Variations and Coordination of Leaflet and Petiole Functional Traits Within Compound Leaves in Three Hardwood Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Leaf Sample Collection
2.3. Leaf Morphology and Anatomy
2.4. Data Analysis
3. Results
3.1. Leaf Vascular Traits Along Phyllotaxy
3.2. Leaf Mesophyll Traits Along Phyllotaxy
3.3. Relative Share of Biomass and Leaflet Area Within Compound Leaf
4. Discussion
4.1. Vascular Network in Compound Leaves
4.2. Leaf Morphology and Anatomy Differentiation Along Phyllotaxy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mommer, L.; Weemstra, M. The role of roots in the resource economics spectrum. New Phytol. 2012, 195, 725–727. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Fan, X.; Yan, X.; Qian, C.; Bachir, D.G.; Yin, X.; Sun, P.; Ma, X.F. Leaf size variations in a dominant desert shrub, Reaumuria soongarica, adapted to heterogeneous environments. Ecol. Evol. 2020, 10, 10076–10094. [Google Scholar] [CrossRef]
- Baird, A.S.; Taylor, S.H.; Pasquet-Kok, J.; Vuong, C.; Zhang, Y.; Watcharamongkol, T.; Scoffoni, C.; Edwards, E.J.; Christin, P.; Osborne, C.P.; et al. Developmental and biophysical determinants of grass leaf size worldwide. Nature 2021, 592, 242–247. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Warman, L.; Moles, A.T.; Edwards, W. Not so simple after all: Searching for ecological advantages of compound leaves. Oikos 2011, 120, 813–821. [Google Scholar] [CrossRef]
- Bharathan, G.; Sinha, N.R. The regulation of compound leaf development. Plant Physiol. 2001, 127, 1533–1538. [Google Scholar] [CrossRef]
- Friedman, W.E.; Moore, R.C.; Purugganan, M.D. The evolution of plant development. Am. J. Bot. 2004, 91, 1726–1741. [Google Scholar] [CrossRef]
- Mzoughi, O.; Yahiaoui, I.; Boujemaa, N.; Zagrouba, E. Multiple leaflets-based identification approach for compound leaf species. In Proceedings of the International Workshop on Environmental Multimedia Retrieval (EMR 2014), Glasgow, UK, 1–4 April 2014; Available online: http://ceur-ws.org (accessed on 24 December 2024).
- Niinemets, U.; Portsmuth, A.; Tobias, M. Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol. 2006, 171, 91–104. [Google Scholar] [CrossRef]
- Niinemets, U. Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs. Plant Ecol. 1998, 134, 1–11. [Google Scholar] [CrossRef]
- Niinemets, U.; Kull, O. Biomass investment in leaf lamina versus lamina support in relation to growth irradiance and leaf size in temperate deciduous trees. Tree Physiol. 1999, 19, 349–358. [Google Scholar] [CrossRef]
- Levionnois, S.; Coste, S.; Nicolini, E.; Stahl, C.; Morel, H.; Heuret, P. Scaling of petiole anatomies, mechanics and vasculatures with leaf size in the widespread Neotropical pioneer tree species Cecropia obtusa Trécul (Urticaceae). Tree Physiol. 2020, 40, 245–258. [Google Scholar] [CrossRef]
- Niklas, K.J. Research review: A mechanical perspective on foliage leaf form and function. New Phytol. 1999, 143, 19–31. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Zhou, J.; Zhao, Z.; Zhang, S.; Bu, H.; Qi, W. Geographic variation in the petiole-lamina relationship of 325 eastern Qinghai-Tibetan woody species: Analysis in three dimensions. Front. Plant Sci. 2021, 12, 748125. [Google Scholar] [CrossRef]
- Rutishauser, R.; Peisl, P. Phyllotaxy. In Encyclopedia of Life Sciences; Macmillan Publishers Ltd.: Basingstoke, UK, 2001. [Google Scholar] [CrossRef]
- Vidaković, A.; Matijašević, S.; Tumpa, K.; Poljak, I. Leaf phenotypic plasticity of European ash (Fraxinus excelsior) at its northern range in Dinaric Alps. Acta Bot. Croat. 2025, 84, 1–18. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, G.; Liu, Z. Effects of phyllotaxy on variation and inner relationships of leaflet traits in compound-leaved plants. Chin. J. Appl. Ecol. 2023, 34, 577–587. (In Chinese) [Google Scholar]
- Wang, G.; Chen, B.; Huang, Y. Effects of growing position on leaflet trait variations and its correlations in Fraxinus mandshurica. Chin. J. Plant Ecol. 2022, 46, 712–721. (In Chinese) [Google Scholar] [CrossRef]
- McCulloh, K.A.; Sperry, J.S.; Meinzer, F.C.; Lachenbruch, B.; Atala, C. Murray’s law, the ‘Yarrum’ optimum, and the hydraulic architecture of compound leaves. New Phytol. 2009, 184, 234–244. [Google Scholar] [CrossRef]
- Franklin, O.; Fransson, P.; Hofhansl, F.; Jansen, S.; Joshi, J. Optimal balancing of xylem efficiency and safety explains plant vulnerability to drought. Ecol. Lett. 2023, 26, 1485–1496. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Mitchell, R.J.; Han, W.; Hendricks, J.J.; Fahey, T.J.; Hendrick, R.L. Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol. 2008, 177, 443–456. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. An Implementation of the Grammar of Graphics; Package ‘ggplot2’: Tokyo, Japan, 2016. [Google Scholar]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the structure and allometry of plant vascular systems. Nature 1999, 400, 664–667. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Water Balance of Plants. In Plant Physiology and Development; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Song, J.; Trueba, S.; Yin, X.; Cao, K.; Brodribb, T.J.; Hao, G. Hydraulic vulnerability segmentation in compound-leaved trees: Evidence from an embolism visualization technique. Plant Physiol. 2022, 189, 204–214. [Google Scholar] [CrossRef]
- Rimer, I.M.; McAdam, S.A.M. Within-leaf variation in embolism resistance is not a rule for compound-leaved angiosperms. Am. J. Bot. 2024, 111, e16447. [Google Scholar] [CrossRef]
- Klepsch, M.; Zhang, Y.; Kotowska, M.M.; Lamarque, L.J.; Nolf, M.; Schuldt, B.; Torres-Ruiz, J.M.; Qin, D.; Choat, B.; Delzon, S.; et al. Is xylem of angiosperm leaves less resistant to embolism than branches? Insights from microCT, hydraulics, and anatomy. J. Exp. Bot. 2018, 69, 5611–5623. [Google Scholar] [CrossRef]
- Peters, J.M.R.; Choat, B. Out on a limb: Testing the hydraulic vulnerability segmentation hypothesis in trees across multiple ecosystems. Plant Cell Environ. 2024, 1–16. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Skelton, R.P.; McAdam, S.A.; Bienaime, D.; Lucani, C.J.; Marmottant, P. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol. 2016, 209, 1403–1409. [Google Scholar] [CrossRef]
- Martín-Sánchez, R.; Sancho Knapik, D.; Ferrio, J.P.; Alonso Forn, D.; Losada, J.M.; Peguero Pina, J.J.; Mencuccini, M.; Gil Pelegrín, E. Xylem and phloem in petioles are coordinated with leaf gas exchange in oaks with contrasting anatomical strategies depending on leaf habit. Plant Cell Environ. 2024, 48, 1–18. [Google Scholar] [CrossRef]
- Ocheltree, T.W.; Gleason, S.M. Grass veins are leaky pipes: Vessel widening in grass leaves explain variation in stomatal conductance and vessel diameter among species. New Phytol. 2024, 241, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017, 40, 831–845. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yoda, K.; Kira, K.H.A.T. A quantitative analysis of plant form—The pipe model theory. Jpn. J. Ecol. 1964, 14, 97–105. [Google Scholar]
- Song, Y.; Zhu, J.; Yu, L.; Wang, K. Photosynthetic characteristics of Juglans mandshurica, Fraxinus mandshurica and Phellodendron amurense under different light regimes. Sci. Silvae Sin. 2009, 45, 29–36. (In Chinese) [Google Scholar]
- Li, G.; Yang, D.; Sun, S. Allometric relationships between lamina area, lamina mass and petiole mass of 93 temperate woody species vary with leaf habit, leaf form and altitude. Funct. Ecol. 2008, 22, 557–564. [Google Scholar] [CrossRef]
- Yoshinaka, K.; Nagashima, H.; Yanagita, Y.; Hikosaka, K. The role of biomass allocation between lamina and petioles in a game of light competition in a dense stand of an annual plant. Ann. Bot. 2018, 121, 1055–1064. [Google Scholar] [CrossRef]
- Perez, R.P.A.; Dauzat, J.; Pallas, B.; Lamour, J.; Verley, P.; Caliman, J.; Costes, E.; Faivre, R. Designing oil palm architectural ideotypes for optimal light interception and carbon assimilation through a sensitivity analysis of leaf traits. Ann. Bot. 2017, 121, 909–926. [Google Scholar] [CrossRef]
- Roig-Villanova, I.; Martínez-García, J.F. Plant responses to vegetation proximity: A whole life avoiding shade. Front Plant Sci. 2016, 7, 236. [Google Scholar] [CrossRef]
- Bell, D.L.; Galloway, L.F. Plasticity to neighbour shade: Fitness consequences and allometry. Funct. Ecol. 2007, 21, 1146–1153. [Google Scholar] [CrossRef]
- Santiago, L.S.; Kitajima, K.; Wright, S.J.; Mulkey, S.S. Coordinated changes in photosynthesis, water relations and leaf nutritional traits of canopy trees along a precipitation gradient in lowland tropical forest. Oecologia 2004, 139, 495–502. [Google Scholar] [CrossRef]
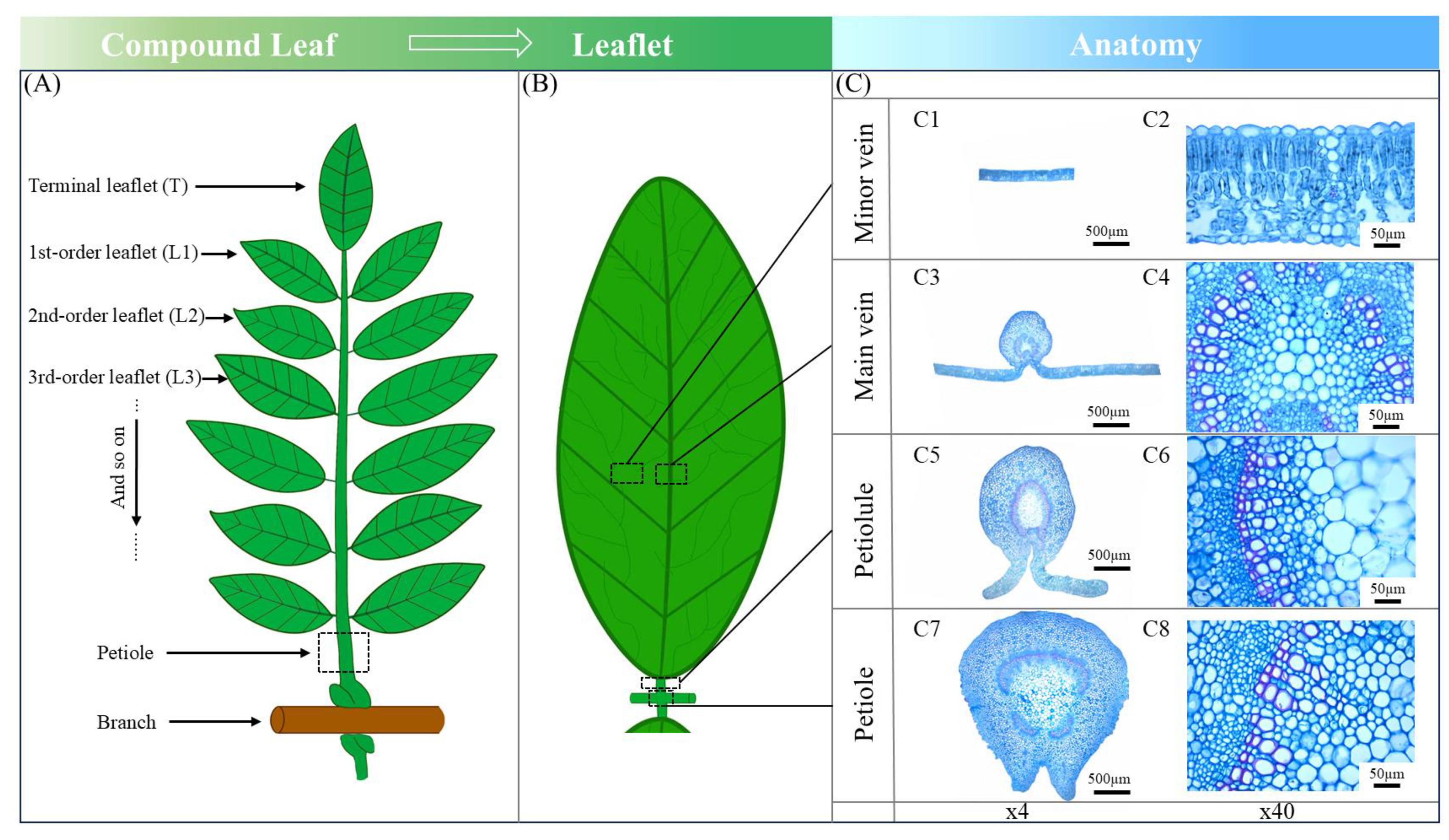
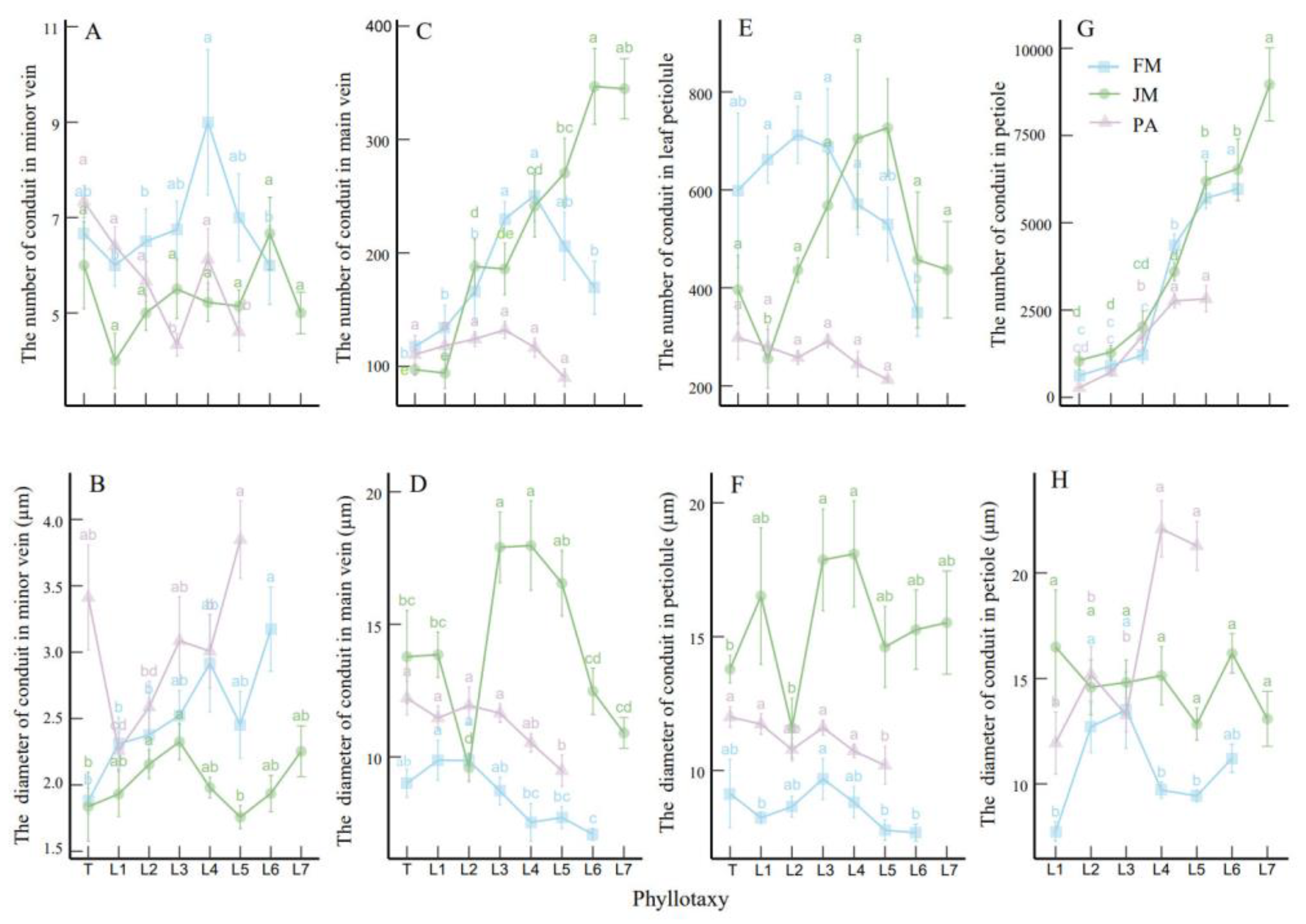



| Trait | Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraxinus mandshurica | Juglans mandshurica | Phellodendron amurense | ||||||||||
| Mean | Maximum | Minimum | CV | Mean | Maximum | Minimum | CV | Mean | Maximum | Minimum | CV | |
| NC-minor | 6.85 | 9.00 | 6.00 | 0.149 | 5.32 | 6.67 | 4.00 | 0.148 | 5.74 | 7.30 | 4.60 | 0.189 |
| NC-main | 181.82 | 250.71 | 117.33 | 0.270 | 220.98 | 347.78 | 94.00 | 0.447 | 115.17 | 132.11 | 90.00 | 0.125 |
| NC-PL | 587.07 | 712.50 | 348.80 | 0.211 | 497.66 | 726.67 | 255.00 | 0.321 | 264.00 | 298.33 | 212.60 | 0.123 |
| NC-P | 3127.58 | 5974.00 | 614.67 | 0.798 | 4229.52 | 8963.67 | 1037.80 | 0.720 | 1666.25 | 2821.68 | 277.00 | 0.696 |
| DC-minor | 2.52 | 3.17 | 1.87 | 0.861 | 2.02 | 2.33 | 1.76 | 0.100 | 3.03 | 3.85 | 2.26 | 0.187 |
| DC-main | 8.54 | 9.88 | 9.00 | 0.085 | 14.13 | 17.97 | 13.78 | 0.222 | 11.56 | 11.94 | 12.22 | 0.055 |
| DC-PL | 8.57 | 9.69 | 9.14 | 0.131 | 15.41 | 18.09 | 13.79 | 0.140 | 11.17 | 12.00 | 10.20 | 0.063 |
| DC-P | 10.72 | 13.51 | 7.74 | 0.039 | 14.73 | 16.49 | 16.19 | 0.095 | 16.75 | 22.10 | 11.94 | 0.278 |
| PT | 79.70 | 83.53 | 72.67 | 0.053 | 51.64 | 60.03 | 38.19 | 0.140 | 56.84 | 67.89 | 49.97 | 0.114 |
| ST | 49.36 | 51.52 | 46.09 | 0.035 | 21.55 | 24.87 | 15.74 | 0.134 | 56.84 | 67.89 | 49.97 | 0.114 |
| PT/ST | 1.62 | 1.72 | 1.49 | 0.055 | 2.43 | 2.94 | 1.77 | 0.155 | 1.07 | 1.22 | 1.87 | 0.121 |
| CTR | 0.50 | 0.52 | 0.48 | 0.033 | 0.56 | 0.60 | 0.49 | 0.066 | 0.40 | 0.44 | 0.37 | 0.062 |
| SR | 0.31 | 0.34 | 0.30 | 0.039 | 0.23 | 0.29 | 0.20 | 0.115 | 0.38 | 0.43 | 0.34 | 0.075 |
| LT | 157.68 | 162.02 | 150.27 | 0.032 | 92.21 | 105.56 | 77.53 | 0.110 | 146.99 | 157.58 | 132.35 | 0.061 |
| PLB | 12.50 | 21.26 | 4.61 | 0.004 | 12.35 | 17.85 | 5.50 | 0.004 | 14.29 | 18.84 | 7.83 | 0.003 |
| PLA | 14.29 | 19.74 | 6.20 | 0.004 | 14.29 | 21.53 | 6.40 | 0.004 | 16.67 | 21.59 | 10.46 | 0.003 |
| SLA | 23.30 | 25.25 | 20.02 | 0.077 | 17.73 | 19.03 | 17.06 | 0.040 | 20.45 | 22.03 | 18.62 | 0.062 |
| Source of Variation | F Values | p Values | ||
|---|---|---|---|---|
| Number of Conduit | Diameter of Conduit | Number of Conduit | Diameter of Conduit | |
| Species | 31.057 | 114.606 | <0.001 | <0.001 |
| LP | 1482.648 | 453.755 | <0.001 | <0.001 |
| PI | 130.217 | 21.732 | <0.001 | <0.001 |
| Species × LP | 13.453 | 148.993 | <0.001 | <0.001 |
| Species × PI | 4.623 | 27.124 | <0.001 | <0.001 |
| LP × PI | 110.554 | 13.038 | <0.001 | <0.001 |
| Species × LP × PI | 3.531 | 21.499 | <0.001 | <0.001 |
| Source of Variation | F Values | p Values | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | ST | PT/ST | CTR | SR | LT | PLB | PLA | SLA | PT | ST | PT/ST | CTR | SR | LT | PLB | PLA | SLA | |
| Species | 169.713 | 322.066 | 184.806 | 144.976 | 214.479 | 390.521 | 1.197 | 1.575 | 33.523 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.139 | 0.208 | <0.001 |
| PI | 5.009 | 2.495 | 3.944 | 3.940 | 2.806 | 4.587 | 32.618 | 21.266 | 3.304 | <0.001 | 0.020 | <0.001 | <0.001 | 0.010 | <0.001 | <0.001 | <0.001 | 0.021 |
| Species × PI | 2.400 | 1.607 | 3.570 | 2.822 | 2.615 | 2.106 | 3.462 | 2.724 | 0.386 | 0.009 | 0.105 | <0.001 | 0.003 | 0.005 | 0.025 | <0.001 | 0.002 | 0.957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhang, J.; Gu, J.; Li, Z.; Wang, Y. Variations and Coordination of Leaflet and Petiole Functional Traits Within Compound Leaves in Three Hardwood Species. Forests 2025, 16, 139. https://doi.org/10.3390/f16010139
Guo X, Zhang J, Gu J, Li Z, Wang Y. Variations and Coordination of Leaflet and Petiole Functional Traits Within Compound Leaves in Three Hardwood Species. Forests. 2025; 16(1):139. https://doi.org/10.3390/f16010139
Chicago/Turabian StyleGuo, Xiaohui, Jinshan Zhang, Jiacun Gu, Zhongyue Li, and Yan Wang. 2025. "Variations and Coordination of Leaflet and Petiole Functional Traits Within Compound Leaves in Three Hardwood Species" Forests 16, no. 1: 139. https://doi.org/10.3390/f16010139
APA StyleGuo, X., Zhang, J., Gu, J., Li, Z., & Wang, Y. (2025). Variations and Coordination of Leaflet and Petiole Functional Traits Within Compound Leaves in Three Hardwood Species. Forests, 16(1), 139. https://doi.org/10.3390/f16010139







