Improving Carbon Sequestration in Wetlands Using Native Poplar Genotypes for Reforestation Purposes
Abstract
:1. Introduction
2. Materials and Methods
Surveys and Statistical Analysis
- V is the total stem volume plus the branches up to 10 cm in diameter;
- D is the diameter at breast height over bark;
- H is the total height;
- s is the average spacing at planting;
- a is the age of plantation.
3. Results
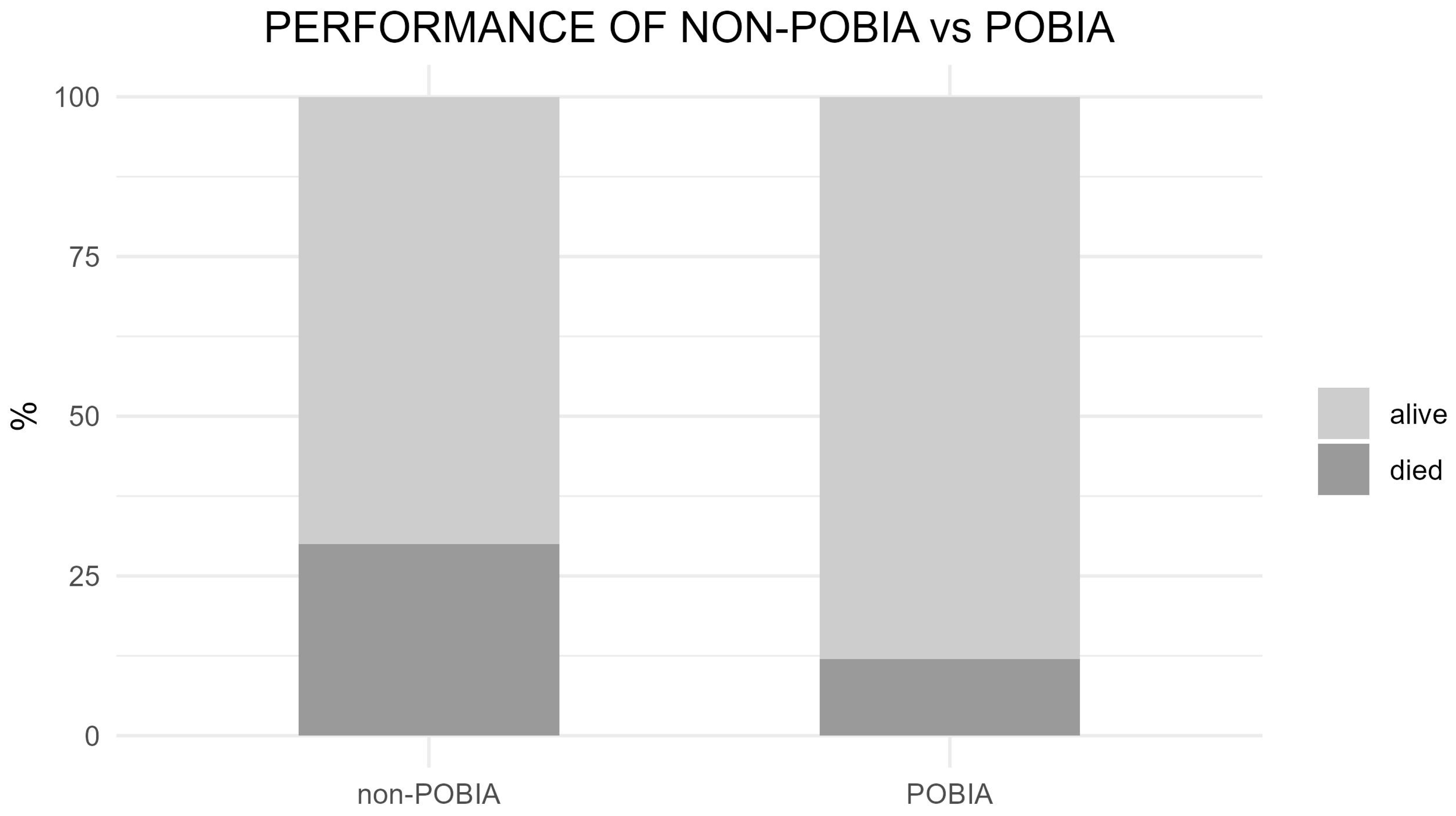
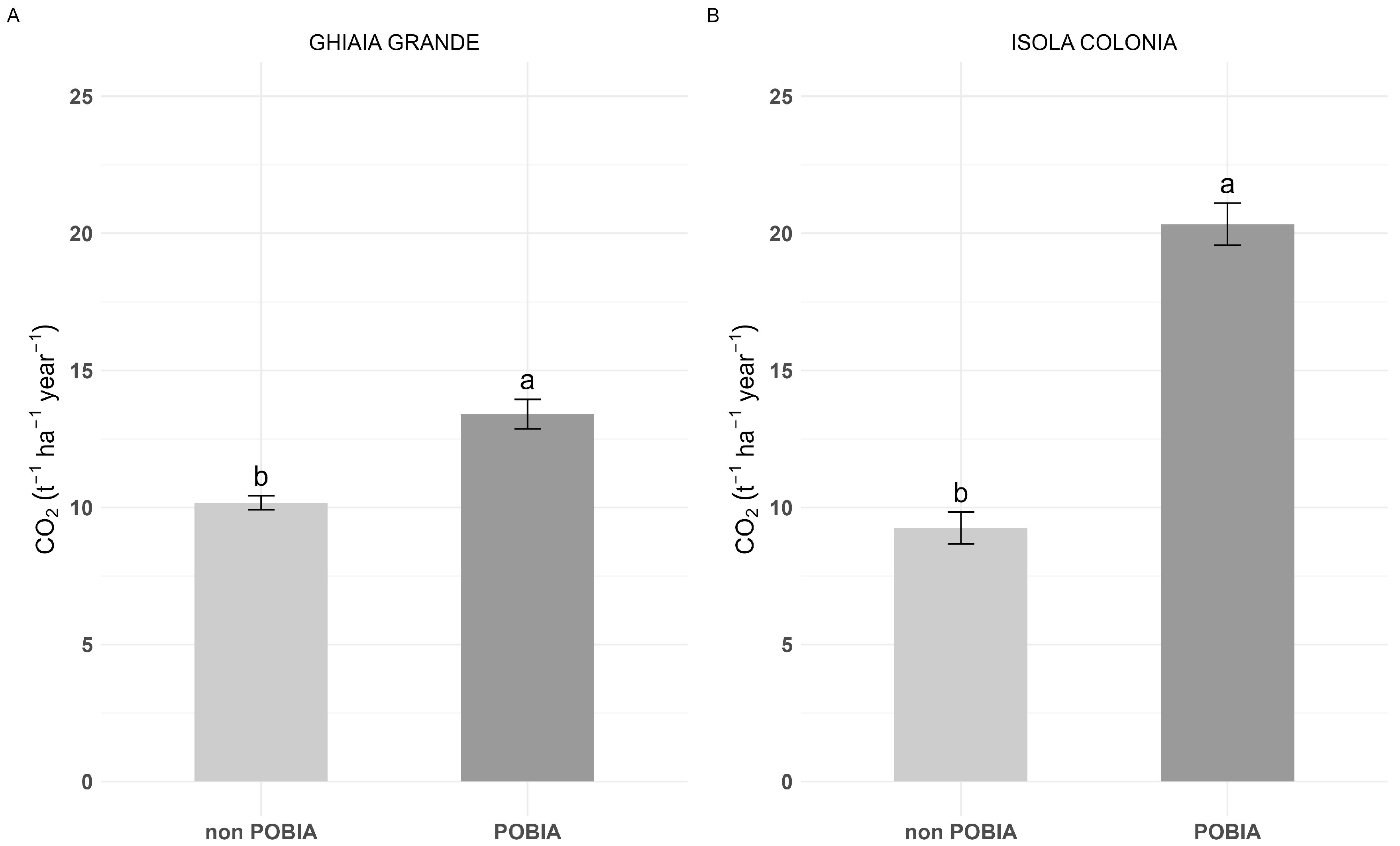
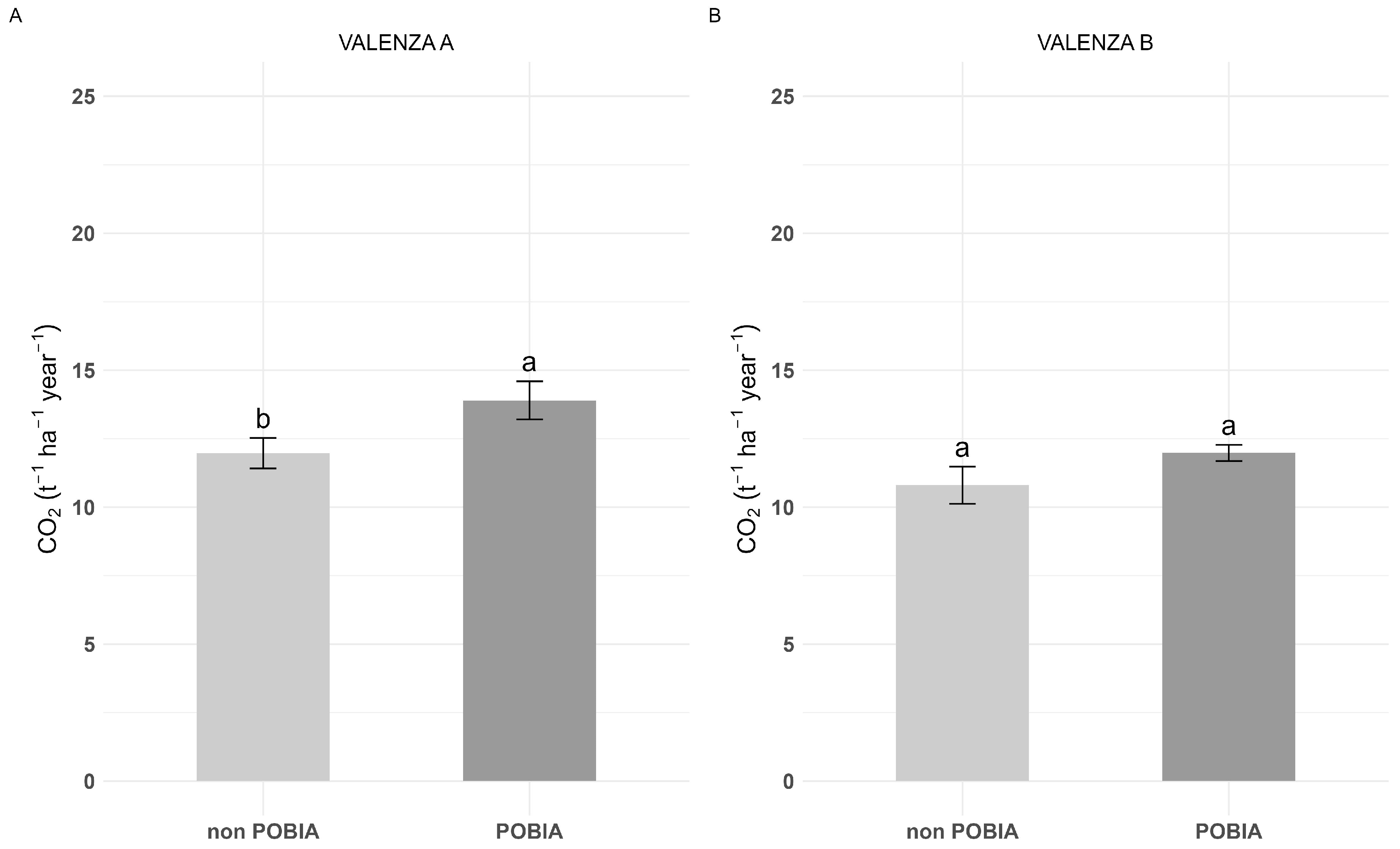
3.1. Poplar Growth and C Sink: ‘POBIA’ Mixture vs. Non-POBIA Clones
3.2. Poplar C Sink vs. Other Species
3.3. Influence of Climatic Factors on Unmanaged Plantations’ Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, R.B.; Randerson, J.T.; Canadell, J.G.; Anderson, R.G.; Avissar, R.; Baldocchi, D.D.; Bonan, G.B.; Caldeira, K.; Diffenbaugh, N.S.; Field, C.B.; et al. Protecting climate with forests. Environ. Res. Lett. 2008, 3, 044006. [Google Scholar]
- Dufour, S.; Rinaldi, M.; Piégay, H.; Michalon, A. How do river dynamics and human influences affect the landscape pattern of fluvial corridors? Lessons from the Magra River, Central-Northern Italy. Landsc. Urban Plan. 2015, 134, 107–118. [Google Scholar]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar]
- Moatar, M.M.; Camen, D.D.; Dragomir, P.I.; Stefan, C.; Panici, P.A. The Importance of Poplar Species in the Ecological Process of Strongly Polluted Industrial Waters. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, Albena, Bulgaria, 3–9 July 2023; Volume 23, pp. 335–342. [Google Scholar]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Potential for hybrid poplar riparian buffers to provide ecosystem services in three watersheds with contrasting agricultural land use. Forests 2016, 7, 37. [Google Scholar] [CrossRef]
- Blujdea, V.N.B.; Viñas, R.A.; Federici, S.; Grassi, G. The EU greenhouse gas inventory for the LULUCF sector: I. Overview and comparative analysis of methods used by EU member states. Carbon Manag. 2015, 6, 247–259. [Google Scholar]
- EU. Regulation (EU) 2023/839 of the European Parliament and of the Council of 19 April 2023 amending Regulation (EU) 2018/841 as regards the scope, simplifying the reporting and compliance rules, and setting out the targets of the Member States for 2030, and Regulation (EU) 2018/1999 as regards improvement in monitoring, reporting, tracking of progress and review. Off. J. Eur. Union 2023, 107, 1–28. [Google Scholar]
- Di Lallo, G.; Chiriacò, M.V.; Tarasova, E.; Köhl, M.; Perugini, L. The Land Sector in the Low Carbon Emission Strategies in the European Union: Role and Future Expectations. Clim. Policy 2023, 24, 586–600. [Google Scholar]
- Vietto, L.; Chiarabaglio, P.M. Restoration of floodplain woodlands with native Poplars (Populus nigra and Populus alba) in Italy: Some case studies on the Po river. In Proceedings of the 3rd International Conference on River Restoration in Europe (River Restoration 2004. Principles, Processes, Practices), Zagreb, Croatia, 17–21 May 2004; pp. 375–381. Available online: https://www.ecrr.org/About/History-ECRR-RESTORE/ECRR-Past-events/River-Restoration-Conference-2004 (accessed on 29 July 2024).
- Gonzälez del Tänago, M.; Garcia de Jalön, D. River restoration in spain, case study: Llobregat river. In Proceedings of the Conference on River Restoration (River Restoration in Europe, Practical Approaches), Wageningen, The Netherlands, 15–19 May 2000; pp. 293–296. [Google Scholar]
- Huybrechts, W.; Becker, P.D.; Raymaeker, F.; Saey, F. Floodplain restoration of the Dijle river. In River Restoration in Europe: Proceedings of the Conference on River Restoration Wageningen, The Netherlands 2000; Institute for Inland Water Management and Waste Water Treatment/RIZA: Lelystad, The Netherlands, 2001; 263p, ISBN 90-369-53774. [Google Scholar]
- Winfield, M.; Wetlands, F.H. Variation in Populus nigra clones: Implications for river restoration projects in the United Kingdom. Wetlands 2002, 1, 33–48. [Google Scholar]
- Broeck, A.V. EUFORGEN Technical Guidelines for Genetic Conservation and Use of European Black Poplar (Populus nigra); International Plant Genetic Resources Institute: Rome, Italy, 2003. [Google Scholar]
- Scholz, M.; Ilg, C.; Rupp, H. Floodplain restoration by dike relocation along the Elbe river and the need to monitor the effects. In Proceedings of the 4th ECRR International Conferences on River Restoration, Venice, Italy, 16–21 June 2008. [Google Scholar]
- Sistema Delle Aree Protette Della Fascia Fluviale del Po-Istituzione. Legge Regione Piemonte 17 Aprile 1990, n. 28. Available online: http://arianna.consiglioregionale.piemonte.it/base/leggi/l1990028.html (accessed on 17 April 1990).
- Zsuffa, L. The genetics of Populus nigra L. Ann. For. 1974, 6, 29–53. [Google Scholar]
- Herpka, I. A survey of development and possibilities of growing: Natural forests of poplars and willows. In Poplars and Willows in Yugoslavia; Poplar Research Institute: Novi Sad, Serbia, 1986; pp. 21–36. [Google Scholar]
- Lefèvre, F.; Kajba, D.; Heinze, B.; Rotach, P.; De Vries, S.M.G.; Turok, J. Black poplar: A model for gene resource conservation in forest ecosystems. For. Chron. 2001, 77, 239–244. [Google Scholar]
- Cagelli, L.; Lefevre, F. The conservation of Populus nigra L. and gene flow with cultivated poplars in Europe. For. Genet. 1995, 2, 135–144. [Google Scholar]
- Distribution Map of Black Poplar (Populus nigra) EUFORGEN 2015. Available online: www.euforgen.org (accessed on 13 January 2015).
- Harvey-Brown, Y.; Barstow, M.; Mark, J.; Rivers, M.C. Populus nigra. In IUCN Red List Threatened Species; IUCN Red List: Cambridge, UK, 2017. [Google Scholar]
- Alimpić, F.; Milovanović, J.; Pielech, R.; Hinkov, G.; Jansson, R.; Dufour, S.; Beza, M.; Bilir, N.; del Blanco, L.S.; Božič, G.; et al. The status and role of genetic diversity of trees for the conservation and management of riparian ecosystems: A European experts’ perspective. J. Appl. Ecol. 2022, 59, 2476–2485. [Google Scholar]
- Arbez, M.; Lefèvre, F. The European Forest Genetic Resource Programme. Objectives and Generai Concept. A Case Study Concerning the Black Poplar (Populus nigra L.). Bocconea 1997, 7, 389–398. [Google Scholar]
- White, J. Black Poplar: The Most Endangered Native Timber Tree in Britain; Forestry Commission Research Information Note 239; Forestry Commission: Edinburgh, UK, 1993.
- Broeck, A.V.; Storme, V.; Cottrell, J.E.; Boerjan, W.; Van Bockstaele, E.; Quataert, P.; Van Slycken, J. Gene flow between cultivated poplars and native black poplar (Populus nigra L.): A case study along the river Meuse on the Dutch-Belgian border. For. Ecol. Manag. 2004, 197, 307–310. [Google Scholar]
- de Vries, S.M.G.; Alan, M.; Bozzano, M.; Burianek, V.; Collin, E.; Cottrell, J.; Ivankovic, M.; Kelleher, C.T.; Koskela, J.; Rotach, P.; et al. Pan-European Strategy for Genetic Conservation of Forest Trees and Establishment of a Core Network of Dynamic Conservation Units; European Forest Genetic Resources Programme: Barcelona, Spain, 2015; ISBN 978-92-9255-029-5. [Google Scholar]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.; Ewers, R.M.; Harms, K.E.; Luizão, R.C.C.; Ribeiro, J.E. Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2007, 2, e1017. [Google Scholar]
- Jentsch, A.; Beierkuhnlein, C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. Comptes Rendus-Geosci. 2008, 340, 621–628. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar]
- Bisoffi, S. Poplar breeding strategies between conventional methods and new techniques. In Proceedings of the 1 Simposio del Chopo, Zamora, Spain, 9–11 May 2001. [Google Scholar]
- Cagelli, L.; Bisoffi, S.; Vietto, L. Ex SitU conservation: Update on the EUFORGEN core collection and the database of clones. In Proceedings of the the Fifth Meeting, Kyiv, Ukraine, 5–8 May 1998; Turok, J., Lefèvre, F., de Vries, S.M.G., Heinze, B., Volosyanchuk, R., Lipman, E., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1999; p. 70. [Google Scholar]
- Vietto, L.; Castro, G.; Chiarabaglio, P.M.; Nervo, G. Conservation of native poplars (Populus nigra L. and Populus alba L.). In Presentato a’La Conservazione Delle Risorse Genetiche Delle Specie Spontanee’APAT; Agenzia per la Protezione Dell’ambiente e per i Servizi Tecnici: Rome, Italy, 2006. [Google Scholar]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55. [Google Scholar]
- Coaloa, D.; Chiarabaglio, P.M. Continuous Poplar Inventory Through Netv/Ork Of Permanent Sample Plots. In Proceedings of the IUFRO Conference, Copenhagen, Denmark, 14–17 June 1993; pp. 18–24. [Google Scholar]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C.; Hoehn, R.E.; Walton, J.T.; Bond, J. A ground-based method of assessing urban forest structure and ecosystem services. Arboric. Urban For. 2008, 34, 347–358. [Google Scholar]
- Giordano, G. Tecnología del Legno: Il Legno e le sue Caratteristiche; Transformazioni Meccaniche e Miglioramenti; U. Hoepli: Milan, Italy, 1951; Volume 759. [Google Scholar]
- Eggleston, H.; Leandro, B.; Kyoko, M.; Todd, N.; Kiyoto, T. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 2006.
- Čomic, D.R. Regression models of Carbon and CO2 sequestration of hybrid poplar plantations in northern serbia. Can. J. For. Res. 2021, 51, 1859–1874. [Google Scholar]
- Riccioli, F.; Nissim, W.G.; Masi, M.; Palm, E.; Mancuso, S.; Azzarello, E. Modeling the ecosystem services related to phytoextraction: Carbon sequestration potential using willow and poplar. Appl. Sci. 2020, 10, 8011. [Google Scholar] [CrossRef]
- Rotach, P. Poplars and biodiversity. In Report of the Seventh (25–27 October 2001, Osijek, Croatia) and Eighth Meetings (22–24 May 2003, Treppein, Germany); International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2004; pp. 79–100. [Google Scholar]
- Xu, S.; Eisenhauer, N.; Ferlian, O.; Zhang, J.; Zhou, G.; Lu, X.; Liu, C.; Zhang, D. Species richness promotes ecosystem Carbon storage: Evidence from biodiversity-ecosystem functioning experiments: Species richness promotes C storage. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202063. [Google Scholar]
- Vacek, Z.; Bílek, L.; Remeš, J.; Vacek, S.; Cukor, J.; Gallo, J.; Šimůnek, V.; Bulušek, D.; Brichta, J.; Vacek, O.; et al. Afforestation suitability and production potential of five tree species on abandoned farmland in response to climate change, Czech Republic. Trees-Struct. Funct. 2022, 36, 1369–1385. [Google Scholar]
- Šimůnek, V.; Sharma, R.P.; Vacek, Z.; Vacek, S.; Hůnová, I. Sunspot area as unexplored trend inside radial growth of European beech in Krkonoše Mountains: A forest science from different perspective. Eur. J. For. Res. 2020, 139, 999–1013. [Google Scholar]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar]
- Bergante, S.; Facciotto, G.; Minotta, G. Identification of the main site factors and management intensity affecting the establishment of Short-Rotation-Coppices (SRC) in Northern Italy through stepwise regression analysis. Cent. Eur. J. Biol. 2010, 5, 522–530. [Google Scholar]
- Marchi, M.; Bergante, S.; Ray, D.; Barbetti, R.; Facciotto, G.; Chiarabaglio, P.M.; Hynynen, J.; Nervo, G. Universal reaction norms for the sustainable cultivation of hybrid poplar clones under climate change in Italy. iForest 2022, 15, 47–55. [Google Scholar]
- Toochi, E.C. Carbon sequestration: How much can forestry sequester CO2? For. Res. Eng. Int. J. 2018, 2, 148–150. [Google Scholar]
- Thomas, A.D.; Elliott, D.R.; Dougill, A.J.; Stringer, L.C.; Hoon, S.R.; Sen, R. The influence of trees, shrubs, and grasses on microclimate, soil Carbon, nitrogen, and CO2 efflux: Potential implications of shrub encroachment for Kalahari rangelands. Land Degrad. Dev. 2018, 29, 1306–1316. [Google Scholar]
- Höhl, M.; Ahimbisibwe, V.; Stanturf, J.A.; Elsasser, P.; Kleine, M.; Bolte, A. Forest landscape restoration-What generates failure and success? Forests 2020, 11, 938. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Stanturf, J.A.; Gardiner, E.S.; Perdue, J.H.; Young, T.M.; Coyle, D.R.; Headlee, W.L.; Bañuelos, G.S.; Hass, A. Ecosystem Services of Woody Crop Production Systems. Bioenergy Res. 2016, 9, 465–491. [Google Scholar]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar]
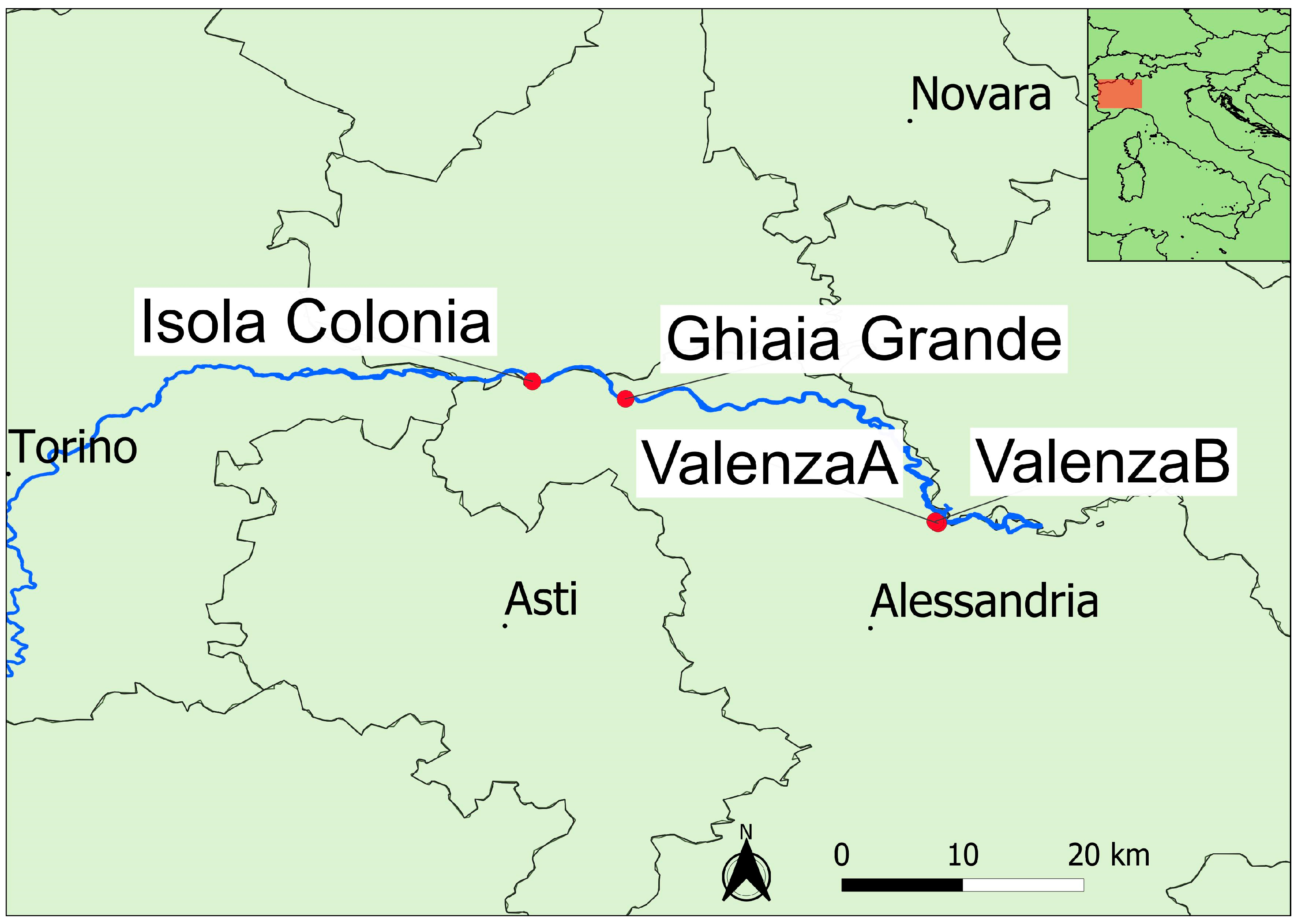
| Site | Pl. Year | P. nigra Density ha−1 | P. alba Density ha−1 | Other Species Density ha−1 |
|---|---|---|---|---|
| Ghiaia Grande | 2008 | 204 | 204 | 408 |
| Isola Colonia | 2005 | 286 | 0 | 285 |
| ValenzaA | 2003 | 417 | 0 | 416 |
| ValenzaB | 2004 | 416 | 417 | 208 |
| Site | Species | Average per Tree |
|---|---|---|
| Ghiaia Grande | Other | 4 |
| P. alba | 36 | |
| P. nigra | 73 | |
| Isola Colonia | Other | 3 |
| P. nigra | 49 | |
| ValenzaA | Other | 5 |
| P. nigra | 88 | |
| ValenzaB | Other | 9 |
| P. alba | 62 | |
| P. nigra | 57 |
| Estimate | Std. Error | t Value | Pr (>|t|) | |
|---|---|---|---|---|
| Intercept | −82.415156 | 19.260119 | −4.279 | <0.001 *** |
| SiteIC | −0.890619 | 0.749378 | −1.188 | 0.235 |
| Site VA | −3.993094 | 0.789902 | −5.053 | <0.001 *** |
| Site VB | −2.760110 | 0.848865 | −3.252 | 0.001 ** |
| PAn | 0.015304 | 0.002586 | 5.919 | <0.001 *** |
| EtpAn | −0.068710 | 0.040420 | −1.700 | 0.090 |
| Tsv | 7.617842 | 1.882087 | 4.048 | <0.001 *** |
| Defsv | −0.015844 | 0.008429 | −1.880 | 0.061 |
| Sursv | 0.004230 | 0.008783 | 0.482 | 0.630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantamessa, S.; Chiarabaglio, P.M.; Rizza, D.; Debernardi, G.; Bergante, S. Improving Carbon Sequestration in Wetlands Using Native Poplar Genotypes for Reforestation Purposes. Forests 2024, 15, 1641. https://doi.org/10.3390/f15091641
Cantamessa S, Chiarabaglio PM, Rizza D, Debernardi G, Bergante S. Improving Carbon Sequestration in Wetlands Using Native Poplar Genotypes for Reforestation Purposes. Forests. 2024; 15(9):1641. https://doi.org/10.3390/f15091641
Chicago/Turabian StyleCantamessa, Simone, Pier Mario Chiarabaglio, Daniele Rizza, Giacomo Debernardi, and Sara Bergante. 2024. "Improving Carbon Sequestration in Wetlands Using Native Poplar Genotypes for Reforestation Purposes" Forests 15, no. 9: 1641. https://doi.org/10.3390/f15091641








