Effects of Thinning Practices on Soil Properties and Arbuscular Mycorrhizal Fungi in Natural Pure Oriental Beech Forests
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Design Description
2.2. Field Conditions and Soil Sampling
2.3. Spore Isolation and Identification of AM Fungi
2.4. Bioassay with Host Plant to Access Root Colonization by AMF
2.5. Statistical Analyses
3. Results
3.1. Thinning Intensities Influence on Soil Physical–Chemical Properties of a Ultisol Covered by Monospecific Stands of F. orientalis
3.2. Thinning Intensities Influence Arbuscular Mycorrhizal Fungi Community Composition from Ultisol and Root Colonization by AM Fungi
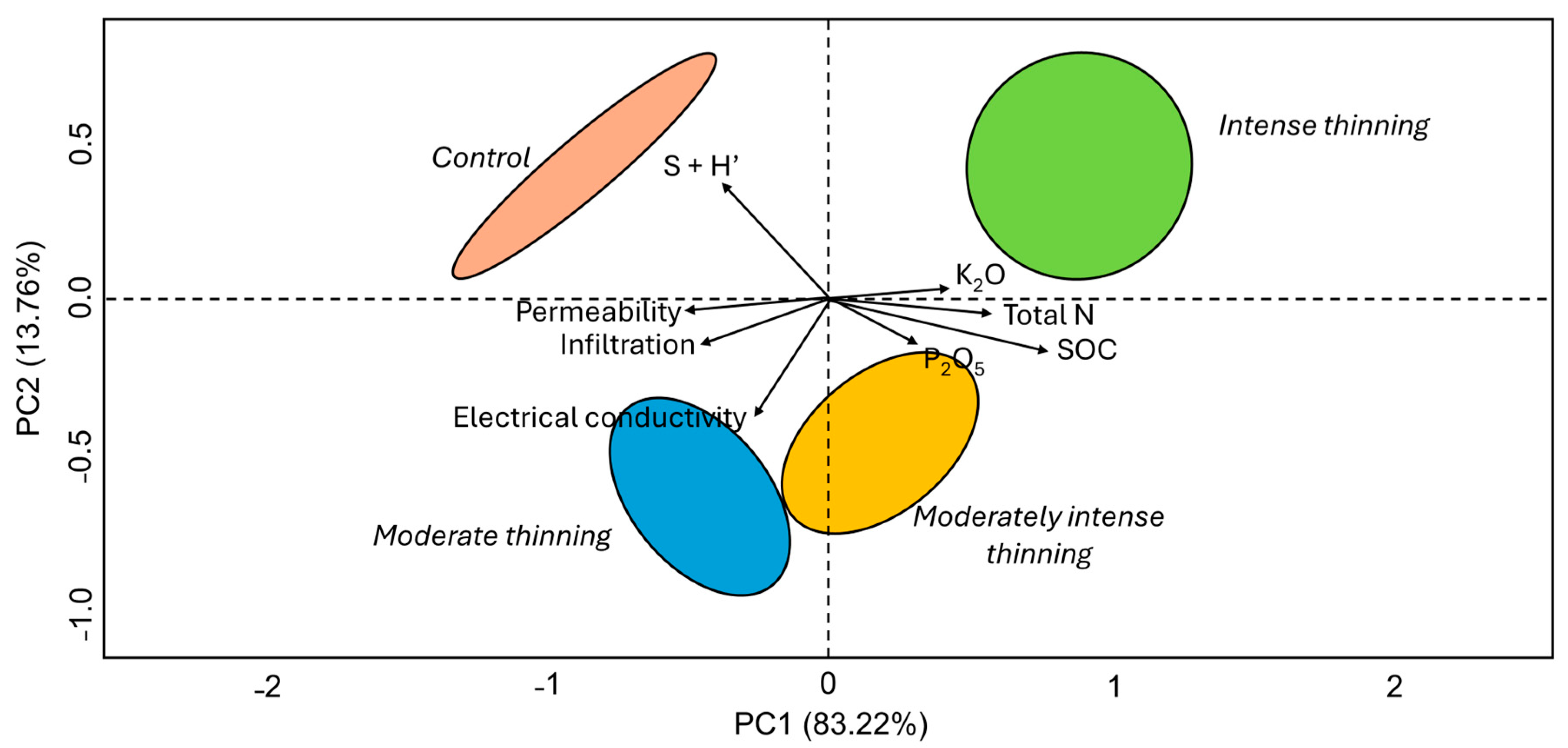
3.3. Multivariate Analysis: A Deeper View of Thinning İntensities’ İnfluence on Soil Properties and AM Fungi Community Composition
4. Discussion
4.1. Relationship among Soil Properties, AM Fungi, and Thinning Intensities in F. orientalis Stands
4.2. The İnfluence of Different Thinning İntensities on AM Fungi Community Composition: Evidence of AM Fungi–Host Pairing Specificity
4.3. Thinning Intensities Changing F. orientalis Rhizosphere: Evidence for Changes in Soil Physical–Chemical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, T.A.F.; Nascimento, G.S.; da Silva, L.J.R.; Welter, L.J. Root-mycorrhizae species and variety pairing matters: A study case with arbuscular mycorrhizal fungi communities and Vitis vinifera varieties in the southern Brazil. Rhizosphere 2024, 29, e100870. [Google Scholar] [CrossRef]
- Lanzas, M.; Pou, N.; Bota, G.; Pla, M.; Villero, D.; Brotons, L.; de la Maza, P.S.; Bach, J.; Pont, S.; Marc, A.; et al. Detecting management gaps for biodiversity conservation: An integrated assessment. J. Environ. Manag. 2024, 354, e120247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mohammed, S.A.; Devi, N.; Varts, G.; Tuli, H.S.; Saini, A.K.; Dhir, Y.W.; Dhir, S.; Singh, B. Unveiling the dynamic relationship of viruses and/or symbiotic bacteria with plant resilience in abiotic stress. Stress Biol. 2024, 4, e10. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.; Dobner, M., Jr.; Schmitt, D.E.; da Silva, L.J.R.; Schneider, K. Soil biotic and abiotic traits as driven factors for site quality of Araucaria angustifolia plantations. Biologia 2022, 77, 1219–1230. [Google Scholar] [CrossRef]
- Latterini, F.; Mederski, P.S.; Jaeger, D.; Venanzi, R.; Tavankar, F.; Picchio, R. The Influence of Various Silvicultural Treatments and Forest Operations on Tree Species Biodiversity. Curr. For. Rep. 2023, 9, 59–71. [Google Scholar] [CrossRef]
- Limaki, M.K.; Nimvari, M.E.-H.; Alavi, S.J.; Mataji, A.; Kazemnezhad, F. Potential elevation shift of oriental beech (Fagus orientalis L.) in Hyrcanian mixed forest ecoregion under future global warming. Ecol. Model. 2021, 455, e109637. [Google Scholar] [CrossRef]
- Dogan Ciftci, N.; Şahin, A.D.; Yousefpour, R.; Christen, A. Effects of climate trends and variability on tree health responses in the Black Sea and Mediterranean forests of Türkiye. Theor. Appl. Climatol. 2024, 155, 3969–3991. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Tan, S.; Yu, L.; Tang, C.; You, Y. Overview of vegetation factors related to the diversity of arbuscular mycorrhizal fungi and their interactions in karst areas. Appl. Soil Ecol. 2024, 198, e105387. [Google Scholar] [CrossRef]
- Sun, W.; Li, Q.; Qiao, B.; Jia, K.; Li, C.; Zhao, C. Advances in Plant–Soil Feedback Driven by Root Exudates in Forest Ecosystems. Forests 2024, 15, e515. [Google Scholar] [CrossRef]
- Tüfekçioğlu, A.; Güner, S.; Tilki, F. Thinning Effects on Production, Root Biomass and Soil Properties in a Young Oriental Beech Stand in Artvin, Turkey. J. Environ. Biol. 2005, 26, 91–95. [Google Scholar]
- Barbosa, L.S.; Souza, T.A.F.; Lucena, E.O.; Silva, L.J.R.; Laurindo, L.K.; Nascimento, G.S.; Santos, D. Arbuscular mycorrhizal fungi diversity and transpiratory rate in long-term field cover crop systems from tropical ecosystem, northeastern Brazil. Symbiosis 2021, 85, 207–216. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, G.; Singh, P.; Ghuman, R.S.; Singh, P.; Vyas, P. Distinct changes in soil organic matter quality, quantity and biochemical composition in response to land-use change to diverse cropping systems and agroforestry in north-western India. Agrofor. Syst. 2024, 98, 1049–1073. [Google Scholar] [CrossRef]
- WRB—IUSS Working Group. World Reference Base for Soil; World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Black, C.A. Methods of soil analysis, part 2. In Agronomy Monograph; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 771–1572. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Klute, A.; Dirksen, C. Hydraulic Conductivity and Diffusivity: Laboratory Methods. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; Klute, A., Ed.; SSA Book Series: 5; John and Wiley and Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- Tüzüner, A. Toprak ve Su Analiz Laboratuvarları El Kitabı; T.C. Tarım Orman ve Köy İşleri Bakanlığı Köy Hizmetleri Genel Müd. Yay.: Ankara, Turkey, 1990; 375p.
- Walkley, A.; Black, A.I. An examination of the method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Rhoades, J.D. Soluble Salts. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; SSSA Book series, No: 9; John and Wiley and Sons: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Kaçar, B. Bitki ve toprağın kimyasal analizleri, III. Toprak Analizleri; AÜ Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı Yayınları No 3: Ankara, Turkey, 1995; 705p. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U. S. Department of Agriculture Circular: Washington, DC, USA, 1954; p. 939.
- Palta, Ş.; Genç-Lermi, A.; Öztürk, H. Determination of arbuscular mycorrhizal fungi at different altitudinal gradients. Fresenius Env. Bull. 2018, 27, 7045–7053. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal endogonespecies extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 1, 43–66. [Google Scholar]
- Jenkins, W.R. A rapid centrifugal flotation technique forseparating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Schenck, N.C.; Perez, Y. Manual for the Identification of VA Mycorrhizal Fungi, 3rd ed.; Synergistic Publications: Gainesville, FL, USA, 1990. [Google Scholar]
- Leopold, H.J. Beimfung von Klee Mit VA—Mykorrhiza und Rhizobium zur Ertags und Qualittssteigerung. Ph.D. Thesis, Giessen University, Giessen, Germany, 1990. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedure for cleaning roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assesment of infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovanetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- SPSS. SPSS for Windows, Version 18.0.; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Zhang, W.P.; Surigaoge, S.; Yang, H.; Yu, R.P.; Wu, J.P.; Xing, Y.; Chen, Y.; Li, L. Diversified cropping systems with complementary root growth strategies improve crop adaptation to and remediation of hostile soils. Plant Soil 2024, 1–24. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Siqueira, J.O. Species richness and spore abundance of arbuscular mycorrhizal fungi across distinct land uses in Western Brazilian Amazon. Mycorrhiza 2011, 21, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Anshumali, S.K. Biogeochemical appraisal of carbon fractions and carbon stock in riparian soils of the Ganga River basin. Appl. Soil Ecol. 2023, 182, e104687. [Google Scholar] [CrossRef]
- Scarciglia, F.; Sauer, D.; Zerboni, A. Pleistocene paleosols of Italy: Pedostratigraphy, genesis, paleoclimate and geoarchaeology. Alp. Mediterr. Quat. 2023, 36, 149–183. [Google Scholar] [CrossRef]
- Caihong, Z.; Nier, S.; Hao, W.; Honglin, X.; Hailong, S.; Ling, Y. Effects of thinning on soil nutrient availability and fungal community composition in a plantation medium aged pure forest of Picea karaiensis. Sci. Rep. 2023, 13, e2492. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Leyva, J.F.; Segura-Castruita, M.A.; Hernández-Cuevas, L.V.; Íñiguez-Rivas, M. Arbuscular mycorrhizal fungi associated with maize (Zea mays L.) in the formation and stability of aggregates in two types of soil. Microorganisms 2023, 11, e2615. [Google Scholar] [CrossRef]
- Palta, Ş.; Lermi, A.G.; Beki, R. The effect of different land uses on arbuscular mycorrhizal fungi in the northwestern Black Sea Region. Environ. Monit. Assess. 2016, 188, e350. [Google Scholar] [CrossRef]
- Masebo, N.; Birhane, E.; Takele, S.; Belay, Z.; Lucena, J.J.; Perenz-Sanz, A.; Anjulo, A. Diversity of arbuscular mycorrhizal fungi under different agroforestry practices in the drylands of Southern Ethiopia. BMC Plant Biol. 2023, 23, e634. [Google Scholar] [CrossRef]
- Mohamed, O.Z.; Said, E.K.; Miloud, S.; Abdellatif, H.; El Hassan, A.; Rachid, B. Effect of agricultural management practices on diversity, abundance, and infectivity of arbuscular mycorrhizal fungi: A review. Symbiosis 2023, 91, 33–44. [Google Scholar] [CrossRef]
- Fors, R.O.; Sorci-Uhmann, E.; Santos, E.S.; Silva-Flores, P.; Abreu, M.M.; Viegas, W.; Nogales, A. Influence of soil type, land use, and rootstock genotype on root-associated arbuscular mycorrhizal fungi communities and their impact on grapevine growth and nutrition. Agriculture 2023, 13, e2163. [Google Scholar] [CrossRef]
- Liu, K.L.; Chen, B.Y.; Zhang, B.; Wang, R.H.; Wang, C.S. Understory vegetation diversity, soil properties and microbial community response to different thinning intensities in Cryptomeria japonica var. sinensis plantations. Front. Microbiol. 2023, 14, 1117384. [Google Scholar] [CrossRef]
- Laurindo, L.K.; Souza, T.A.F.; da Silva, L.J.R.; Nascimento, G.S.; Cruz, S.P. Pinus taeda L. changes arbuscular mycorrhizal fungi communities in a Brazilian subtropical ecosystem. Symbiosis 2022, 87, 269–279. [Google Scholar] [CrossRef]
- Nascimento, G.S.; Souza, T.A.F.; da Silva, L.J.R.; Santos, D. Soil physico-chemical properties, biomass production, and oot density in a green manure farming system from tropical ecosystem, North-eastern Brazil. J. Soil Sed. 2021, 21, 2203–2211. [Google Scholar] [CrossRef]
- Nungula, E.Z.; Mugwe, J.; Massawe, B.H.J.; Gitari, H.I. Morphological, Pedological and Chemical Characterization and Classification of Soils in Morogoro District, Tanzania. Agric. Res. 2024, 13, 266–276. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Q.; Zhang, B.; Gao, D.; Zhang, Y.; Jiang, J.; Zuo, H. Effects of thinning and understory removal on water use efficiency of Pinus massoniana: Evidence from photosynthetic capacity and stable carbon isotope analyses. J. For. Res. 2024, 35, 41. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, T.; Yang, F.; Li, Q.; Wang, Q.; Xu, M.; Li, S.; Wang, H. Effects of thinning on forest soil and stump respiration in a subtropical pine plantation. For. Ecol. Manag. 2023, 531, e120797. [Google Scholar] [CrossRef]
- Gondim, J.E.F.; de Souza, T.A.F.; Portela, J.C.; Santos, D.; Batista, R.O.; Nascimento, G.D.S.; Dias, P.M.S. Land uses shifts the abundance and structure of soil biota and soil chemical traits in tropical ecosystem, Apodi Plateau, Brazil. Trop. Ecol. 2024, 65, 179–190. [Google Scholar] [CrossRef]
- Gondim, J.E.F.; Souza, T.; Portela, J.C.; Santos, D.; Nascimento, G.D.S.; Da Silva, L.J.R. Soil Physical-chemical Traits and Soil Quality Index in a Tropical Cambisol as Influenced by Land Uses and Soil Depth at Apodi Plateau, Northeastern Brazil. Int. J. Plant Prod. 2023, 17, 491–501. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Plaza-Alvarez, P.A.; Xu, X.; Carra, B.G.; Zema, D.A. Exploring the factors influencing the hydrological response of soil after low and high-severity fires with post-fire mulching in Mediterranean forests. Int. Soil Water Conserv. Res. 2023, 11, 169–182. [Google Scholar] [CrossRef]
- Silva, S.I.A.; Souza, T.A.F.; Lucena, E.O.; Silva, L.J.R.; Laurindo, L.K.; Nascimento, G.S.; Santos, D. High phosphorus availability promotes the diversity of arbuscular mycorrhizal spores’ community in different tropical crop systems. Biologia 2021, 76, 3211–3220. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, L.; Shao, Y.; Wang, J. Root and hyphal interactions influence N transfer by arbuscular mycorrhizal fungi in soybean/maize intercropping systems. Fungal. Ecol. 2023, 64, e101240. [Google Scholar] [CrossRef]
- Ma, X.; Qu, H.; Liu, X.; Zhang, Y.; Chao, L.; Liu, H.; Bao, Y. Changes of root AMF community structure and colonization levels under distribution pattern of geographical substitute for four Stipa species in arid steppe. Microbiol. Res. 2023, 271, e127371. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Xie, M.M.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Arbuscular mycorrhizal fungi and rhizobia synergistically promote root colonization, plant growth, and nitrogen acquisition. Plant Growth Regul. 2023, 100, 691–701. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Badji, A.; Ndiaye, A.; Ndiaye, M.; Kyakuwa, P.; Anyoni, O.G.; Kabaseke, C.; et al. Combined effects of indigenous arbuscular mycorrhizal fungi (AMF) and NPK fertilizer on growth and yields of maize and soil nutrient availability. Sustainability 2023, 15, e2243. [Google Scholar] [CrossRef]
- Audisio, M.; Sennhenn-Reulen, H.; Schott, I.; Paligi, S.S.; Mrak, K.; Hertel, D.; Leuschner, C.; Polle, A. Mycorrhization, root tip vitality and biomass of Fagus sylvatica, Picea abies and Pseudotsuga menziesii in monospecific and mixed combinations under water reduction and nitrogen addition. Trees 2024, 38, 695–708. [Google Scholar] [CrossRef]
- Metzler, P.; Ksiazek-Mikenas, K.; Chaudhary, V.B. Tracking arbuscular mycorrhizal fungi to their source: Active inoculation and passive dispersal differentially affect community assembly in urban soils. New Phytol. 2024, 242, 1814–1824. [Google Scholar] [CrossRef]
- Nazari, M.; Pausch, J.; Bickel, S.; Bilyera, N.; Rashtbari, M.; Razavi, B.S.; Zamanian, K.; Sharrififar, A.; Shi, L.; Dippold, M.A.; et al. Keeping thinning-derived deadwood logs on forest floor improves soil organic carbon, microbial biomass, and enzyme activity in a temperate spruce forest. Eur. J. For. Res. 2023, 142, 287–300. [Google Scholar] [CrossRef]
- Wahab, A.; Batool, F.; Muhammad, M.; Zaman, W.; Mikhlef, R.M.; Qaddoori, S.M.; Ulah, S.; Abdi, G.; Saqib, S. Unveiling the complex molecular dynamics of arbuscular mycorrhizae: A comprehensive exploration and future perspectives in harnessing phosphate-solubilizing microorganisms for sustainable progress. Environ. Exp. Bot. 2023, 219, 105633. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S. Soil Physical Productivity and Plant Growth. In Soil Physical Environment and Plant Growth; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Xu, T.; Jonhson, D.; Bardgett, R.D. Defoliation modifies the response of arbuscular mycorrhizal fungi to drought in temperate grassland. Soil Biol. Biochem. 2024, 192, e109386. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Qiu, T.; He, H.; Liu, J.; Duan, C.; Cui, Y.; Huang, M.; Wu, C.; Fang, L. High nitrogen fertilizer input enhanced the microbial network complexity in the paddy soil. Soil Ecol. Lett. 2024, 6, 230205. [Google Scholar] [CrossRef]
- Wu, J.; Fu, X.; Zhao, L.; Lv, J.; Lv, S.; Shang, J.; Lv, J.; Du, S.; Guo, H.; Ma, F. Biochar as a partner of plants and beneficial microorganisms to assist in-situ bioremediation of heavy metal contaminated soil. Sci. Total Environ. 2024, 923, 171442. [Google Scholar] [CrossRef]
- Liu, F.; Qian, J.; Zhu, Y.; Wang, P.; Hu, J.; Lu, B.; He, S.T.; Shen, J.; Liu, Y.; Li, F. Phosphate solubilizing microorganisms increase soil phosphorus availability: A review. Geomicrobiol. J. 2024, 41, 1–16. [Google Scholar] [CrossRef]
- Kaya, C. Microbial modulation of hormone signaling, proteomic dynamics, and metabolomics in plant drought adaptation. Food Ener. Sec. 2023, 13, e513. [Google Scholar] [CrossRef]
- Qin, X.; Xu, J.; An, X.; Yang, J.; Wang, Y.; Dou, M.; Wang, M.; Huang, J.; Fu, Y. Insight of endophytic fungi promoting the growth and development of woody plants. Crit. Rev. Biotechnol. 2024, 44, 78–99. [Google Scholar] [CrossRef] [PubMed]



| Descriptors | Control | Moderate Thinning | Moderately Intense Thinning | Intense Thinning |
|---|---|---|---|---|
| Stand type | Kncd2 | Kncd2 | Kncd2 | Kncd2 |
| Bonitet class | III | III | III | III |
| Elevation (m) | 557 | 558 | 563 | 552 |
| Exposure | South | South | South | South |
| Slope status | Middle slope | Middle slope | Middle slope | Middle slope |
| Canopy | 0.8–0.9 | 0.7–0.8 | 0.6–0.7 | 0.5–0.6 |
| Density | 0.8 | 0.7 | 0.6 | 0.5 |
| Stratification | Single layer | Single layer | Single layer | Single layer |
| Mix status | Pure beech | Pure beech | Pure beech | Pure beech |
| Average age (year) | 58 | 56 | 56 | 58 |
| Average diameter (cm) | 26.7 | 27.4 | 28.5 | 29.3 |
| Average height (m) | 18.5 | 17.1 | 17.8 | 18.2 |
| Average initial number of trees (number/ha) | 1328.4 | 1216.7 | 1040.3 | 783.9 |
| Average volume (m3/ha) | 500.81 | 472.17 | 437.96 | 375.49 |
| Average annual increment (m3/ha/year) | 8.63 | 8.43 | 7.82 | 6.47 |
| Soil texture | ||||
| Sand (%) | 71.14 | 65.28 | 62.50 | 52.97 |
| Silt (%) | 13.72 | 20.98 | 18.74 | 27.11 |
| Clay (%) | 15.14 | 13.74 | 18.76 | 19.92 |
| Soil Properties | Control | MT | MIT | IT |
|---|---|---|---|---|
| Bulk density (g cm−3) | 1.42 ± 0.01 a | 1.39 ± 0.01 b | 1.38 ± 0.01 c | 1.35 ± 0.01 d |
| Grain density | 2.85 ± 0.21 a | 2.74 ± 0.24 a | 2.56 ± 0.19 b | 2.53 ± 0.15 b |
| Soil pore volume | 80.69 ± 5.47 a | 78.56 ± 6.24 a | 72.43 ± 4.71 b | 67.32 ± 5.35 b |
| SFC (%) | 19.51 ± 0.89 d | 20.53 ± 1.04 c | 21.89 ± 1.86 b | 23.70 ± 1.03 a |
| PWP (%) | 10.57 ± 0.64 b | 10.53 ± 0.49 b | 11.82 ± 0.81 a | 12.04 ± 0.97 a |
| SAW (%) | 8.93 ± 0.33 c | 10.50 ± 1.41 b | 10.06 ± 1.27 b | 11.65 ± 1.18 a |
| Soil permeability (mm/sa) | 103.65 ± 19.01 a | 85.60 ± 7.25 b | 71.92 ± 5.15 c | 55.15 ± 5.32 d |
| Infiltration | 15.59 ± 2.57 b | 15.95 ± 2.72 a | 9.42 ± 2.98 c | 8.01 ± 3.01 d |
| Soil pH (1:2.5, v:v) | 4.09 ± 0.10 b | 4.16 ± 0.15 b | 4.32 ± 0.65 a | 4.33 ± 0.12 a |
| CaCO3 (%) | 1.08 ± 0.09 a | 1.18 ± 0.15 a | 1.17 ± 0.33 a | 1.20 ± 0.24 a |
| Electrical conductivity (dS m−1) | 0.16 ± 0.07 b | 0.23 ± 0.11 a | 0.16 ± 0.06 b | 0.11 ± 0.04 c |
| SOC (%) | 0.78 ± 0.36 d | 1.37 ± 0.45 c | 1.71 ± 0.85 b | 2.27 ± 1.77 a |
| Total nitrogen (%) | 0.05 ± 0.02 d | 0.07 ± 0.03 c | 0.10 ± 0.05 b | 0.12 ± 0.08 a |
| P2O5 (kg/da) | 2.48 ± 0.40 d | 2.79 ± 0.65 c | 3.25 ± 0.40 a | 2.92 ± 0.34 b |
| K2O (kg/da) | 13.80 ± 3.18 c | 17.39 ± 4.75 b | 18.77 ± 5.79 b | 26.20 ± 13.2 a |
| AMF Species | FOi 1 (Classification 2) | Species Classification 3 | |||
|---|---|---|---|---|---|
| Control | MT | MIT | IT | ||
| Order Diversisporales | |||||
| Family Acaulosporaceae | |||||
| Acaulospora dilatata | 4.3 (R) | - | - | - | Exclusive |
| A. bireticulata | - | 8.3 (R) | - | - | Exclusive |
| Acaulospora sp. | 17.4 (C) | - | - | - | Exclusive |
| Order Gigasporales | |||||
| Family Dentiscutataceae | |||||
| Dentiscutata heterogama | - | 15.7 (C) | - | - | Exclusive |
| Family Gigasporaceae | |||||
| Racocetra coralloidea | - | 9.7 (R) | - | - | Exclusive |
| Scutellospora calospora | 4.8 (R) | - | - | - | Exclusive |
| S. rubra | 4.5 (R) | 8.5 (R) | 8.7 (R) | 17.5 (C) | Generalists |
| Scutellospora sp. | - | - | - | 9.1 (R) | Exclusive |
| Order Glomerales | |||||
| Family Entrophosporaceae | |||||
| Claroideoglomus claroideum | 13.0 (C) | - | - | - | Exclusive |
| C. etunicatum | 8.7 (R) | - | 18.7 (C) | - | Intermediate |
| C. geosporum | 5.6 (R) | - | 9.3 (R) | 9.1 (R) | Intermediate |
| Claroideoglomus sp. | - | - | 8.3 (R) | 8.5 (R) | Intermediate |
| Family Glomeraceae | |||||
| Funneliformis geosporum | 4.9 (R) | 16.3 (C) | - | - | Intermediate |
| F. mosseae | 9.1 (R) | - | - | - | Exclusive |
| Funneliformis sp. | - | - | - | 16.2 (C) | Exclusive |
| Glomus multicaule | - | - | - | 9.7 (R) | Exclusive |
| Glomus sp. | 19.5 (C) | 24.8 (C) | 23.7 (C) | - | Intermediate |
| Rhizophagus irregularis | 7.5 (R) | 16.7 (C) | 21.9 (C) | 17.6 (C) | Generalists |
| R. intraradices | - | - | - | 12.3 (C) | Exclusive |
| Sclerocystis coremioides | - | - | 9.4 (R) | - | Exclusive |
| Ecological indices | |||||
| Species richness (S) | 11.00 a | 7.00 b | 7.00 b | 8.00 b | |
| Simpson index (D) | 0.93 a | 0.94 a | 0.91 a | 0.94 a | |
| Shannon index (H′) | 2.31 a | 1.89 c | 1.83 c | 2.02 b | |
| Hurlbert’s PIE | 0.93 b | 0.94 b | 1.07 a | 0.95 b | |
| Pielou index (J) | 0.93 a | 0.97 a | 0.94 a | 0.97 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palta, Ş.; Özel, H.B.; Souza, T.A.F.d.; Baş, E. Effects of Thinning Practices on Soil Properties and Arbuscular Mycorrhizal Fungi in Natural Pure Oriental Beech Forests. Forests 2024, 15, 1643. https://doi.org/10.3390/f15091643
Palta Ş, Özel HB, Souza TAFd, Baş E. Effects of Thinning Practices on Soil Properties and Arbuscular Mycorrhizal Fungi in Natural Pure Oriental Beech Forests. Forests. 2024; 15(9):1643. https://doi.org/10.3390/f15091643
Chicago/Turabian StylePalta, Şahin, Halil Barış Özel, Tancredo Augusto Feitosa de Souza, and Eren Baş. 2024. "Effects of Thinning Practices on Soil Properties and Arbuscular Mycorrhizal Fungi in Natural Pure Oriental Beech Forests" Forests 15, no. 9: 1643. https://doi.org/10.3390/f15091643







