Moss Cover Modulates Soil Fungal Functional Communities and Nutrient Cycling in Alpine Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Site and Sampling
2.2. Soil Physicochemical Properties
2.3. DNA Extraction, Sequencing, and Data Analysis
3. Results
3.1. Physicochemical Properties of Soil
3.2. Soil Fungal Alpha, Beta Diversity, and Co-Occurrence Network Changes
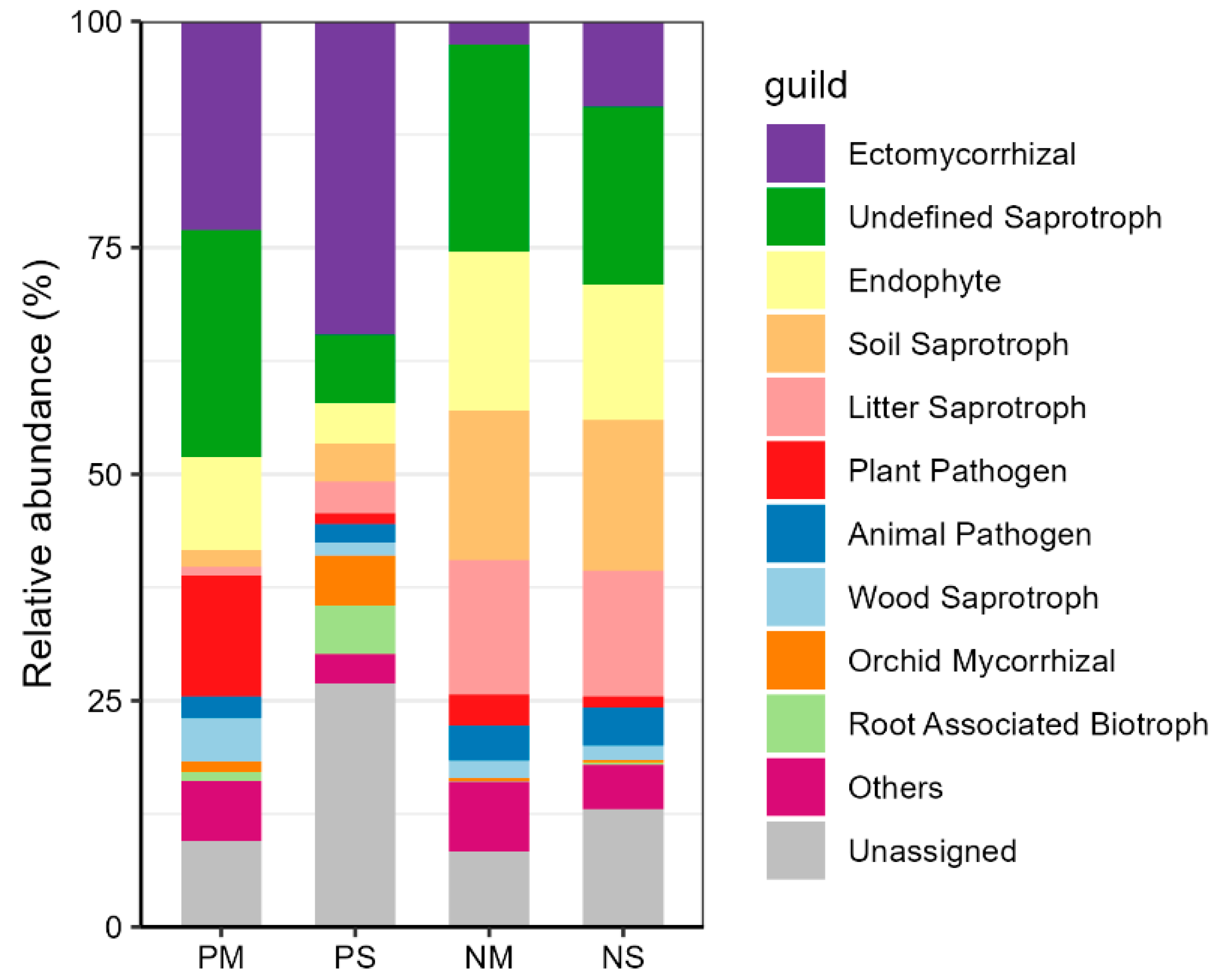
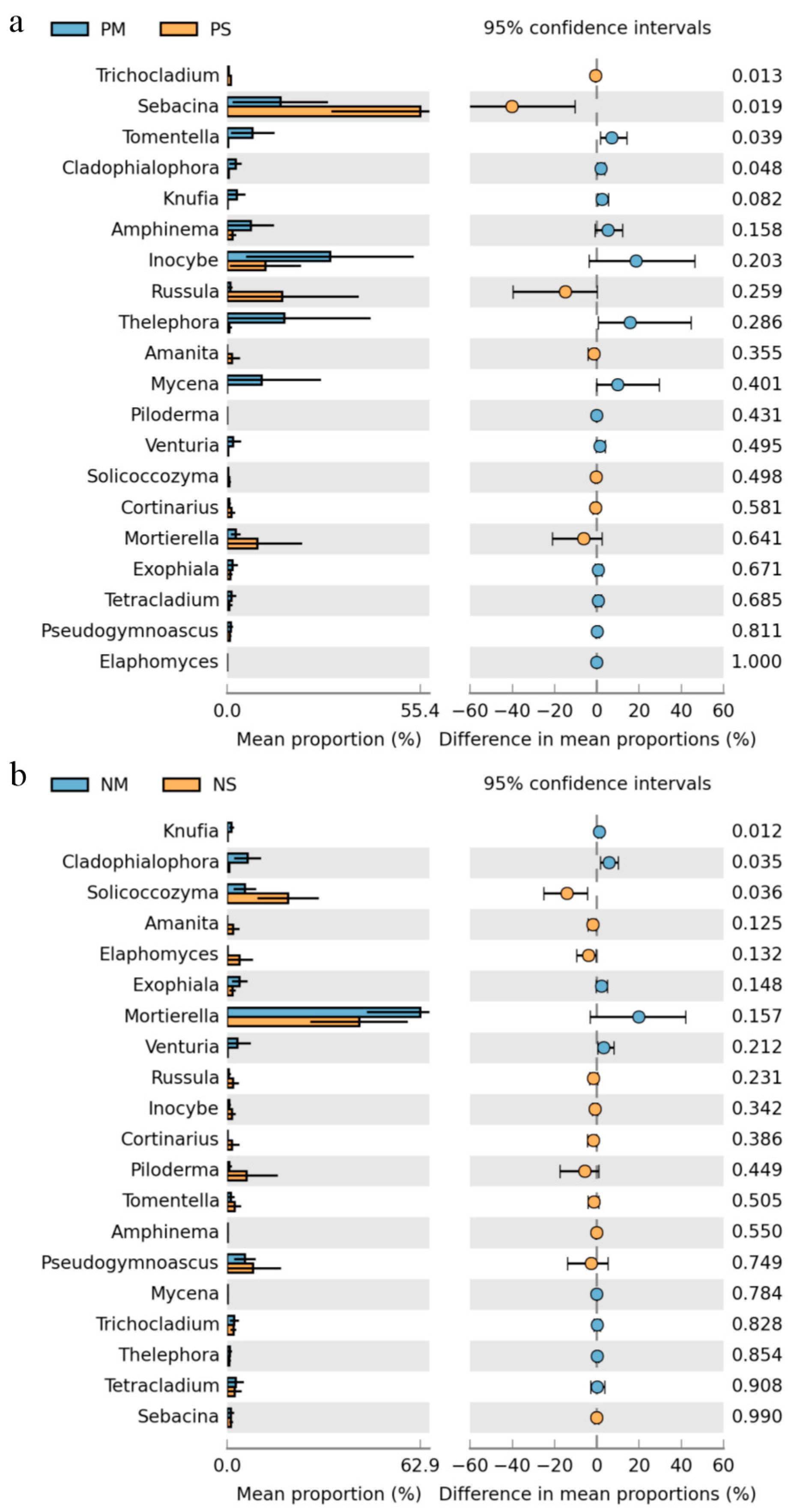
3.3. Dominant Fungal Phylum and Trophic Group Changes
3.4. Dominant Fungal Functional Group Changes
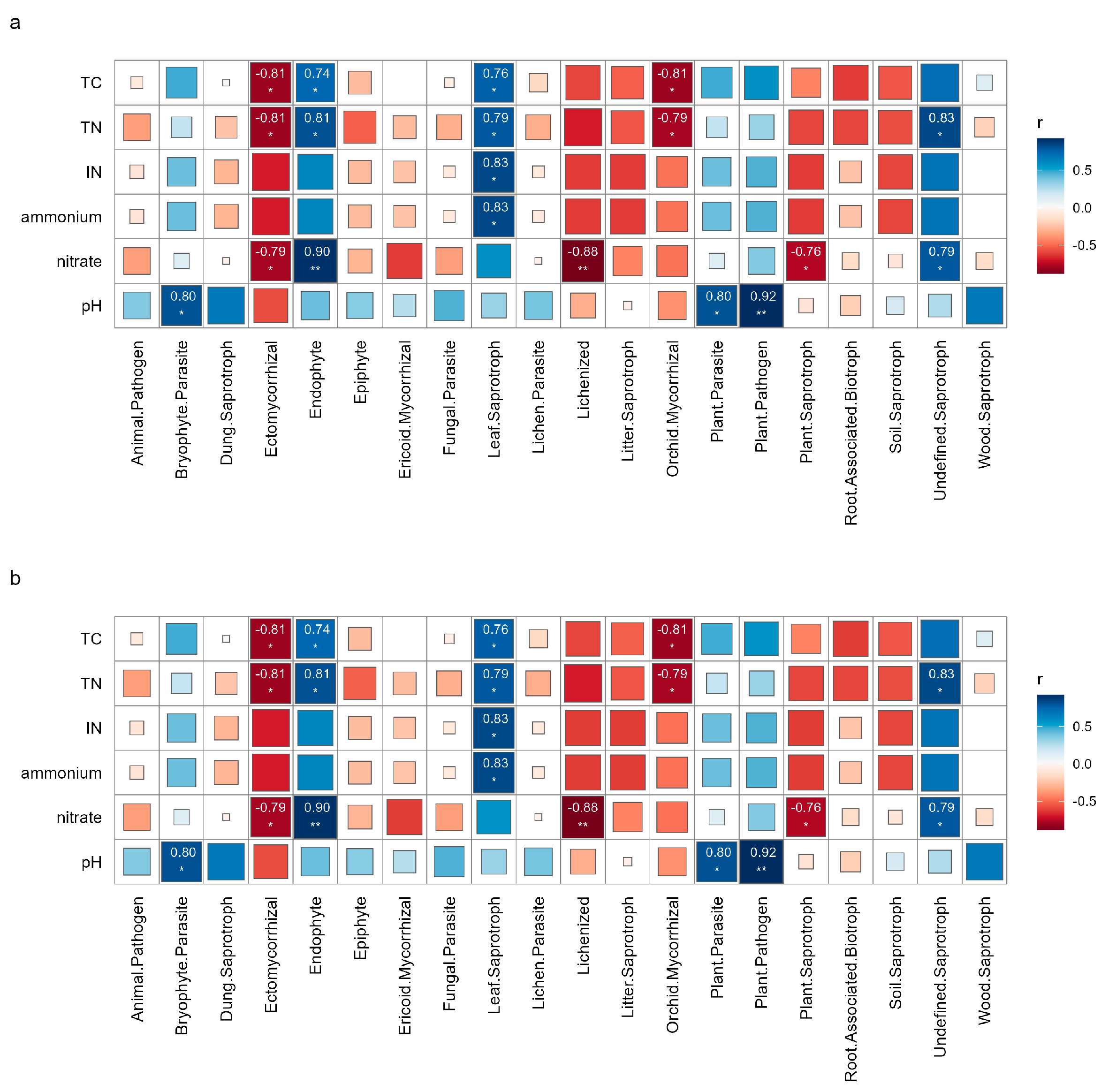
3.5. The Relationship Between Fungal Functions and Soil Physicochemical Properties
4. Discussion
4.1. Effect of Moss Cover on Soil Physicochemical Properties
4.2. Effects of Moss Cover on Fungal Community Diversity and Composition Structure
4.3. Effects of Moss Cover on Fungal Functional Guilds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berdugo Moreno, M.; Quant, J.; Wason, J.; Dovčiak, M. Latitudinal Patterns and Environmental Drivers of Moss Layer Cover in Extratropical Forests. Glob. Ecol. Biogeogr. 2018, 27, 1213–1224. [Google Scholar] [CrossRef]
- Rousk, K.; Jones, D.L.; Deluca, T.H. Moss-Cyanobacteria Associations as Biogenic Sources of Nitrogen in Boreal Forest Ecosystems. Front. Microbiol. 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- Rousk, K.; Michelsen, A. Ecosystem Nitrogen Fixation throughout the Snow-Free Period in Subarctic Tundra: Effects of Willow and Birch Litter Addition and Warming. Glob. Change Biol. 2017, 23, 1552–1563. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Nilsson, M.-C.; Sun, S.; Arróniz-Crespo, M. Long-Term Fate of Nitrogen Fixation in Pleurozium schreberi Brid (Mit.) Moss Carpets in Boreal Forests. Appl. Soil Ecol. 2022, 169, 104215. [Google Scholar] [CrossRef]
- Renaudin, M.; Blasi, C.; Bradley, R.L.; Bellenger, P. New Insights into the Drivers of Moss-associated Nitrogen Fixation and Cyanobacterial Biomass in the Eastern Canadian Boreal Forest. J. Ecol. 2022, 110, 1403–1418. [Google Scholar] [CrossRef]
- Renaudin, M.; Laforest-Lapointe, I.; Bellenger, J.-P. Unraveling Global and Diazotrophic Bacteriomes of Boreal Forest Floor Feather Mosses and Their Environmental Drivers at the Ecosystem and at the Plant Scale in North America. Sci. Total Environ. 2022, 837, 155761. [Google Scholar] [CrossRef]
- Calabria, L.M.; Petersen, K.S.; Bidwell, A.; Hamman, S.T. Moss-Cyanobacteria Associations as a Novel Source of Biological N2-Fixation in Temperate Grasslands. Plant Soil 2020, 456, 307–321. [Google Scholar] [CrossRef]
- Groß, C.; Hossen, S.; Dittrich, S.; Knorr, K.-H.; Borken, W.; Noll, M. Biological Nitrogen Fixation, Diversity and Community Structure of Diazotrophs in Two Mosses in 25 Temperate Forests. Environ. Microbiol. 2024, 26, e16555. [Google Scholar] [CrossRef]
- Mayer, M.; Rewald, B.; Matthews, B.; Sandén, H.; Rosinger, C.; Katzensteiner, K.; Gorfer, M.; Berger, H.; Tallian, C.; Berger, T.W.; et al. Soil Fertility Relates to Fungal-Mediated Decomposition and Organic Matter Turnover in a Temperate Mountain Forest. New Phytol. 2021, 231, 777–790. [Google Scholar] [CrossRef]
- Liu, T.; Wu, X.; Li, H.; Alharbi, H.; Wang, J.; Dang, P.; Chen, X.; Kuzyakov, Y.; Yan, W. Soil Organic Matter, Nitrogen and pH Driven Change in Bacterial Community Following Forest Conversion. For. Ecol. Manag. 2020, 477, 118473. [Google Scholar] [CrossRef]
- Slate, M.; Sullivan, B.; Callaway, R. Desiccation and Rehydration of Mosses Greatly Increases Resource Fluxes That Alter Soil Carbon and Nitrogen Cycling. J. Ecol. 2019, 107, 1767–1778. [Google Scholar] [CrossRef]
- Siwach, A.; Kaushal, S.; Sarma, K.; Baishya, R. Interplay of Moss Cover and Seasonal Variation Regulate Soil Physicochemical Properties and Net Nitrogen Mineralization Rates in Central Himalayas, India. J. Environ. Manag. 2023, 345, 118839. [Google Scholar] [CrossRef] [PubMed]
- Koranda, M.; Michelsen, A. Mosses Reduce Soil Nitrogen Availability in a Subarctic Birch Forest via Effects on Soil Thermal Regime and Sequestration of Deposited Nitrogen. J. Ecol. 2021, 109, 1424–1438. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, G.; DeLuca, T.H.; Sun, S.; Zhang, W.; Chen, P.; Wu, A.; Sun, X.; Sun, H.; Hu, Z. Soil Nitrogen Transformations Differ under Sedge and Bryophyte Communities in a Subalpine Forest on the Eastern Tibetan Plateau. Geoderma 2023, 430, 116309. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Gao, H.; Li, H.; Liu, Y.; Zhang, L.; Li, H.; You, C.; Liu, S.; Tan, B.; et al. Linking Fungal Community Structure with Soil Nitrogen Dynamics Following Forest Conversion in a Subalpine Forest in China. Geoderma 2023, 433, 116448. [Google Scholar] [CrossRef]
- Hu, P.; Xiao, J.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K. Response of Soil Microbial Communities to Natural and Managed Vegetation Restoration in a Subtropical Karst Region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Yang, L.; Luo, P.; Xiao, K.; Chen, H.; Zhang, W.; He, X.; Chen, H.; Wang, K. Dynamics of Soil Organic Carbon and Nitrogen Following Agricultural Abandonment in a Karst Region. JGR Biogeosci. 2017, 122, 230–242. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Jiang, S.; Song, T.; Wang, K. Responses of Soil Nutrients and Microbial Communities to Three Restoration Strategies in a Karst Area, Southwest China. J. Environ. Manag. 2018, 207, 456–464. [Google Scholar] [CrossRef]
- Arróniz-Crespo, M.; Bougoure, J.; Murphy, D.V.; Cutler, N.A.; Souza-Egipsy, V.; Chaput, D.L.; Jones, D.L.; Ostle, N.; Wade, S.C.; Clode, P.L.; et al. Revealing the Transfer Pathways of Cyanobacterial-Fixed N into the Boreal Forest through the Feather-Moss Microbiome. Front. Plant Sci. 2022, 13, 1036258. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal Types Differ in Ecophysiology and Alter Plant Nutrition and Soil Processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef]
- Cao, T.; Luo, Y.; Shi, M.; Tian, X.; Kuzyakov, Y. Microbial Interactions for Nutrient Acquisition in Soil: Miners, Scavengers, and Carriers. Soil Biol. Biochem. 2024, 188, 109215. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Zhang, W.; Zhu, J.; Chen, B.; Jiang, L.; Xu, D.; Li, W.; Liu, J.; He, Z. Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests. Forests 2024, 15, 643. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Sun, X.; He, L.; Zhou, W.; Zhao, G.; Yu, J.; Bai, X.; Zhang, J. Characteristics of Bacterial Communities under Different Tree Species and Their Response to Soil Physicochemical Properties. Forests 2024, 15, 740. [Google Scholar] [CrossRef]
- Menkis, A.; Burokienė, D.; Gaitnieks, T.; Uotila, A.; Johannesson, H.; Rosling, A.; Finlay, R.D.; Stenlid, J.; Vasaitis, R. Occurrence and Impact of the Root-Rot Biocontrol Agent Phlebiopsis Gigantea on Soil Fungal Communities in Picea Abies Forests of Northern Europe. FEMS Microbiol. Ecol. 2012, 81, 438–445. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Agerer, R. Exploration Types of Ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Hobbie, E.A.; Horton, T.R. Conservation of Ectomycorrhizal Fungi: Exploring the Linkages between Functional and Taxonomic Responses to Anthropogenic N Deposition. Fungal Ecol. 2011, 4, 174–183. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, H.; Oksanen, M. The Vegan Package. Vegan: Community Ecology Package. 2010. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 23 July 2024).
- Macias Fauria, M.; Helle, T.; Niva, A.; Posio, H.; Timonen, M. Removal of the Lichen Mat by Reindeer Enhances Tree Growth in a Northern Scots Pine Forest. Can. J. For. Res. 2008, 38, 2981–2993. [Google Scholar] [CrossRef]
- Gao, L.; Paré, D.; Chavardès, R.D.; Bergeron, Y. Initiating the Transition from Open-Canopy Lichen Woodland to Productive Forest by Transplanting Moss, Results from a 10-Year Experiment. Plant Soil 2023, 488, 363–376. [Google Scholar] [CrossRef]
- García-Carmona, M.; Arcenegui, V.; García-Orenes, F.; Mataix-Solera, J. The Role of Mosses in Soil Stability, Fertility and Microbiology Six Years after a Post-Fire Salvage Logging Management. J. Environ. Manag. 2020, 262, 110287. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, R.; Boucher, J.-F.; Tremblay, P.; Lord, D. Comparing Soil Profiles of Adjacent Forest Stands with Contrasting Tree Densities: Lichen Woodlands vs. Black Spruce–Feathermoss Stands in the Continuous Boreal Forest. Can. J. Soil Sci. 2018, 98, 458–468. [Google Scholar] [CrossRef]
- Pacé, M.; Paré, D.; Fenton, N.J.; Bergeron, Y. Effects of Lichen, Sphagnum Spp. and Feather Moss Leachates on Jack Pine and Black Spruce Seedling Growth. Plant Soil 2020, 452, 441–455. [Google Scholar] [CrossRef]
- Ma, S.; Chen, X.; Su, H.; Xing, A.; Chen, G.; Zhu, J.; Zhu, B.; Fang, J. Phosphorus Addition Decreases Soil Fungal Richness and Alters Fungal Guilds in Two Tropical Forests. Soil Biol. Biochem. 2022, 175, 108836. [Google Scholar] [CrossRef]
- Gao, L.; Paré, D.; Martineau, C.; Yin, X.; Rodríguez-Rodríguez, J.C.; Gagné, P.; Bergeron, Y. Response of the Soil Microbial Communities to Forest Ground Cover Manipulation in a Boreal Forest. For. Ecol. Manag. 2024, 553, 121615. [Google Scholar] [CrossRef]
- Sweeney, C.J.; de Vries, F.T.; van Dongen, B.E.; Bardgett, R.D. Root Traits Explain Rhizosphere Fungal Community Composition among Temperate Grassland Plant Species. New Phytol. 2021, 229, 1492–1507. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil Bacterial Networks Are Less Stable under Drought than Fungal Networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The Ecology of the Microbiome: Networks, Competition, and Stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental Stress Destabilizes Microbial Networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Brandström Durling, M.; Clemmensen, K.E.; Lindahl, B.D. Changes in Fungal Communities along a Boreal Forest Soil Fertility Gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef]
- Nie, S.; Lei, X.; Zhao, L.; Brookes, P.C.; Wang, F.; Chen, C.; Yang, W.; Xing, S. Fungal Communities and Functions Response to Long-Term Fertilization in Paddy Soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Shen, C.; Gunina, A.; Luo, Y.; Wang, J.; He, J.-Z.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting Patterns and Drivers of Soil Bacterial and Fungal Diversity across a Mountain Gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Cao, G.; Zhang, Z.; Zhang, J.; Yan, X. Soil Microbial Community Diversity and Distribution Characteristics under Three Vegetation Types in the Qilian Mountains, China. J. Arid Land 2023, 15, 359–376. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, T.; Wang, W.; Sun, S.; Zhang, M.; Song, F. Nitrogen Addition Changed Soil Fungal Community Structure and Increased the Biomass of Functional Fungi in Korean Pine Plantations in Temperate Northeast China. Sci. Total Environ. 2024, 927, 172349. [Google Scholar] [CrossRef]
- Cheng, M.; Song, J.; Li, W.; Zhao, Y.; Zhang, G.; Chen, Y.; Gao, H. Potentilla Parvifolia Strongly Influenced Soil Microbial Community and Environmental Effect along an Altitudinal Gradient in Central Qilian Mountains in Western China. Ecol. Evol. 2023, 13, e10685. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular Mycorrhizal Symbiosis and Abiotic Stress in Plants: A Review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Kolaříková, Z.; Kohout, P.; Krüger, C.; Janoušková, M.; Mrnka, L.; Rydlová, J. Root-Associated Fungal Communities along a Primary Succession on a Mine Spoil: Distinct Ecological Guilds Assemble Differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Crowther, T.W.; Boddy, L.; Hefin Jones, T. Functional and Ecological Consequences of Saprotrophic Fungus–Grazer Interactions. ISME J. 2012, 6, 1992–2001. [Google Scholar] [CrossRef]
- Nehls, U.; Plassard, C. Nitrogen and Phosphate Metabolism in Ectomycorrhizas. New Phytol. 2018, 220, 1047–1058. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E. Mycorrhizal and Saprotrophic Fungal Guilds Compete for the Same Organic Substrates but Affect Decomposition Differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Cheeke, T.; Phillips, R.; Brzostek, E.; Rosling, A.; Bever, J.; Fransson, P. Dominant Mycorrhizal Association of Trees Alters Carbon and Nutrient Cycling by Selecting for Microbial Groups with Distinct Enzyme Function. New Phytol. 2016, 214, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Microbial Activity and the Dynamics of Ecosystem Processes in Forest Soils. Curr. Opin. Microbiol. 2017, 37, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Yuan, Y.; Liu, Q.; Yin, H. Rejoinder to Jones et al.’s Comments on: “Plant Nitrogen Acquisition from Inorganic and Organic Sources via Root and Mycelia Pathways in Ectomycorrhizal Alpine Forests”. Soil Biol. Biochem. 136: 107517. Soil Biol. Biochem. 2020, 149, 107941. [Google Scholar] [CrossRef]
- Högberg, P.; Högberg, M.N.; Göttlicher, S.G.; Betson, N.R.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Schindlbacher, A.; Hurry, V.; Lundmark, T.; et al. High Temporal Resolution Tracing of Photosynthate Carbon from the Tree Canopy to Forest Soil Microorganisms. New Phytol. 2008, 177, 220–228. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Ji, Y.; Zhao, J.; Gao, J. Nitrogen Deposition Affects the Productivity of Planted and Natural Forests by Modulating Forest Climate and Community Functional Traits. For. Ecol. Manag. 2024, 563, 121970. [Google Scholar] [CrossRef]
- Chen, Q.; Strashnov, I.; van Dongen, B.; Johnson, D.; Cox, F. Environmental Dependency of Ectomycorrhizal Fungi as Soil Organic Matter Oxidizers. New Phytol. 2024, 244, 2536–2547. [Google Scholar] [CrossRef]

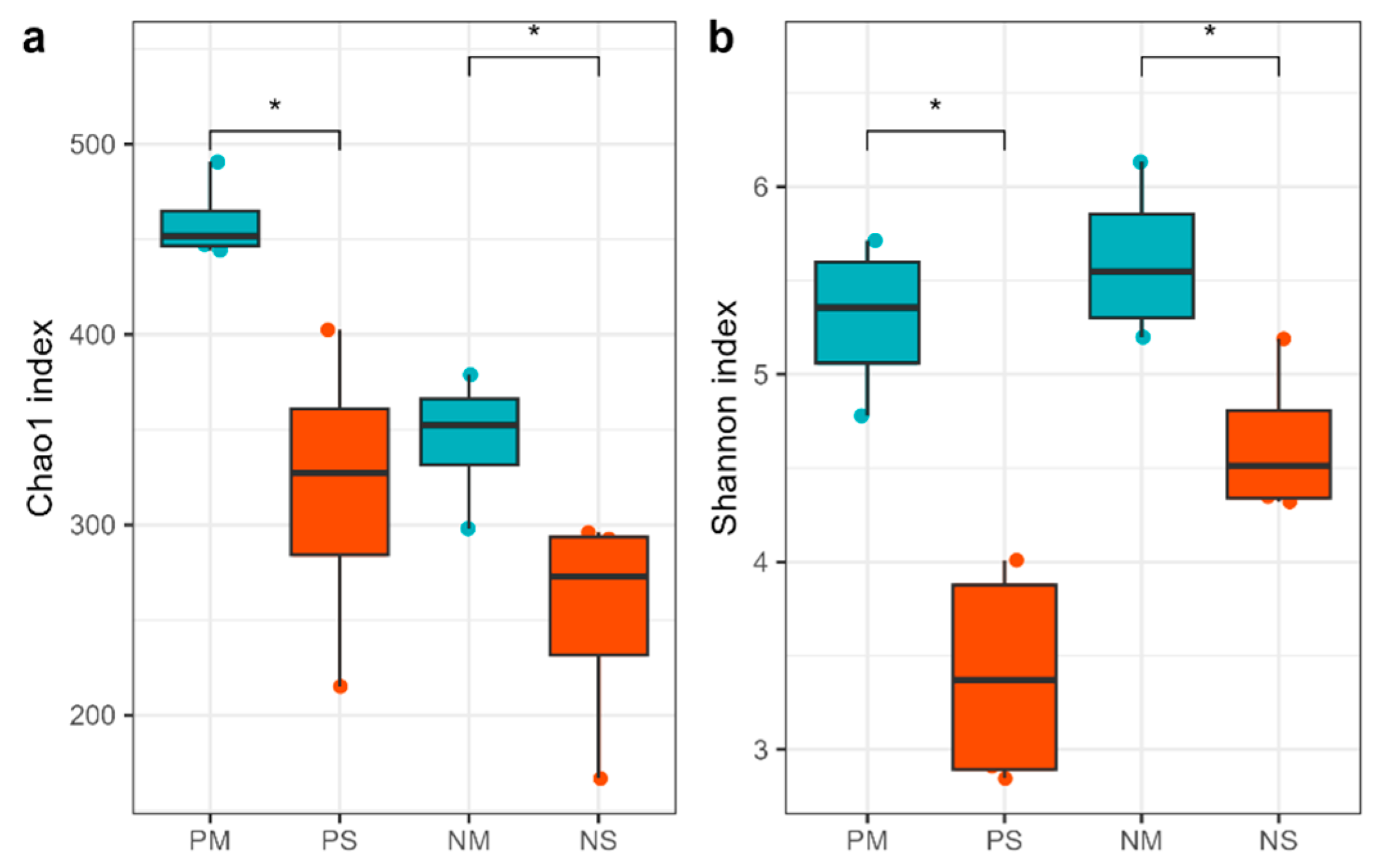
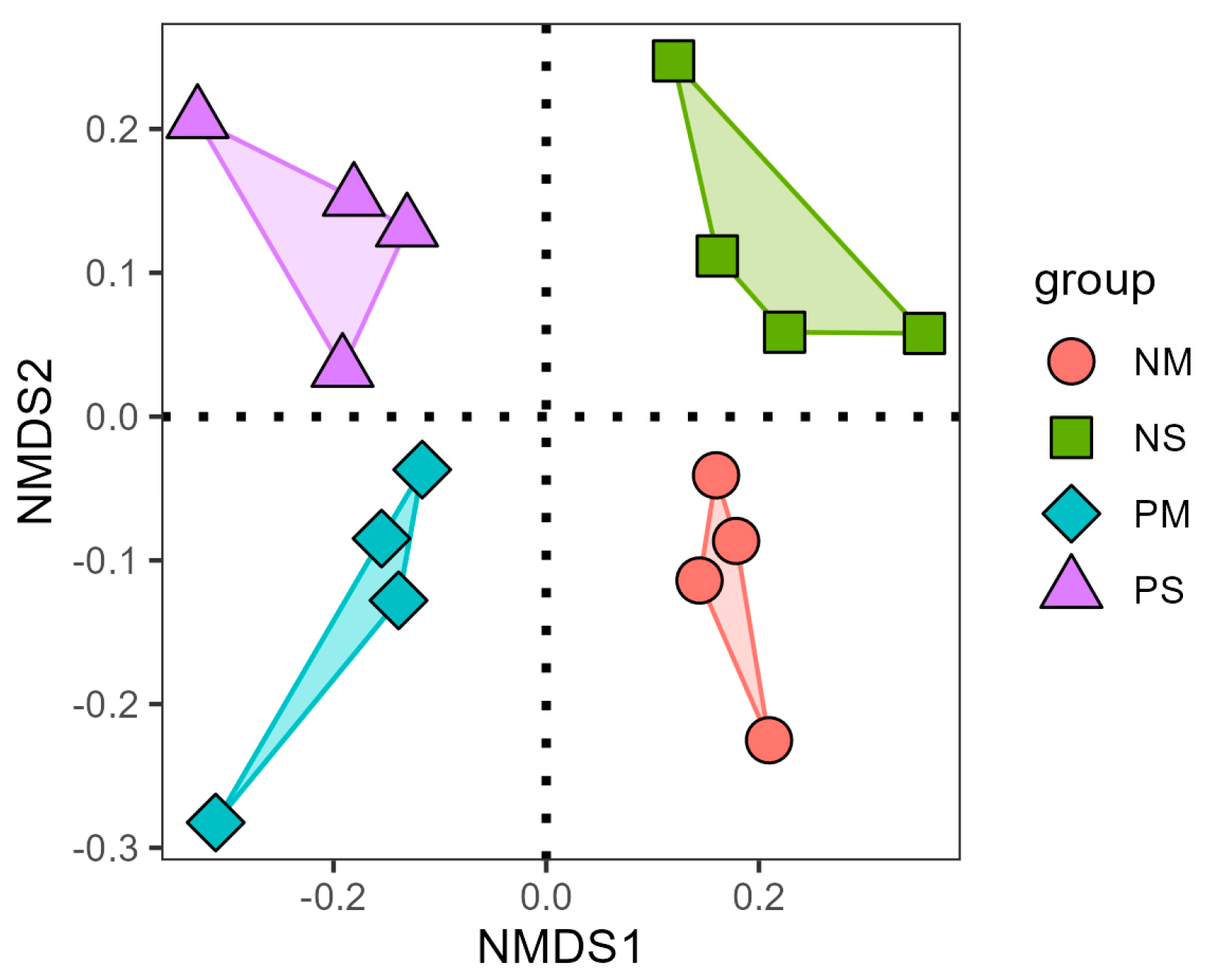
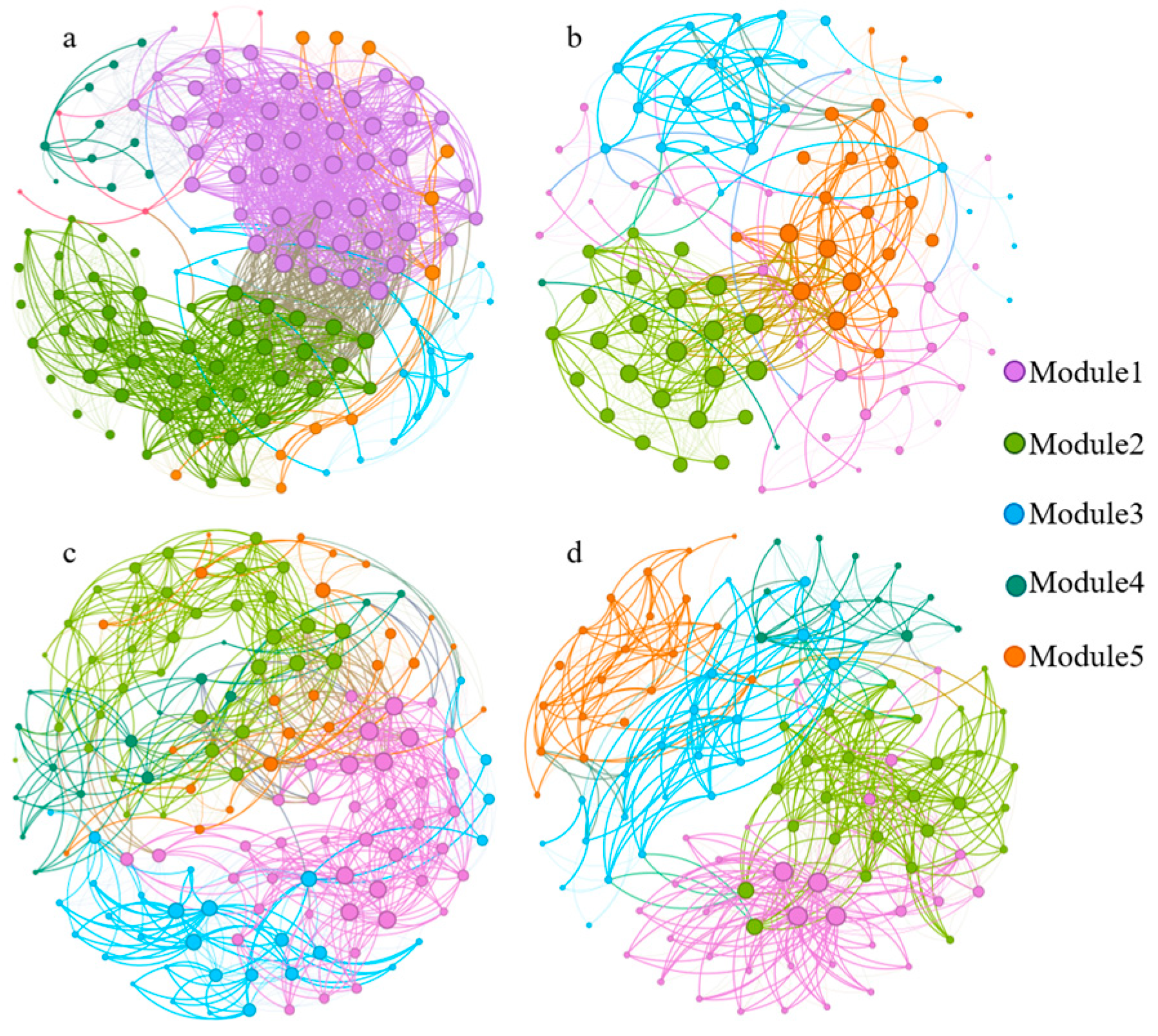
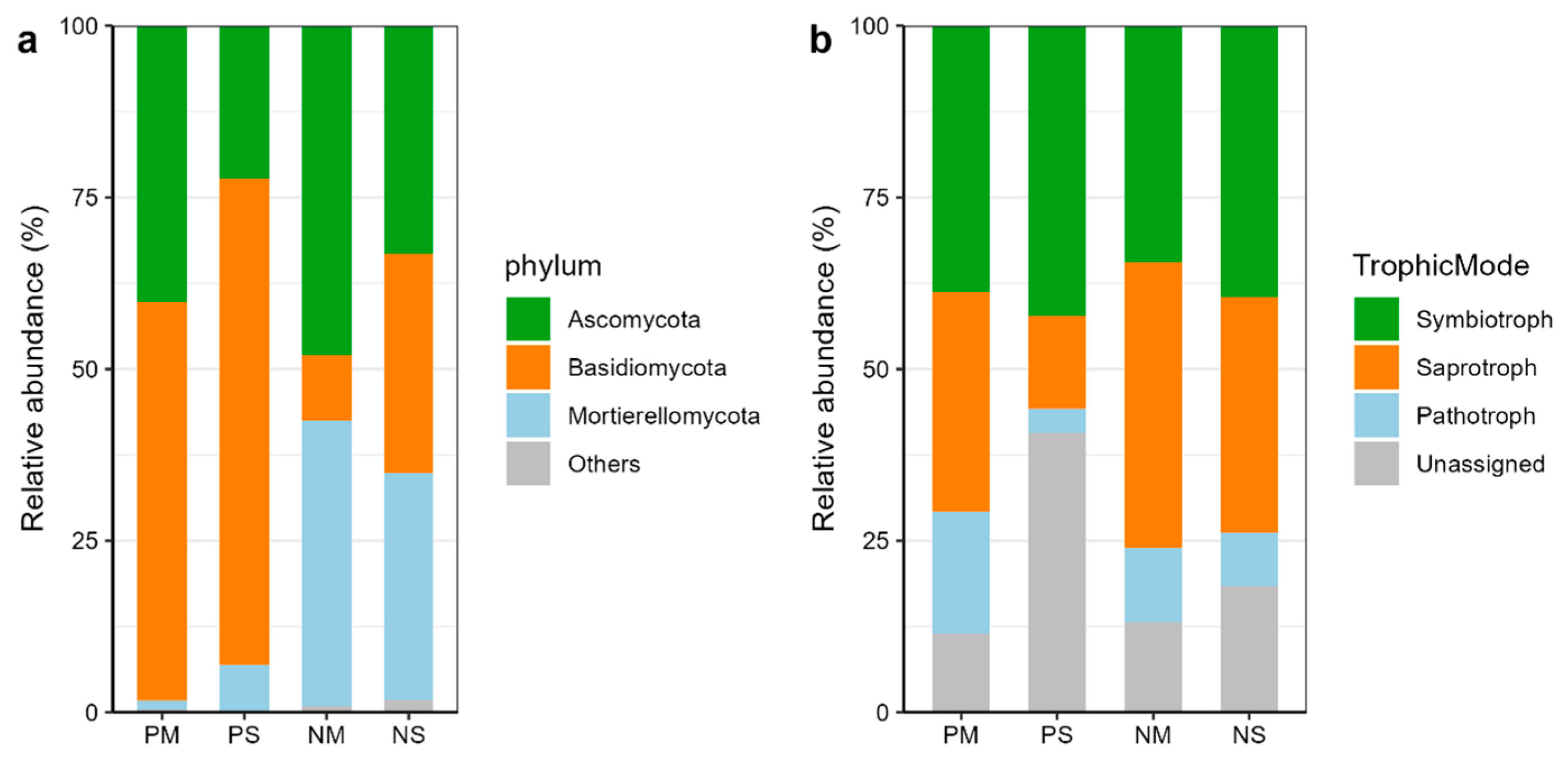
| Nodes | Edges | Positive Correlation | Negative Correlation | Average Degree | Diameter | RM | Average CC | |
|---|---|---|---|---|---|---|---|---|
| PM | 135 | 1308 | 1301 | 7 | 19.38 | 0.35 | 0.35 | 0.08 |
| PS | 100 | 439 | 360 | 79 | 8.78 | 0.22 | 0.50 | 0.28 |
| NM | 145 | 900 | 671 | 229 | 12.41 | 0.20 | 0.55 | 0.17 |
| NS | 113 | 641 | 410 | 231 | 11.35 | 0.19 | 0.62 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Sun, Q.; Liu, D. Moss Cover Modulates Soil Fungal Functional Communities and Nutrient Cycling in Alpine Forests. Forests 2025, 16, 138. https://doi.org/10.3390/f16010138
Wei M, Sun Q, Liu D. Moss Cover Modulates Soil Fungal Functional Communities and Nutrient Cycling in Alpine Forests. Forests. 2025; 16(1):138. https://doi.org/10.3390/f16010138
Chicago/Turabian StyleWei, Maolu, Qian Sun, and Dongyan Liu. 2025. "Moss Cover Modulates Soil Fungal Functional Communities and Nutrient Cycling in Alpine Forests" Forests 16, no. 1: 138. https://doi.org/10.3390/f16010138
APA StyleWei, M., Sun, Q., & Liu, D. (2025). Moss Cover Modulates Soil Fungal Functional Communities and Nutrient Cycling in Alpine Forests. Forests, 16(1), 138. https://doi.org/10.3390/f16010138







