Detection of Hymenoscyphus fraxineus in Leaf Rachises from European Ash (Fraxinus excelsior) in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Sampling
2.2. Fungal Isolation
2.3. DNA Extraction, PCR and Sequencing
2.4. Phylogeny
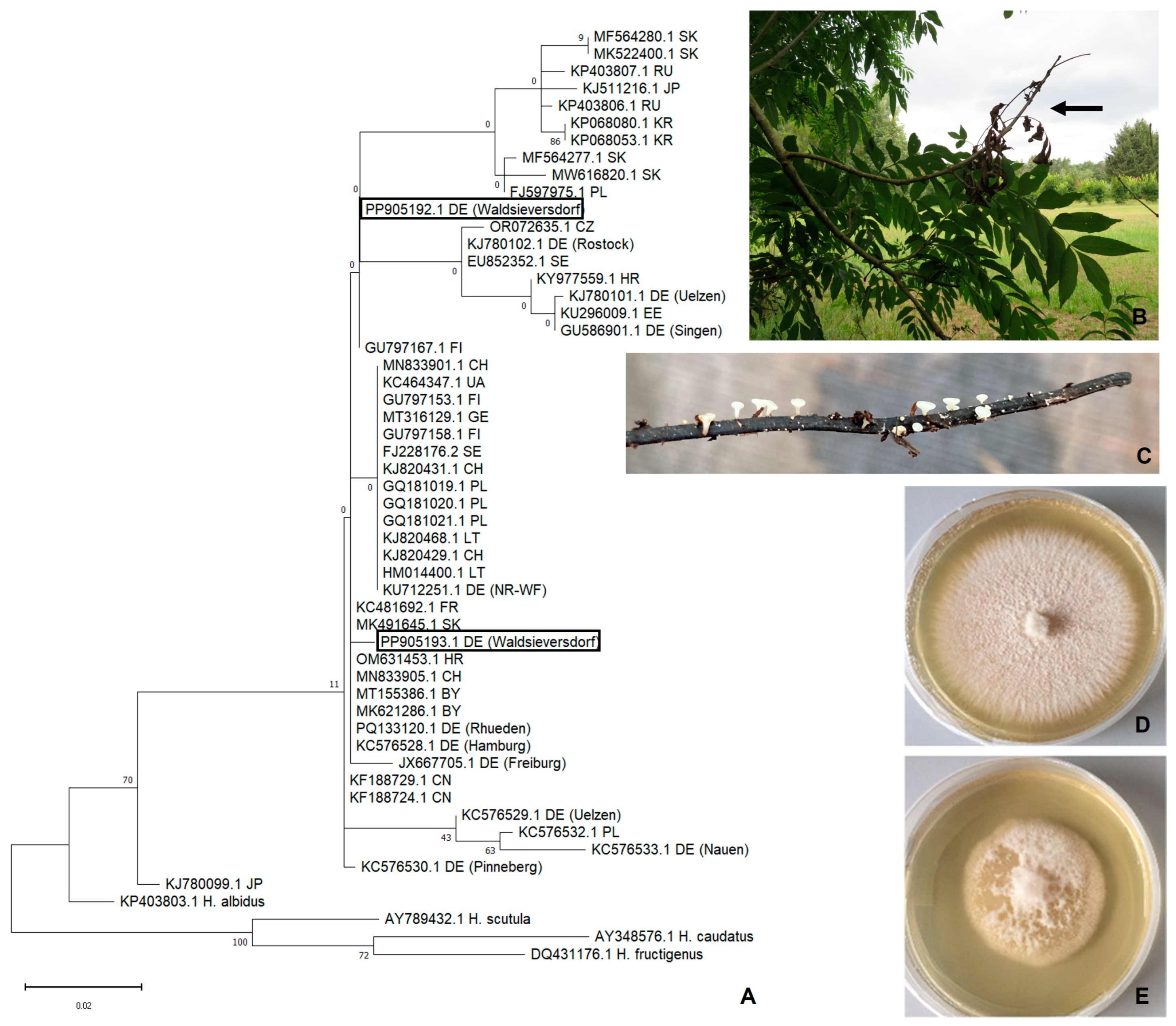
3. Results
3.1. Fungal Isolates
3.2. Sequence Analysis
3.3. Phylogeny
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalski, T. Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior) in Poland. For. Pathol. 2006, 36, 264–270. [Google Scholar] [CrossRef]
- Kowalski, T.; Holdenrieder, O. The teleomorph of Chalara fraxinea, the causal agent of ash dieback. For. Pathol. 2009, 39, 304–308. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef]
- McMullan, M.; Rafiqi, M.; Kaithakottil, G.; Clavijo, B.J.; Bilham, L.; Orton, E.; Percival-Alwyn, L.; Ward, B.J.; Edwards, A.; Saunders, D.G.O.; et al. The ash dieback invasion of Europe was founded by two genetically divergent individuals. Nat. Ecol. Evol. 2018, 2, 1000–1008. [Google Scholar] [CrossRef]
- Carroll, D.; Boa, E. Ash dieback: From Asia to Europe. Plant Pathol. 2024, 73, 741–759. [Google Scholar] [CrossRef]
- Elfstrand, M.; Chen, J.; Cleary, M.; Halecker, S.; Ihrmark, K.; Karlsson, M.; Davydenko, K.; Stenlid, J.; Stadler, M.; Brandström Durling, M. Comparative analyses of the Hymenoscyphus fraxineus and Hymenoscyphus albidus genomes reveals potentially adaptive differences in secondary metabolite and transposable element repertoires. BMC Genom. 2021, 22, 503. [Google Scholar] [CrossRef] [PubMed]
- Lygis, V.; Vasiliauskas, R.; Larsson, K.H.; Stenlid, J. Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Schumacher, J.; Kehr, R.; Leonhard, S.; Wulf, A. New details on the pathogenesis of ash dieback. J. Cultiv. Plants 2010, 62, 1–9. [Google Scholar]
- Marçais, B.; Giraudel, A.; Husson, C. Ability of the ash dieback pathogen to reproduce and to induce damage on its host are controlled by different environmental parameters. PLoS Pathog. 2024, 19, e1010558. [Google Scholar] [CrossRef]
- Kirisitis, T.; Cech, T.L. Beobachtungen zum sexuellen Stadium des Eschentriebsterben-Erregers Chalara fraxinea in Österreich. Forstsch. Aktuell 2009, 48, 21–25. [Google Scholar]
- Cleary, M.R.; Daniel, G.; Stenlid, J. Light and scanning electron microscopy studies of the early infection stages of Hymenoscyphus pseudoalbidus on Fraxinus excelsior. Plant Pathol. 2013, 62, 1294–1301. [Google Scholar] [CrossRef]
- Langer, G. Collar Rots in Forests of Northwest Germany Affected by Ash Dieback. Balt. For. 2017, 23, 4–19. [Google Scholar]
- Enderle, R.; Fussi, B.; Lenz, H.; Langer, G.; Nagel, R.; Metzler, B. Ash dieback in Germany: Research on disease development, resistance and management options. In Dieback of European Ash (Fraxinus spp.): Consequences and Guidelines for Sustainable Management; Vaisatis, R., Enderle, R., Eds.; SLU: Uppsala, Sweden, 2017; pp. 89–105. [Google Scholar]
- Griffiths, S.M.; Galambao, M.; Rowntree, J.; Goodhead, I.; Hall, J.; O’Brien, D.; Atkinson, N.; Antwis, R.E. Complex associations between cross—Kingdom microbial endophytes and host genotype in ash dieback disease dynamics. J. Ecol. 2020, 108, 291–309. [Google Scholar] [CrossRef]
- Becker, R.; Ulrich, K.; Behrendt, U.; Kube, M.; Ulrich, A. Analyzing ash leaf-colonizing fungal communities for their biological control of Hymenoscyphus fraxineus. Front. Microbiol. 2020, 11, 590944. [Google Scholar] [CrossRef]
- Ulrich, K.; Becker, R.; Behrendt, U.; Kube, M.; Ulrich, A. A Comparative Analysis of Ash Leaf-Colonizing Bacterial Communities Identifies Putative Antagonists of Hymenoscyphus fraxineus. Front. Microbiol. 2020, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Ulrich, K.; Behrendt, U.; Schneck, V.; Ulrich, A. Genomic characterization of Aureimonas altamirensis C2P003—A specific member of the microbiome of Fraxinus excelsior trees tolerant to ash dieback. Plants 2022, 11, 3487. [Google Scholar] [CrossRef] [PubMed]
- Heydeck, P.; Bemmann, M.; Kontzog, H.G. Triebsterben an Gemeiner Esche (Fraxinus excelsior) im nordostdeutschen Tiefland. Forst Holz 2005, 60, 505–506. [Google Scholar]
- Wulf, A.; Schumacher, J. Die Waldschutzsituation 2005 in der Bundesrepublik Deutschland. Forst Holz 2005, 60, 503–505. [Google Scholar]
- Schumacher, J.; Wulf, A.; Leonhard, S. Erster Nachweis von Chalara fraxinea T. Kowalski sp. nov. in Deutschland-ein Verursacher neuartiger Schäden an Eschen. Nachrichtenblatt Dtsch. Pflanzenschutzd. 2007, 59, 121–123. [Google Scholar]
- Leonhard, S.; Straßer, L.; Nannig, A.; Blaschke, M.; Schumacher, J.; Immler, T. Neues Krankheitsphänomen an der Esche.Das von Chalara fraxinea verursachte Eschentriebsterben ist auch in Bayern nachgewiesen. LWF Aktuell 2009, 71, 60–63. [Google Scholar]
- Metzler, B.; Enderle, R.; Karopka, M.; Töpfner, K.; Aldinge, E. Entwicklung des Eschentriebsterbens in einem Herkunftsversuch an verschiedenen Standorten in Süddeutschland. Allg. Forst Jagdztg. 2012, 183, 168–180. [Google Scholar]
- Enderle, R.; Peters, F.; Nakou, A.; Metzler, B. Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of Fraxinus excelsior. Eur. J. For. Res. 2013, 132, 865–876. [Google Scholar] [CrossRef]
- Fussi, B.; Konnert, M. Genetic analysis of European common ash (Fraxinus excelsior L.) populations affected by ash dieback. Silvae Gen. 2014, 63, 198–212. [Google Scholar] [CrossRef]
- Enderle, R.; Metzler, B.; Riemer, U.; Kändler, G. Ash dieback on sample points of the national forest inventory in South-Western Germany. Forests 2018, 9, 25. [Google Scholar] [CrossRef]
- Langer, G.J.; Fuchs, S.; Osewold, J.; Peters, S.; Schrewe, F.; Ridley, M.; Kätzel, R.; Bubner, B.; Grüner, J. FraxForFuture—Research on European ash dieback in Germany. J. Plant Dis. Prot. 2022, 129, 1285–1295. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Drenkhan, R.; Riit, T.; Adamson, K.; Hanso, M. The earliest samples of Hymenoscyphus albidus vs. H. fraxineus in Estonian mycological herbaria. Mycol. Prog. 2016, 15, 835–844. [Google Scholar] [CrossRef]
- Drenkhan, R.; Solheim, H.; Bogacheva, A.; Riit, T.; Adamson, K.; Drenkhan, T.; Maaten, T.; Hietala, A.M. Hymenoscyphus fraxineus is a leaf pathogen of local Fraxinus species in the Russian Far East. Plant Pathol. 2017, 66, 490–500. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Aime, M.C.; Rokas, A.; Pandey, B.; Li, Y.; Pfister, D.H. Extensive intragenomic variation in the internal transcribed spacer region of fungi. iScience 2023, 26, 107317. [Google Scholar] [CrossRef]
- Paloi, S.; Luangsa-ard, J.J.; Mhuantong, W.; Stadler, M.; Kobmoo, N. Intragenomic variation in nuclear ribosomal markers and its implication in species delimitation, identification and barcoding in fungi. Fungal Biol. Rev. 2022, 42, 1–33. [Google Scholar] [CrossRef]
- Bengtsson, S.B.K.; Vasaitis, R.; Kirisitis, T.; Solheim, H.; Stenlid, J. Population structure of Hymenoscyphus pseudoalbidus and its genetic relationship to Hymenoscyphus albidus. Fungal Ecol. 2012, 5, 147–153. [Google Scholar] [CrossRef]
- Kowalski, T.; Kraj, W.; Bednarz, B. Fungi on stems and twigs in initial and advanced stages of dieback of European ash (Fraxinus excelsior) in Poland. Eur. J. For. Res. 2016, 135, 565–579. [Google Scholar] [CrossRef]
- Cross, H.; Sønstebø, J.H.; Nagy, N.E.; Timmermann, V.; Solheim, H.; Børja, I.; Kauserud, H.; Carlsen, T.; Rzepka, B.; Wasak, K.; et al. Fungal diversity and seasonal succession in ash leaves infected by the invasive ascomycete Hymenoscyphus fraxineus. New Phytol. 2016, 213, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Orton, E.S.; Brasier, C.M.; Bilham, L.J.; Bansal, A.; Webber, J.F.; Brown, J.K.M. Population structure of the ash dieback pathogen, Hymenoscyphus fraxineus, in relation to its mode of arrival in the UK. Plant Pathol. 2018, 67, 255–264. [Google Scholar] [CrossRef]
- Kowalski, T.; Bartnik, C. Morphological variation in colonies of Chalara fraxinea isolated from ash (Fraxinus excelsior) stems with symptoms of dieback and effects of temperature on colony growth and structure. Acta Agrobot. 2010, 63, 99–106. [Google Scholar] [CrossRef]
- Johansson, S.B.K.; Vasaitis, R.; Ihrmark, K.; Barklund, P.; Stenlid, J. Detection of Chalara fraxinea from tissue of Fraxinus excelsior using species—Specific ITS primers. For. Pathol. 2010, 40, 111–115. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organisation. Hymenoscyphus pseudoalbidus. Bull. OEPP/EPPO Bull. 2013, 43, 449–461. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Enderle, R.; Nakou, A.; Thomas, K.; Metzler, B. Susceptibility of autochthonous German Fraxinus excelsior clones to Hymenoscyphus pseudoalbidus is genetically determined. Ann. For. Sci. 2015, 72, 183–193. [Google Scholar] [CrossRef]
- Kraj, W.; Kowalski, T. Genetic variability of Hymenoscyphus pseudoalbidus on ash leaf rachises in leaf litter of forest stands in Poland. J. Phytopathol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Stadler, M.; Lambert, C.; Wibberg, D.; Kalinowski, J.; Cox, R.J.; Kolařík, M.; Kuhnert, E. Intragenomic polymorphisms in the ITS region of high-quality genomes of the Hypoxylaceae (Xylariales, Ascomycota). Mycol. Prog. 2020, 19, 235–245. [Google Scholar] [CrossRef]
- Burokiene, D.; Prospero, S.; Jung, E.; Marciulyniene, D.; Moosbrugger, K.; Norkute, G.; Rigling, D.; Lygis, V.; Schoebel, C.N. Genetic population structure of the invasive ash dieback pathogen Hymenoscyphus fraxineus in its expanding range. Biol. Invasions 2015, 17, 2743–2756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisold, A.-M.E.; Bubner, B.; Blunk, V.; Schneck, V. Detection of Hymenoscyphus fraxineus in Leaf Rachises from European Ash (Fraxinus excelsior) in Germany. Forests 2025, 16, 149. https://doi.org/10.3390/f16010149
Eisold A-ME, Bubner B, Blunk V, Schneck V. Detection of Hymenoscyphus fraxineus in Leaf Rachises from European Ash (Fraxinus excelsior) in Germany. Forests. 2025; 16(1):149. https://doi.org/10.3390/f16010149
Chicago/Turabian StyleEisold, Anne-Mareen E., Ben Bubner, Viktoria Blunk, and Volker Schneck. 2025. "Detection of Hymenoscyphus fraxineus in Leaf Rachises from European Ash (Fraxinus excelsior) in Germany" Forests 16, no. 1: 149. https://doi.org/10.3390/f16010149
APA StyleEisold, A.-M. E., Bubner, B., Blunk, V., & Schneck, V. (2025). Detection of Hymenoscyphus fraxineus in Leaf Rachises from European Ash (Fraxinus excelsior) in Germany. Forests, 16(1), 149. https://doi.org/10.3390/f16010149





