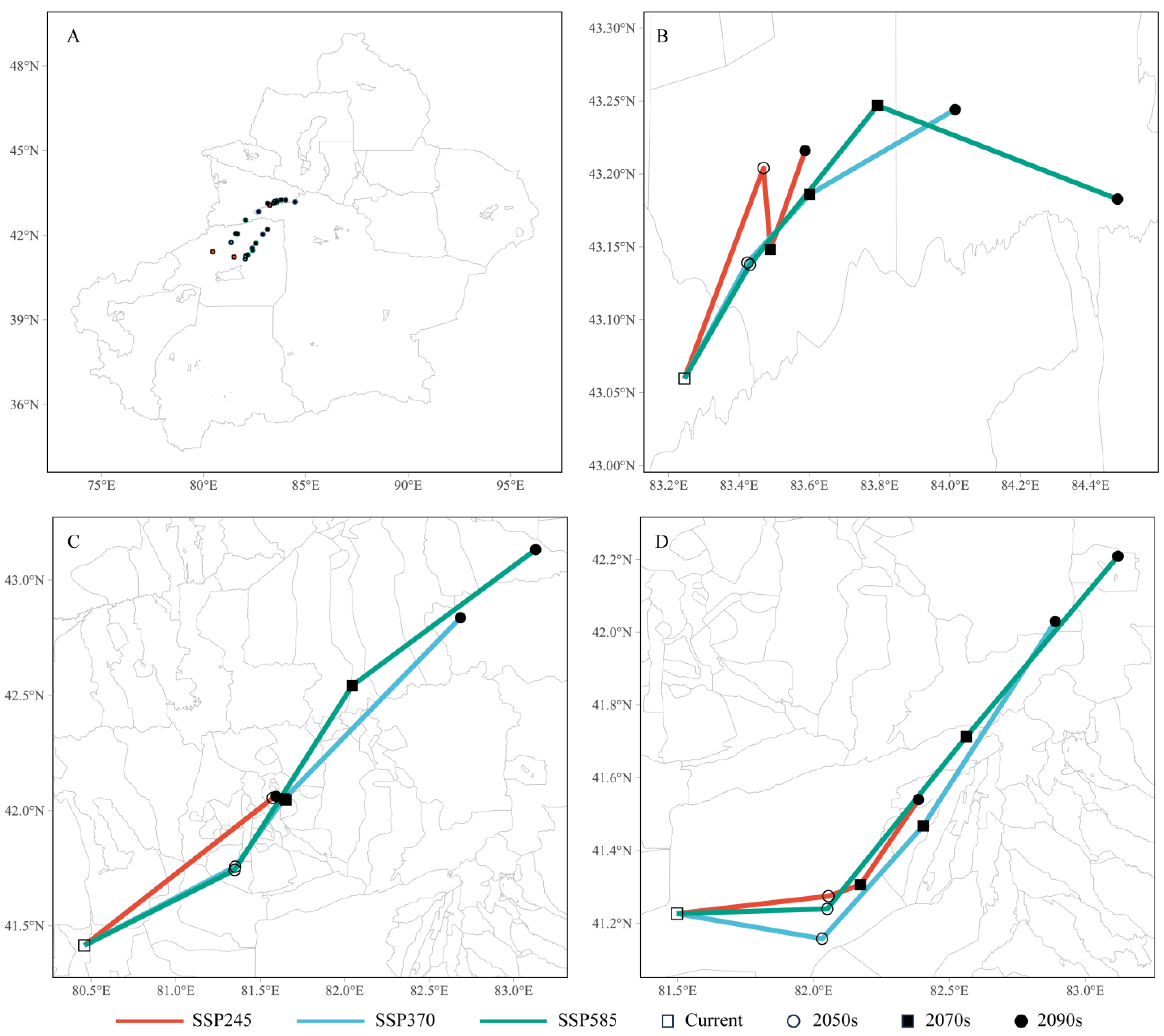
| Specie | Unsuitable Habitat | Lowly Suitable Habitat | Moderately Suitable Habitat | Highly Suitable Habitat |
|---|---|---|---|---|
| C. chrysosperma | 0 ≤ p < 152 | 152 ≤ p < 443 | 443 ≤ p < 787 | 787 ≤ p ≤ 1000 |
| C. nivea | 0 ≤ p < 779.5 | 779.5 ≤ p < 857 | 857 ≤ p < 932 | 932 ≤ p ≤ 1000 |
| C. mali | 0 ≤ p < 547.5 | 547.5 ≤ p < 672 | 672 ≤ p < 802 | 802 ≤ p ≤ 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Cao, S.; Wang, L.; Hou, R.; Sun, W. Impacts of Climate Change on the Potential Distribution of Three Cytospora Species in Xinjiang, China. Forests 2024, 15, 1617. https://doi.org/10.3390/f15091617
Li Q, Cao S, Wang L, Hou R, Sun W. Impacts of Climate Change on the Potential Distribution of Three Cytospora Species in Xinjiang, China. Forests. 2024; 15(9):1617. https://doi.org/10.3390/f15091617
Chicago/Turabian StyleLi, Quansheng, Shanshan Cao, Lei Wang, Ruixia Hou, and Wei Sun. 2024. "Impacts of Climate Change on the Potential Distribution of Three Cytospora Species in Xinjiang, China" Forests 15, no. 9: 1617. https://doi.org/10.3390/f15091617






