Abstract
Forests have a key role in mitigating both non-biological and biological ecological disturbances. However, major disturbances (soil pollution, shift from native forest species to exoticones, forested watersheds and climate changes) can have different impacts on a forest’s soil microbiome. Because the soil microbial community of forests has a key role in a variety of ecosystem services that promote the forest’s health, this review tries to answer the following questions: (i) Which are the main ecological disturbances that drive the responses of the forest soil microbiome? (ii) How can we measure these changes? For this aim, the review summarizes details on the tree vegetation type, the microbial communities in forest ecosystems, and the mutual influence between plants, soil, and microbiomes. Microbial communities are shaped by factors such as soil type and composition, plant and vegetation types, nutrient levels and soil fertility, disturbance patterns, symbiotic associations, biotic interactions, and the progression of forest succession. Anthropogenic activities produce a rapid response in the microbial communities, leading to both short- and long-term alterations. Harvesting processes reduce drastically the microbiome diversity, forcing a shift from specialized to more generalist microorganisms. Restoration scenarios indicate a re-establishment of microbial communities to a level similar to the native forest, but with a high percentage of replaced native microorganisms. This review emphasizes that the forest soil microbiome is shaped by a range of environmental, ecological, and biotic factors. The primary drivers of the soil microbiome in forest ecosystems discussed in this review include soil composition and nutrient availability, plant community structure, microbial interactions within the soil, disturbances, succession, and temporal dynamics. When considered together, these factors interact in complex ways, influencing the diversity, function, and resilience of the soil microbiome in forest ecosystems.
1. Introduction
The biosphere’s health relies mainly on forest ecosystems that regulate essential nutrient and energy patterns, which, in turn, can significantly influence the climate system [1]. Undisturbed forest landscapes, unaffected by human pressures, serve as significant carbon sinks, mitigating greenhouse gas emissions. On the other hand, tropical deforested regions and poorly managed forests can exacerbate climate change [2,3,4,5]. Forest ecosystems are important not only for regulating the climate but also because they include a vast biodiversity that holds crucial ecological and economic value. For this reason, the United Nations General Assembly proclaimed 21 March as the International Day of All Types of Forests in 2012, and the specific 2023 theme was ”Forests and Health”.
The aim is to emphasize the role of healthy forests for healthy people, in the framework also of the “One Health” concept (UN, 2023, https://www.un.org/en/un-chronicle/healthy-forests-are-crucial-human-health-and-sustainable-development, accessed on 1 June 2024) and in line with the sustainable development goals (SDG 15, https://www.un.org/en/chronicle/article/goal-15-seeing-forest-trees-making-most-synergies-achieve-sdgs-constrained-environment#:~:text=A%20case%20in%20point%20is,degradation%20and%20halt%20biodiversity%20loss%E2%80%9D, accessed on 1 June 2024). Current human management practices like deforestation, agriculture on a large scale, small-scale farming, and the development of infrastructures are unfortunately leading to rapid changes in forest biodiversity. Moreover, as forests increasingly become a source of renewable materials and fuels, their economic significance has been growing, forcing their harvesting in several parts of the planet. It is thus imperative to adopt conservation and sustainable management strategies to preserve the functionality of forests, protect global biodiversity and, at the same time, match the socio-economic needs [6,7].
Belowground microbiota, which includes soil, the rhizosphere, and root-associated microbial communities, is crucial for sustaining the physiology and the growth of plants [8]. These beneficial effects depend on nutrient-absorbing processes that increase nutrient uptake efficiency (plant growth enhancement) and protect against various negative abiotic and biotic conditions [8]. Moreover, specific bacteria and fungi interconnected with perennial tree crops interface with soil invertebrate food webs, supporting several regulation mechanisms [9]. On the other hand, trees have also a great influence on soil microbiota diversity, functioning, and fitness. For example, it was recently found that tree diversity has a positive influence on soil microbial resistance to drought [10]. Nevertheless, knowledge of how microbial communities of soil react to forest management is still very scarce. It has been well known that important biomarkers of forest ecosystem status are microbial community structure, their patterns of gene expression, and the metabolic activities. In fact, these factors can increase the capability to observe the health of forest ecosystems, to assess the effects of management practices, and in some way, to identify variations in nutrient and energy flow patterns before they have permanent effects [11].
The use of community profiling approaches such as molecular, biochemical, and physiological techniques have advanced the knowledge on the role of microbial diversity in forest soils. It was recently found that forest type influences microbial community composition and the inter- and intra-ecosystem variability of communities between and within forest ecosystems and that microbial communities respond to disturbance and forest management activities. Finally, numerous works highlight the relationship between microbial community and forest soil processes [12,13,14].
Even though different long-term responses to forest disturbance are observed in bacteria and fungi, several studies validate definite changes in the responses of important bacterial and fungal functional groups (e.g., nitrifying bacteria and mycorrhizal fungi), and indicate that both microbial groups play important functions in the long-term modifications of biogeochemical processes detected as a result of disturbances to forests [15,16].
Understanding the factors that drive the forest soil microbiome has become a prominent research topic worldwide [17]. Soil microbial communities have pivotal functions in promoting soil health by contributing to organic matter turnover and nutrient cycling. As an indispensable part of the soil ecosystem, the soil microbiome drives processes such as soil formation, fertility, plant growth and stress tolerance, as well as the nutrient cycles and carbon storage [18]. In forests, soil microbes contribute to forest ecosystem health by decomposing organic matter, cycling carbon and nutrients, incorporating humic compounds into mineral soils, and connecting plant and ecosystem functions. Moreover, they have a crucial role in carbon storage and are responsible for plant photosynthetic carbon inputs to the soil [19]. However, soil microbial communities are highly sensitive to changes in forest land use, and the impact of such changes on these communities remains poorly understood despite their functional significance [20,21]. Recently, Baldrian et al. [5] indicated that, although significant research progress in the forest microbial ecology framework has taken place, there are still many gaps and challenges that researchers face. Among others, some key limitations and challenges in this field are the complexity of microbial communities, spatial and temporal variation, limited knowledge on microbial functions, methodological constraints, a lack of long-term data, climate change and disturbances, data integration, and collaboration [7]. However, by addressing these issues and working collaboratively, scientists can make significant strides in unraveling the intricate relationships between microorganisms and forest ecosystems, ultimately aiding in forest conservation and management efforts.
The holobiont concept fits into the better understanding of forest ecosystems and their future evolution. The terms holobiont refers to a host organism (in this case the plant) and its associated microbial communities, collectively functioning as a unit [22]. This concept have been challenged the traditional view of organisms as individuals and emphasizes the importance of symbiotic relationships between a host and its microbiome [23]. The microbiome includes bacteria, archaea, viruses, fungi, and other microorganisms that inhabit a specific environment, such as the surface of plant roots or the internal parts of the plants. The holobiont concept suggests that the host and its associated microbiome should be considered as a single ecological and evolutionary unit [24]. Thus, the interactions within the holobiont can play a crucial role in the adaptation and response of the organism to ecological pressures. The microbiome can contribute to the host organism’s ability to adapt to environmental changes. For example, certain microbes in the soil can enhance a plant’s resistance to pathogens or help it acquire nutrients more efficiently [25]. Moreover, symbiotic relationships within the holobiont can provide benefits to the host under different ecological pressures. For instance, gut bacteria in animals can aid in digestion, contribute to the immune system, and even play a role in protecting against harmful pathogens [26]. Based on the frameworks proposed in this concept, new interdisciplinary research areas can be developed, and new functional solutions identified.
Faure et al. [10] consider that the assemblage processes of the microbiota and the function of the host in microbiota selectivity are valuable research topics. The paper highlighted that the exudates from plants can selectively influence the microbiota, causing plants to have contrasting strategies for nutrient resources.
The proposition of interactions between plant and earthworm holobionts for enlisting microbiota that aids in organic matter recycling and mineral nutrition corresponds with the increasing acknowledgment of the intricate relationships between organisms and their microbiomes in ecological processes [12]. The plant–earthworm interaction might entail the enlistment of specific microbial communities by both entities. For instance, plants can release root exudates that attract beneficial microbes engaged in nutrient cycling and promoting plant growth. Earthworms, through their interactions with the soil and organic matter, might harbor specific microbiota in their gut or on their skin. As they traverse the soil, these earthworm-associated microbes could influence the soil microbiome. The concept of holobionts implies that plants, earthworms, and their associated microbiota operate as a cooperative entity. The microbiota linked with both the plant and earthworm could contribute to the overall well-being and functionality of the holobiont in the context of organic matter recycling and nutrient cycling.
Dessaux et al. [27] examined the mutually interdependence between plants and microbiota and their subsequent possible applicability to improve performance and healthy of plants. Holobiont functioning is another important issue when examining holobiont studies. Which functional traits of the holobiont are derive from the microbiota and the host assemblage, and what are these functional traits that support the holobiont’s adaptation in a mutational environment?
The holobiont functional traits resulting from the assembly of microbiota and host can encompass various aspects, all of which contribute to the holobiont’s adaptation in a changing environment [28,29]. Microbiota associated with the host can facilitate nutrient uptake and metabolism, including the acquisition of essential nutrients from the environment and the breakdown of complex organic compounds [30]. This can enhance the holobiont’s ability to thrive in nutrient-limited or variable environments. Certain microbiota can confer resistance or tolerance to pathogens and pests by directly antagonizing them through the production of antimicrobial compounds or by priming the host’s immune system [31]. This contributes to the holobiont’s ability to withstand disease pressure. Microbiota can enhance the host’s tolerance to various abiotic stresses such as drought, salinity, or extreme temperatures by modulating physiological processes or producing stress-protective compounds [32]. This helps the holobiont to survive and thrive in challenging environmental conditions. Beneficial symbiotic relationships between the host and specific microbiota can provide mutualistic benefits, such as the exchange of nutrients or growth-promoting factors. These interactions contribute to the overall fitness of the holobiont in its environment [33]. Its microbiota can influence host development and growth through various mechanisms, including hormone production, nutrient availability, and regulation of gene expression. This can lead to enhanced growth rates or altered developmental patterns that optimize the holobiont’s performance in its environment. Overall, the diverse functional traits resulting from the interaction between host and microbiota contribute to the adaptability and resilience of the holobiont in the face of environmental variability and change [34,35]. Understanding these traits is essential for harnessing the potential of microbiota-mediated adaptations in agriculture, ecosystem management, and other fields.
The diversity of microbial communities within a holobiont can contribute to its resilience in the face of ecological challenges [36]. A diverse microbiome can provide functional redundancy, ensuring that essential functions are maintained even if some microbial species are affected. Understanding the holobiont concept has implications for fields such as medicine, agriculture, and ecology [37,38,39]. It highlights the interconnectedness of organisms and their associated microbiomes and emphasizes the importance of considering this holistic perspective when studying adaptation to ecological pressures.
To understand these relationships is fundamental for sustainable and responsible agriculture and the management of ecosystems. By appreciating the collaborative relationships between different organisms and their microbiomes, researchers and practitioners can develop strategies to enhance soil health, nutrient cycling, and organic matter decomposition in a more holistic manner. This approach aligns with the broader shift towards ecological thinking in agriculture and ecosystem science.
In this review, we explore how bacteria play a role in the complex forest ecosystem, and summarise findings from recent literature to understand the main factors that determine their abundance and diversity. We discuss how microbial populations of forest ecosystems have been responding to climate change, and the implications of this response on ecosystem processes. Finally, current knowledge on the bacteria’s role in addressing ecosystem processes and their capacity to adapt to global change are also discussed.
2. Materials and Methods
This review considers articles published between 2003 and 2023 concerning the main factors that drive the responses of the forest soil microbiome based on an overview of scientific publications on the disturbance responses of forest microbial communities. The articles are original studies as well as literature reviews, including scoping reviews and systematic reviews.
The selection criteria were:
- -
- Peer-reviewed articles;
- -
- Type of publication (only original studies or reviews were considered).
The main directions of the systematic review consisted in reviewing the impact of tree vegetation and tree composition on soil microbial communities; the influence of the soil microbial community on the plant-associated microbiome in the context of forest anthropogenic disturbance; the effect of harvesting on the diversity and structure of soil bacterial and fungal communities; the study of the soil microbial community successional patterns during forest ecosystem restoration; the diversity and response to global change; and the screening of bacterial communities in forest ecosystems.
Exclusion criteria comprised:
- -
- Editorials;
- -
- Published studies other than in English.
ERIC (Education Resources Information Centre) and numerous databases, including Web of Science, Science Direct, SpringerLink and Google Scholar, were used to study key relevant research. The following keywords in the various databases were used: forest soil microbiome, disturbance responses of forest microbial communities, influence of climate change on microbial diversity, drivers of abundance and diversity of forest soil microorganisms, forest anthropogenic disturbance, harvesting, ecosystem restoration.
3. Results and Discussion
The literature review included 102 articles that met the criteria for our analysis. The relevant data extracted from the articles were categorized by continent and research topic (Table 1).

Table 1.
Important data from articles distributed by continent and research topic.
3.1. Bacterial Ecology in Forest Soils
The ecological factors that drive the microbiome in forest soils are diverse and impact the composition, diversity, and function of microbial communities. Forest soils are dynamic environments, and the microbes that inhabit them have key roles in nutrient cycling, decomposition, and the overall health of the ecosystem.
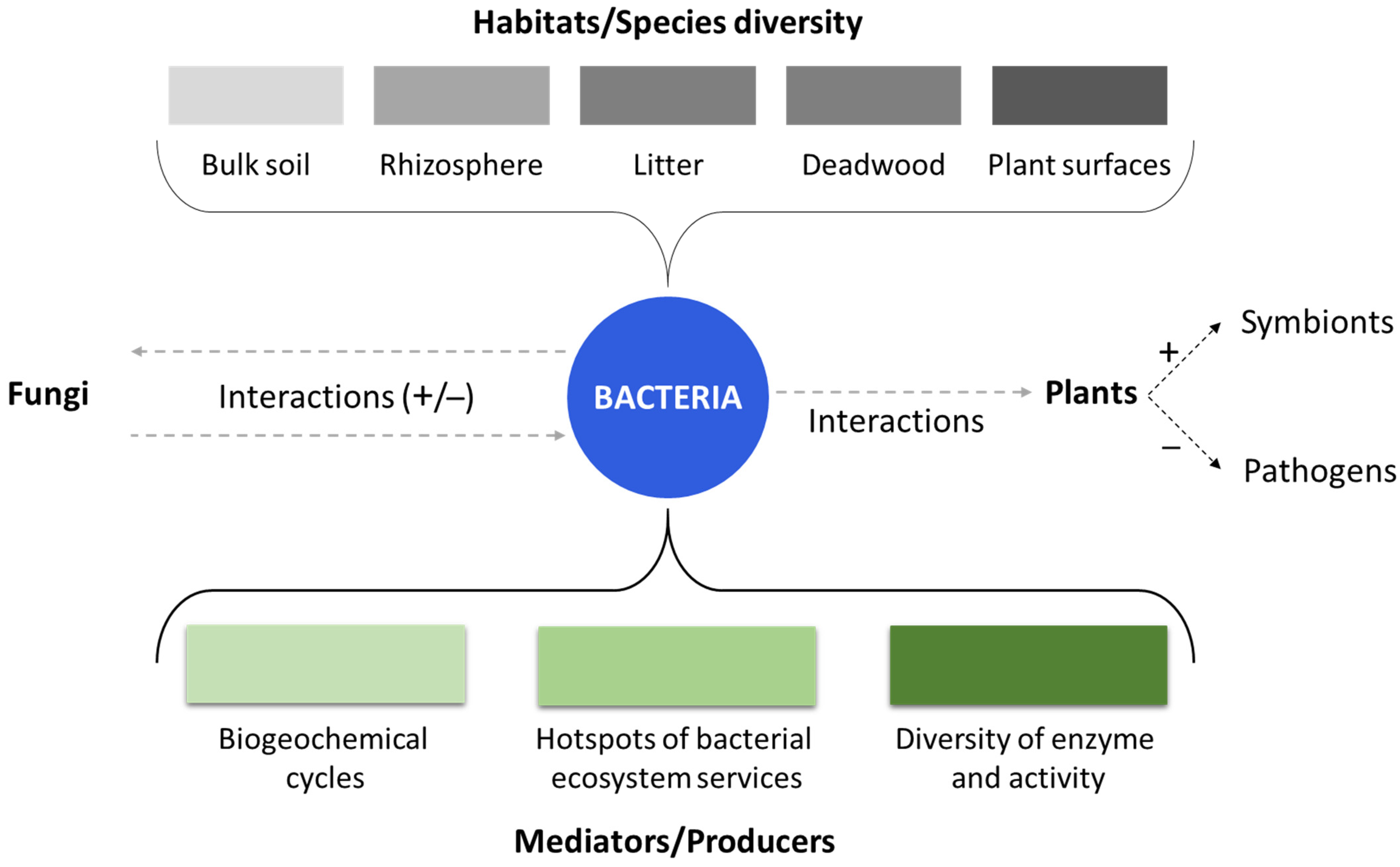
Forest soil ecology studies have predominantly focused on fungi, although bacteria are essential portion of the microbial community in soil of forests [40]. It is important to note that the functions of bacteria and fungi in forest ecosystems do not have to be considered as separate entities, as they interact with each other in various ways (Figure 1). The fungal biomass in forest soils has numerous effects on bacteria, such as the development of specific niches in patches of soil colonized from mycorrhizal fungi (i.e., the mycorrhizosphere) and microbial mats in the soil [41,42]. Moreover, bacteria occupy various habitats within forest soils, including bulk soil, the rhizosphere, litter, and deadwood habitats [42]. Their communities are influenced by soil type and composition, vegetation and plant communities, nutrient availability and soil fertility, disturbance regimes, symbiotic relationships, biotic interactions, and forest succession. Bacteria are involved in essential soil processes such as carbon, nitrogen, and phosphorus cycling, and they contribute to the decomposition of dead plant biomass and fungal mycelia. In fact, recent studies have revealed that bacteria frequently possess genes that encode plant cell wall-degrading enzymes, indicating that they are also involved in the plant material decomposition.
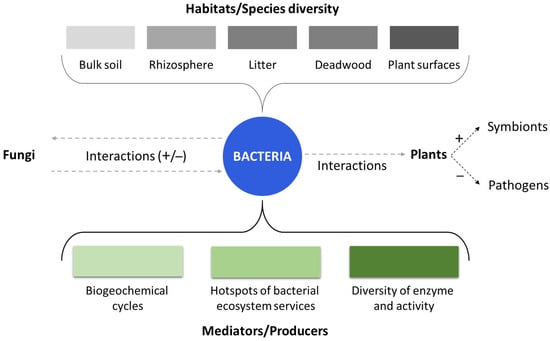
Figure 1.
The role and impact of bacterial communities in forests. Grey dash lines indicate the possible interactions among bacteria, fungi and plants. The different bacterial habitats that influence species diversity are displayed at the top of the figure (in grey). At the bottom of the figure, the different functions of bacteria as mediators or producer are displayed in green.
They interface with the roots of plants and with the mycorrhizal fungi either by acting as commensals or helpers of mycorrhizae. Additionally, bacteria are crucial for several phases in the nitrogen cycle, including nitrogen fixation. The effects of global change, such as climate warming and increased carbon dioxide levels, can impact bacterial communities in forest soils. However, the responses are specific to each forest ecosystem, and it is currently challenging to incorporate bacteria into predictive models. While significant progress has been made in understanding bacterial ecology in forest soils, the exact extent of their contributions to forest ecosystem processes remains incomplete. A comprehensive understanding of all soil community members’ activities is necessary to fully recognize the bacterial role in forest ecosystems [43].
Forest microorganisms, including bacteria and fungi, have been recognized to have crucial roles in the forest ecosystem. As decomposers, symbionts, or pathogens, they affect carbon turnover and retention, as well as the availability of other nutrients [9]. In addition, research has shown that microbial communities are essential for facilitating biogeochemical cycles, and understanding their role in ecosystem processes is crucial for predicting how forests will respond to future environmental conditions [44,45,46]. Fungi are among the most extensively studied microorganisms in temperate and boreal forest soils, where they form diverse communities of saprotrophic and mycorrhizal fungal taxa. They are considered the primary decomposers in forest soils due to their capability to produce several extracellular enzymes that efficiently break down the recalcitrant fraction of dead plant biomass [47,48].
In the context of plant–microbial interactions, negative interactions occur when the collective impact of soil organisms, such as pathogens, mutualists, and decomposers, leads to reduced plant performance. Conversely, positive interactions occur when soil communities provide benefits that enhance plant performance, such as increased biomass production and survival. Due to the crucial role of these interactions in shaping ecosystem properties, it is crucial to understand how soil microbe–microbe and soil microbe–plant relations react to climate change. This research topic will bring insights into important ecosystem functions, including the carbon storage in the soil and net primary productivity [16]. However, it is unclear which way microbial activity will influence carbon feedbacks among plants, soil and the atmosphere [48]. While the direct effects of climate change affecting microbial function have been thoroughly investigated, more indirect effects through changes in the interactions between plants and soil microbes and between microbes and soil microbes are less well known, even though they can influence vital processes such as plant chemistry, plant community composition, and mineralization rates. Plants and microbes can react to climate change by altering their population ranges, symbiotic partners, or timing of phonological actions. The relative abundance and function of soil communities are also affected by climatic changes, as soil community members vary in their physiology, temperature sensitivity, and growth rates. De Angelis et al. [49] demonstrated that a 5 °C warming in a temperate forest changed the relative abundances of soil bacteria and increased the bacterial-to-fungal ratio of the community.
Bacteria are found in various habitats, which represent different layers within forest ecosystems, including plant tissues and surfaces, streams, rocks, and especially the forest floor, soil, and litter. According to Lauber et al. [50], the five most abundant phyla in most soils are Acidobacteria, Actinobacteria, Proteobacteria, Bacteroidetes, and Firmicutes. Besides, the biogeographical patterns of the microbial community show site-uniqueness, and most of the species can be considered as “rare”, due to their site-specific conditions. The pH of the soil appears to be the most significant factor that determines the composition of bacterial communities in the soil, but other factors, such as organic matter content, accessibility of nutrients, climate conditions, and biotic interactions (especially with vegetation), also play crucial roles [51,52,53]. Within the groups described by Lauber et al. [50], only Acidobacteria shows a very high relative abundance at pH between 4–5, while the other ones exhibit a high relative abundance (30%–40%) at neutral and alkaline values. Fungal taxa are 8–10 times less abundant than bacterial ones in forest litter and soil, but have a higher specificity for one or two tree species than bacteria [52]. In general, up to 80% of bacteria are associated with more than six tree species, which indicates the additional effect of litter and pH in their diversity.
Spatial variability of chemical parameters accounts for the presence of hotspots of microbial activity with higher abundance and activity in the soil, such as in and on plant litter and dead wood, or on surrounding plant roots [54,55,56]. Each of these niches has distinct characteristics and, therefore, a characteristic bacterial community. Numerous studies have shown that fungi dominate the litter habitat and play a critical role in litter decomposition, but recent research has also highlighted the active involvement of bacteria in litter transformation. In coniferous forest litter, Betaproteobacteria, Bacteroidetes, and Acidobacteria were found to incorporate relatively more cellulose-derived carbon than fungi. This trend was observed in different pine forest soils in North America as well, where Bacteroidetes (Sphingobacteriales), Proteobacteria (Caulobacteriales, Burkholderiales, Xanthomonadales and Rhizobiales), and subdivision 1 Acidobacteria were identified as the primary accumulators of cellulose-derived carbon. Studies conducted on deciduous forests have also reported that at least 10% of litter bacteria are capable of decomposing cellulose, with the most common groups being Proteobacteria, Actinobacteria, Bacteroidetes, and Acidobacteria [57]. While the acidic soils of coniferous forests are connected to mainly Proteobacteria, Acidobacteria, and Actinobacteria in temperate deciduous forests, litter bacterial communities look especially enriched with Bacteroidetes and Proteobacteria [58,59]. The changes in the chemical structure of litter and root exudates due to the tree species types influence the soil bacterial communities because of the changes in substrate chemistry [60].
Bacterial and fungal communities in the soil ecosystem have varying spatial distributions. Fungal taxa are more confined to either the litter or organic horizon of soil and have a more heterogeneous distribution throughout the ecosystem. Environmental factors are more influential than geographic dispersal limitation in determining the bacterial community structure of forest soils [61].
The impact of soil properties and vegetation types on the structure and diversity of soil bacteria is widely acknowledged. Soil pH is recognized as a crucial factor that regulates the composition of bacterial communities [62]. Additionally, other soil characteristics, such as nutrient availability and plant diversity, also affect the composition and diversity of soil bacterial communities [63,64]. Previous studies have demonstrated that the distribution of bacterial communities is influenced by soil properties, climate, and other factors [64]. However, the specific controlling factors differ across different ecosystem types and spatial scales.
Wei et al. [65] analyzed the structure, diversity, and function of soil bacterial communities in different forest types. They selected two natural and mature forest sites in China and employed high-throughput sequencing to compare bacterial community diversity and function in four different seasons. The authors found that the soil bacterial composition at the phylum level (top 10 phyla) was similar, with differences only observed in relative abundance. The bacterial communities at the two sites exhibited two significant predicted functions related to the carbon cycle and three significant predicted functions related to the nitrogen cycle. This study is among the few to investigate the relationship between bacterial community structure and function and examine bacterial diversity in two typical forest sites under natural conditions in climatically transitional regions of China.
Bacterial communities in forest ecosystems are of significant importance for several reasons, as they play a vital role in maintaining the health, functioning, and sustainability of these ecosystems. Some key points highlighting the importance of bacterial communities in forest ecosystems are nutrient cycling, nitrogen fixation, disease control, and mycorrhizal symbiosis. The forest soil microbiome is shaped by a complex interplay of abiotic (e.g., soil composition, moisture, temperature) and biotic (e.g., plant species, soil fauna) factors. The overall health and functioning of the forest ecosystem are tightly linked to microbial processes driven by these ecological factors. Understanding these interactions is key to improving forest management and conservation efforts. The study and conservation of these communities is essential for the health and sustainability of forest ecosystems, especially in the face of ongoing environmental changes.
3.2. The Impact of Tree Vegetation and Tree Composition on Soil Microbial Communities
It is well known that soil physicochemical properties are strongly correlated with microbial communities. For example, a recent study investigated the seasonal dynamics of six soil enzyme activities (protease, urease, acid phosphatase, cellulase, peroxidase, and polyphenol oxidase) and microorganisms (bacteria, fungi, actinomycetes, and total microorganisms), in different vegetation types, and their reactions to shifts under soil physicochemical properties [66]. Four types of shelter forests (Bambusaoldhamii, Pinuselliottii, Eucalyptus robusta, and Casuarina equisetifolia) in China were chosen to investigate changes in seasonal patterns of enzyme activities (urease, acid phosphatase, cellulase, and polyphenol oxidase) and microorganism presence in soil correlated with soil physico-chemical properties (pH, moisture, temperature, nutrients). The results highlighted that different shelter forests had seasonal differences in the selected microbial activities. The soil bacteria, fungi, and total microbial presence in four shelter forests considerably improved in autumn for Actinomycetes. Soil enzymatic activities were correlated to total microbial presence and soil physicochemical properties. The research also confirmed that soil enzyme activities and total microbial presence had significant seasonal changes among the four shelter forests.
Jin et al. [67] found that bacteria are dominant populations in shelter-forest soils in the extreme arid areas, accounting for more than 80% of total soil microbes, and fungi are not more than 1% of the total soil microbes, although soil microbial community structure changes among three soil layers. Zhang et al. [68] studied bacterial surface soil community diversity and function in the shelterbelts of agricultural soils of five forest types. Dominant forest soil bacterial phyla include Proteobacteria, Actinomycetes, Acidobacteria, Chlorobacteria, and Bacillus, and soil bacteria were found to be better linked and to have more severe competition in the top layer of the soil than in the bottom layer.
On the other hand, Heo et al. [18] showed the capacity of the microbial community as a proactive marker of climate-driven vegetation change. The authors studied the soil bacterial and fungal communities in Korea (Odaesan) over a four-year interval through eDNA meta-barcoding and analyzed the differences in composition and function between forest types (Mongolian oak, Quercusmongolica, forest with, as well as without, Manchurian fir, Abiesholophylla) and sampling years. The results showed that denitrifiers were predominant in Manchurian firs, but no difference in the influence of climate change according to forest type. While the tree vegetation was stable, the microbial communities modified significantly over the course of four years. Climate change alters microbial communities significantly, although it is not sufficient to trigger a change in vegetation, so a microbial indicator can be developed to assess the disturbance of accumulated pressure on the forest ecosystem. The authors showed the impact of Manchurian fir trees and the effects of climate change on soil microbial communities in temperate forests.
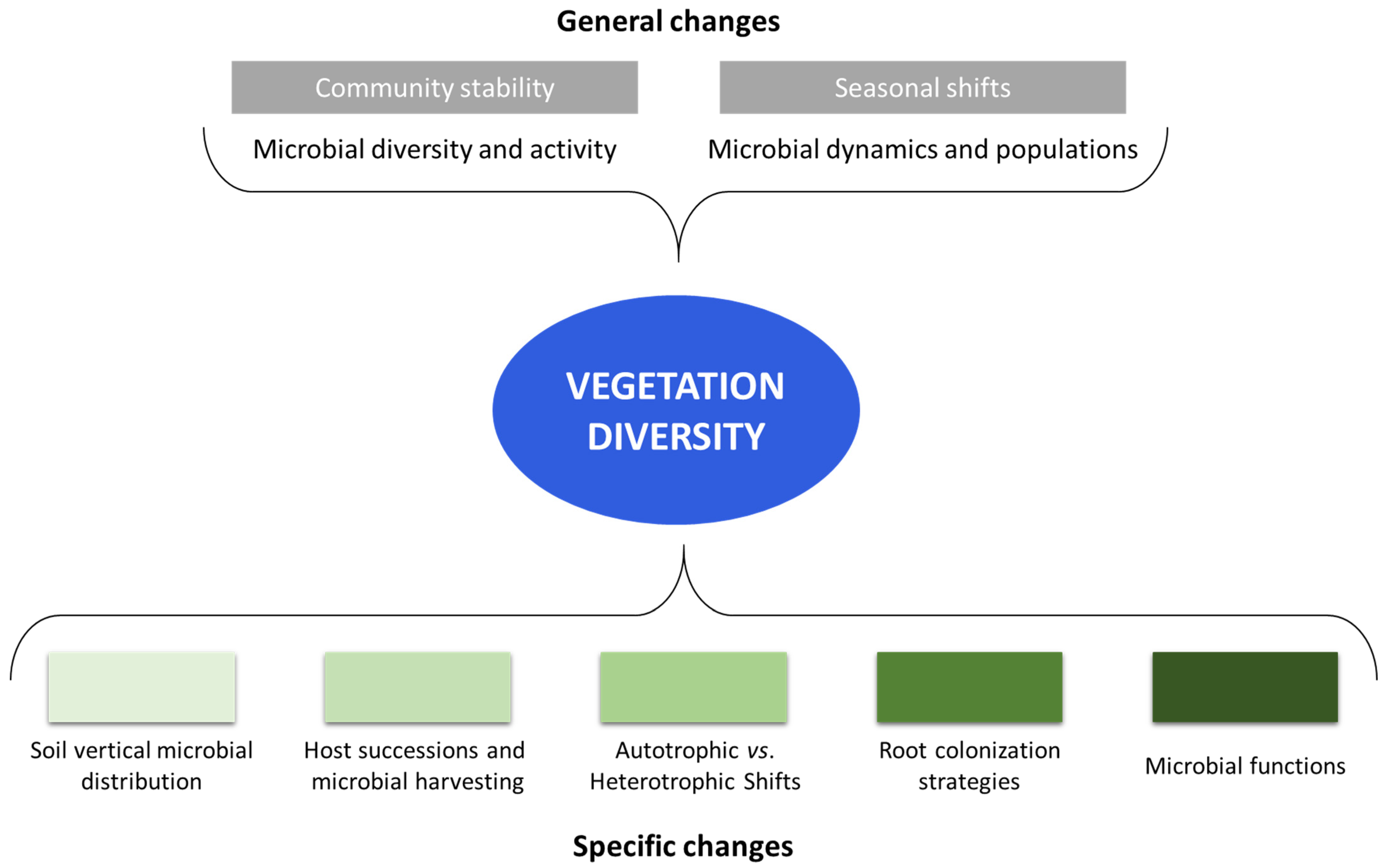
As it is reasonable to understand, not only the activity, but also the microbial community structure, is greatly influenced by the type of vegetation, and in particular, the type of tree species (Figure 2). For example, Prada-Salcedo et al. [69] analyzed the bacterial and fungal diversity in relation to forest composition using amplicon sequencing. They found that microbial Shannon diversity was affected by the proportion of evergreen trees, and the fungi were influenced by forest tree species composition.
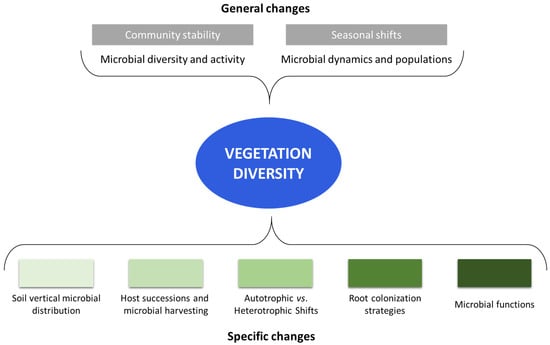
Figure 2.
General (in grey, on the top of the figure) and specific (in green, on the bottom) changes in forest microbial communities due to vegetation type and diversity.
Gillespie et al. [55] analyzed the effect of forest litter and absorptive roots on wide-ranging functions of soil microbial communities under mixed tree species conditions. The work suggested influences indirectly through the effects of tree mixture on surface litter and absorptive root traits and soil parameters. Mixed forests consisting of any three tree species affected soil microbial functioning, influencing nutrient availability in the forest soil litter and resource acquisition by the roots. The mixing of trees also changed soil microbial functioning and catabolic diversity, impacting soil fertility and physicochemical characteristics. Thus, the results indicate an indirect but existing impact of tree species mixing on the microbial heterotrophic soil community activity in four different forest ecosystems, from Mediterranean to boreal forests.
Mycorrhizal communities are greatly influenced by both the composition of tree associations and their cover within the canopy. Symbiotic mycorrhizal fungi sustain the diversity within forest trees, but act with a general-to-specific functionality based on a spatial gradient from large (forest ecosystem) to low scale (host tree) [70]. Arbuscular mycorrhizas in associations with forest ecosystems vary in terms of species distribution: from generalist species with a wide distribution and associated with multiple hosts to specialized species associated with only one or a reduced number of hosts [71]. Tree status and the overall status of the forest plant community are visible in the feedback between mycorrhizas and forest future dynamics, seedling survival, and performance [72]. Mycorrhizal colonization strategy is visible in the diversity of forests [73], with differences induced by arbuscular mycorrhizas or ectomycorrhizas domination [74], both contributing at the establishment of a specific flux of nutrients toward their host, the ecosystem nutrient cycling, and host successions [75,76]. The host species and its physiological traits and status are responsible for harvesting single or multiple mycorrhizal species, either arbuscular, ectomycorrhizal, or their dual colonization [77]. This mechanism is visible in the colonization status related to the type of ecosystem and its tree diversity [78], with the complexity of interactions increasing complementarily to the increase in species composition [79].
Understanding the complex interactions between tree vegetation, composition, and soil microbial communities is essential for sustainable forest management, reforestation efforts, and the conservation of biodiversity [80]. It also has implications for ecosystem functioning and resilience, particularly in the context of changing environmental conditions. Researchers continue to study these relationships to gain a better understanding of the intricate connections between trees and the soil microbiome.
3.3. Interactions Between Soil Microbial Communities and Plant-Associated Microbes Under Forest Anthropogenic Stress
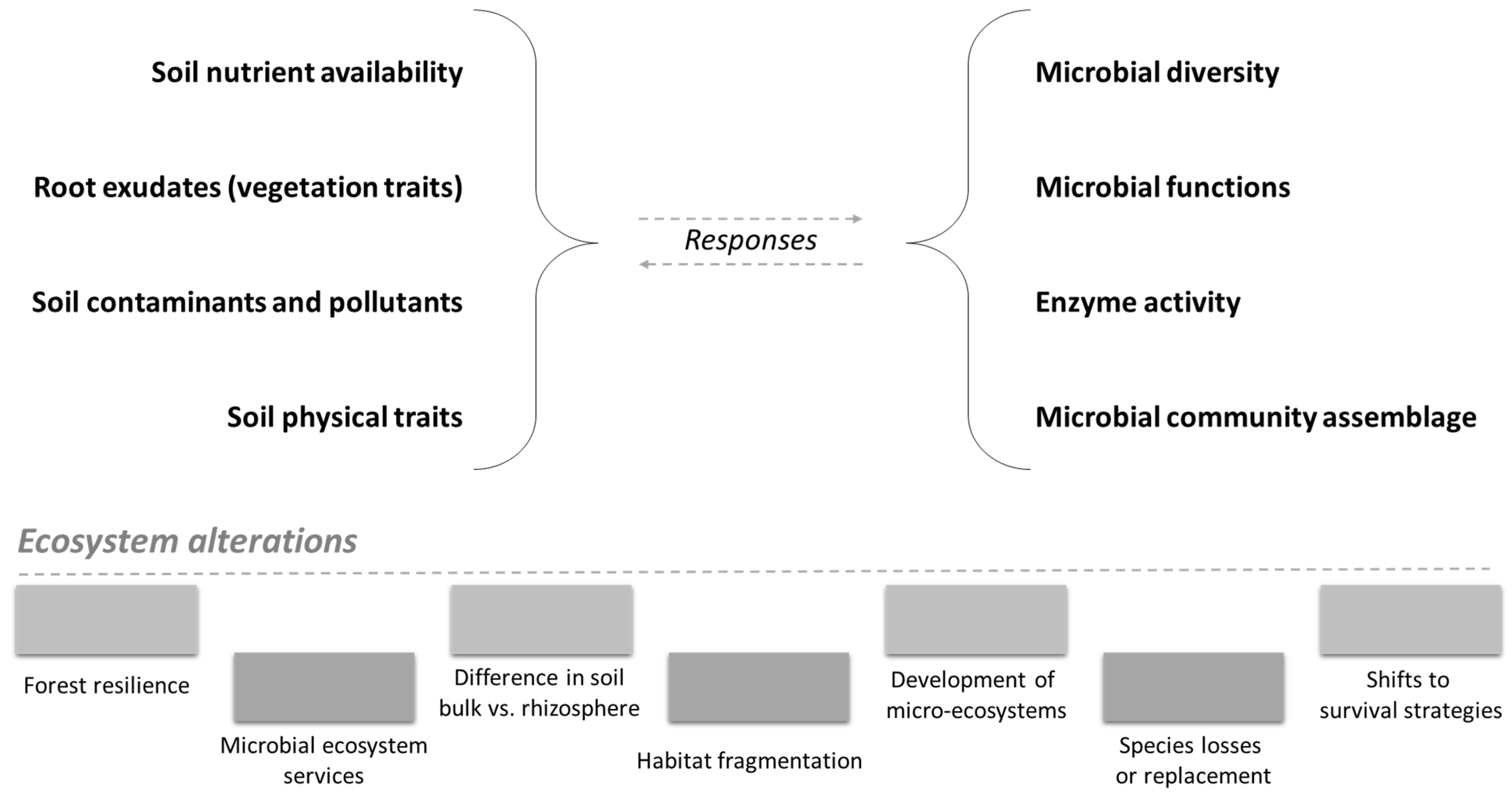
Nutrient availability/presence has a great influence on microbial communities (Figure 3). It is well known that plants release nutrients (e.g., root exudates) in the rhizosphere, which is the main area of the soil that influences the microbial community [6]. The presence of contaminants also has a great influence on the microbial community and activity [81].
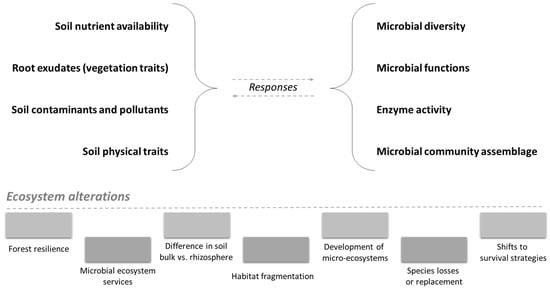
Figure 3.
Effects of ecosystem alterations (due to the combined influence of anthropogenic disturbances and soil–plant conditions) on the various soil factors (on the left) and, consequently, on forest microbiome (on the right).
In particular, Yurong et al. [82] investigated the rhizosphere’s influence on soil characteristics, bacterial communities and enzyme activities in Robinia pseudoacacia L. throughout a gradient of heavy metal content contamination. The authors identified that soil organic matter, accessible nitrogen, and phosphorous contents were clearly higher in the rhizosphere than in the bulk soil at heavy metal contaminated sites. An increase in the activities of four soil enzymes indicative of the C-cycle (β-glucosidase), N-cycle (protease, urease), and P-cycle (alkaline phosphatase) were found in the rhizosphere soil at all study sites compared to the bulk soil. Quantitative PCR (qPCR) and restriction fragment length polymorphism (RFLP) were used to determine the relative abundance, composition, and diversity of bacteria in the bulk and rhizosphere soils, respectively. The bacterial 16S rRNA gene copy number in the bulk soil was markedly less than in the rhizosphere and had distinctly detrimental correlations with total extractable Pb concentrations. At the various study sites, Firmicutes, Gammaproteobacteria, and Alphaproteobacteria were the most prevalent bacteria. The bacterial diversity index of species richness (S) and Margalef (dMa) were both clearly higher in the rhizosphere soil than in the bulk soil, even though no differences were found with Simpson’s index (D) between the bulk and rhizosphere soil. The results of the redundancy analysis revealed that soil pH, electrical conductivity, soil organic matter, and total/extractable Pb contents were the main factors influencing the relative abundance, composition, and diversity of bacteria. The native forest replacement with exotic species can alter microbial-related soil ecosystem services. This was found by Almonacid-Muñozet al. [81] in a Chilean native forest. In particular, the rhizosphere bacterial and fungal communities of the native deciduous tree Nothofagus obliqua (a pioneer species that is dominant in structure and composition after disturbance), grown within an anexotic Pinus radiata forest of a plantation growing nearby, were analysed. β-diversity analysis indicated that soil type (rhizosphere or bulk soil) and location type (native forest or P. radiata plantation) were responsible for most of the change between the bacterial and fungal communities. Proteobacteria and Basidiomycota were the dominant bacterial and fungal phyla in both soil types and sites. Bacteria displayed comparable and abundant taxa at the family level, regardless of soil type or site. The most abundant fungal taxa related to native forests were Tricholomataceae and Cantharellales, while Russulaceae and Hyaloscyphaceae were the dominant families in P. radiata. The key bacterial functional groups were chemoheterotrophic and aerobic chemoheterotrophic, with no significant variation among soil types or sites. Furthermore, the authors showed that the composition and diversity of bacterial and fungal communities typical of the N. obliqua native forest are affected by the adjacent forest and primarily reflect site properties, such as the available source of lignin-rich wood. In this study, the different leaf litter and root exudates between Nothofagus oblique and Pinus radiate probably were key factors that modified chemical and physical parameters of soil, influencing the soil microbial community.
Forest disturbance is recognized for its enduring impact on biogeochemical cycling, particularly on microbially mediated processes such as nitrogen (N) cycling in Appalachian ecosystems. Osburn et al. [20] used sequencing of 16S and ITS to investigate bacterial (16S) and fungal (ITS) soil communities in forested watersheds with varying disturbance histories and in neighbouring reference forests at the Coweeta Hydrologic Laboratory in the Appalachian Mountains of North Carolina. The study revealed that forests that had been altered previously exhibited significant differences in the composition, including increased alpha diversity and abundance of copiotrophic bacterial phyla (e.g., Proteobacteria) and N-cyclophytes (e.g., Nitrospirae). The fungal community structure also revealed detrimental effects, particularly in mycorrhizal taxa.
Nevertheless, fungal alpha diversity was not impacted by disturbance, and disturbance effects were inconsistent at the fungal class level. Co-occurrence networks established for bacteria and fungi displayed that disturbed communities had better-linked and closely clustered networks, suggesting that disturbance impacts not only community structure but similarly potential ecological interactions between taxa. While bacteria and fungi showed various long-term reactions to forest disturbance, the study shows strong responses of relevant bacterial and fungal functional groups (e.g., nitrifying bacteria and mycorrhizal fungi), suggesting both microbial groups have a central role within the long-term modifications of observed biogeochemical pathways resulting from forest disturbance in the region.
Soil fungal communities in forest, especially mycorrhizal symbionts, are greatly affected by anthropogenic disturbances, which often lead to a habitat fragmentation and the development of micro-ecosystems with different environmental conditions, symbiotic functionalities, and health status [83]. The magnitude of anthropogenic pressure is visible in the plant mycorrhizal status, the survival of native species in the competition with invasive (alien) ones, the maintenance of a homogenous native habitat and niche, or the conversion to an artificial heterogeneous one [84]. Fragmentation of forest ecosystems changes the assemblage of mycorrhizal communities, reducing the dimension of fungal networks developed between many different hosts to a simpler and less diverse network between fewer hosts [85]. Along with changes in the mycorrhizal community due to anthropogenic pressure, a shift in the mycorrhizal type and dominance is visible in the rhizosphere of trees, an increase in the incidence of pathogens, and an overall forest decline [86,87,88,89]. The reduction in mycorrhizal diversity due to anthropogenic pollution leads to a decrease in their potential functionality, with the magnitude of the effect caused by single or combined pollutants [90,91]. Currently, the levels of nitrogen and phosphorus, as well as metal pollution, greatly alter the diversity of mycorrhizal communities emerging to a critical load that drastically reduces both fungal species and their hosts [92,93,94]. Human activities in forests alter the resilience of these ecosystems, forcing host species to access mycorrhizal symbionts to a greater level in the roots, changing their specific nutritional function to a larger number of functions, for both survival and protection from abiotic and biotic pressure [95,96].
Overall, the relationship between soil microbial communities and the plant-associated microbiome in the context of forest anthropogenic disturbance is intricate and multifaceted. Research in this area continues to provide insights into how human activities can impact these ecosystems and how to manage and restore them for long-term ecological health and sustainability.
3.4. Influence of Harvesting on the Diversity and Structure of Soil Bacterial and Fungal Communities
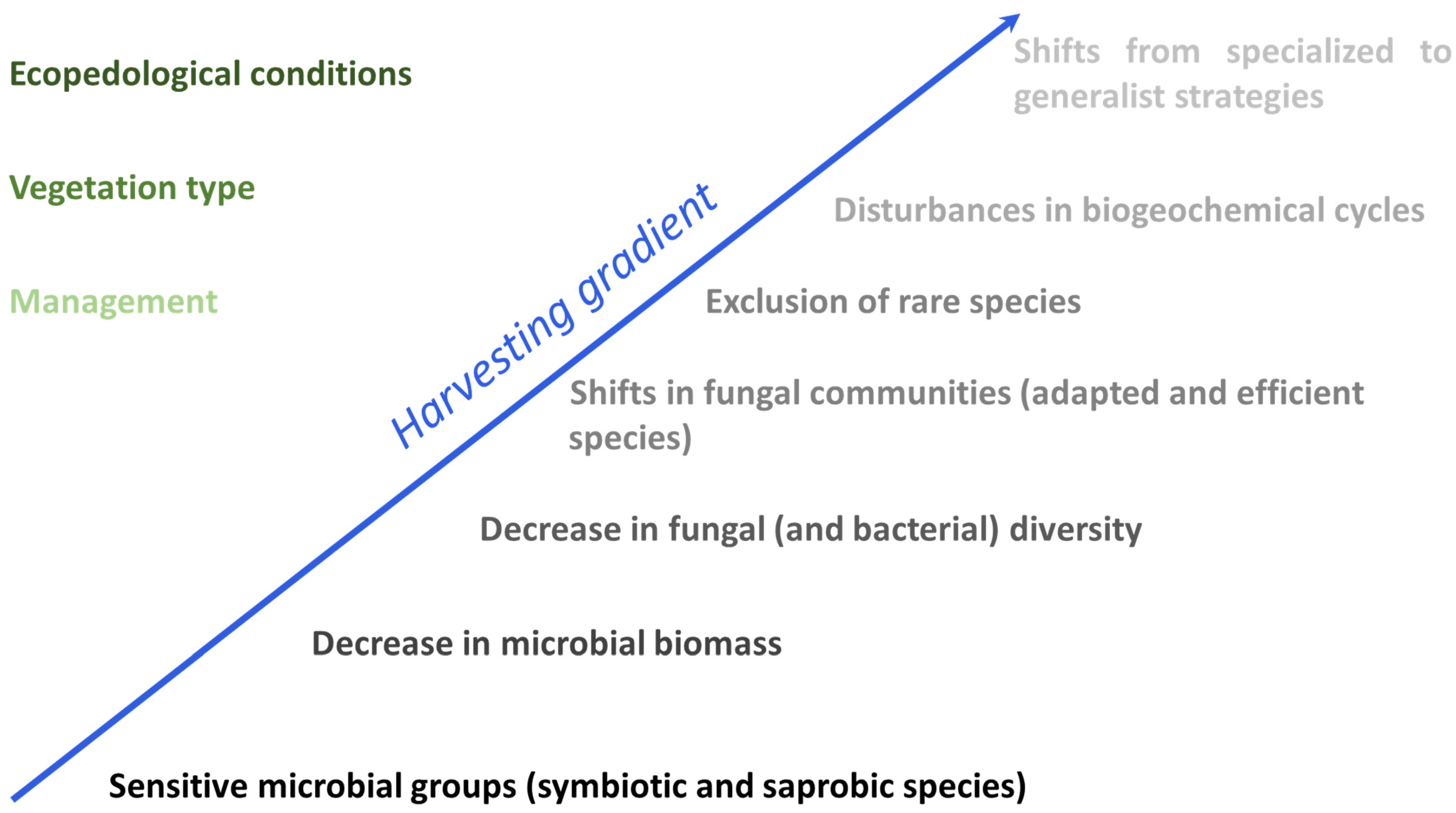
Several studies have shown that all forest harvesting systems have an impact on soils, with significant short-term and long-term effects on microbial communities, particularly in response to organic matter removal and soil compaction, which are considered major disturbances (Figure 4). Different levels of disturbance can result in distinct responses, with some effects persisting for several years after tree harvesting, as demonstrated by Hartman et al. [46]. The most sensitive microbial groups in forest systems are the symbiotic and saprobe species, which can potentially serve as indicators for monitoring recovery. It is necessary to conduct long-term monitoring over the course of a forest stand rotation to assess the resilience of the microbial community structure over time. Although the functional diversity of both bacteria and fungi is high, fungi have demonstrated a much stronger response to harvesting than bacteria.
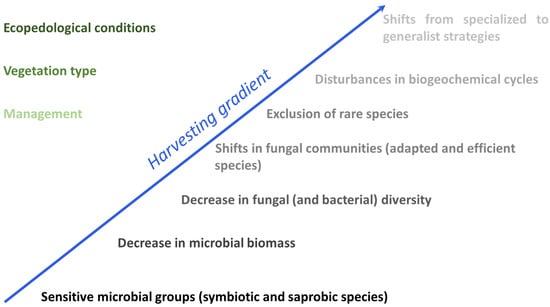
Figure 4.
The impact of harvesting gradient on forest microbial communities (on the right) and on the various factors controlling forest changes (on the left). The higher color intensity indicates the greater impact.
According to Chatterjee et al. [97] the response of forest species to disturbances such as timber harvesting depends on various factors, including the magnitude of the disturbance, soil type, and environmental conditions. Additionally, the biomass of soil microbial communities can decrease after timber harvesting, as found by Smith et al. [98].
The significance of fungal and bacterial communities in forest ecosystems, as highlighted by Dighton et al. [99], means that the removal of overstory trees has a direct impact on these communities. This impact is dependent on carbon inputs, as indicated by several authors, and can also affect the structure of litter inputs through saprotrophic fungi and Actinomycete bacteria [100,101,102,103,104]. However, some studies have contradictory results, suggesting that microbial communities may not respond to perturbations in the same way as trees do [105]. For instance, some studies indicate that microbial biomass C and N content and respiration may not be affected by whole tree harvest, soil compaction [106], or forest floor removal [107]. Meanwhile, other studies have shown that these properties may increase or decrease [108].
Forest ecosystem structure and function are influenced directly or indirectly by stand density. Wang et al. [109] studied the influence of Chinese fir plantation density on the functional diversity of the soil microbial community and concluded that density changes influence the soil physicochemical characteristics, composition, and metabolic functional diversity of microbial communities in Chinese fir plantations. Soil microbial functional diversity was investigated by Biolog ECO technology. Soil pH, content of oxidizable organic carbon, available N, available P, and available K were found to be affected by stand density. Chinese fir plantation density had a considerable influence on the utilization of carbohydrates, amino acids, carboxylic acids, and phenolic acids by the soil microbial community, but did not have a significant effect on the utilization of polymers. Principal component analysis (PCA) showed carbohydrates, polymers, and phenolic acids were susceptible carbon sources, which determined changes in the metabolic functions of soil microbial communities in the studied forest.
Harvesting is a management process that produces, in both the short and long term, a decrease in diversity in fungal communities, along with a reduction in host natural life cycle [110]. Observed shifts in fungal communities are associated with the quantity of mineralizable nitrogen, organic carbon, and lead to an increase in adapted and efficient species, while producing a decrease in the mycelium biomass in soil and in less-represented (rare) species in the community which are excluded from the new formed ecological niche [111,112,113]. In this context, the decomposition process of deadwood and remains suffers a reduction in efficiency, due to the narrowing of fungal diversity [114]. Both management and vegetation type act coupled on soil fungi and their biological processes, with the changes produced in the litter quantity, and its quality, visible in the restriction of nutrient cycling [115,116]. The long-term effect of harvesting on fungal communities causes a rapid reduction in species diversity, with the disappearance of multiple species, followed by a slow re-establishment of fungi to occupy the empty niches [117,118]. This process ends after 30–50 years, and the re-established fungal community presents a different species assemblage and composition compared to the native one. The shift in fungal processes is related to the harvesting intensity, from a high-diversity fungal community with specialized strategies in nutrient acquisition, to a lower-diversity community with generalist strategies.
Understanding the influence of harvesting on soil bacterial and fungal communities is vital for sustainable land and forest management. Properly managed harvesting and land-use practices can help minimize disruption to these microbial communities, ensuring healthy and productive ecosystems in the long term. Research in this area continues to provide insights into the ways in which these impacts can be mitigated and managed.
3.5. Patterns of Soil Microbial Community Succession During the Restoration of Forest Ecosystems
The assessment of soil microbial communities is becoming progressively important for evaluating the sustainability of forest ecosystems and their response to stress and disturbances. However, scant information exists regarding the discernible patterns in microbial community structure and composition during secondary succession or ecosystem restoration. The translation of forests to managed states, such as agriculture or timber plantations, has contributed to the modification of 75% of ice-free terrestrial ecosystems globally [119]. While approximately 50% of the earth’s land surface was afforested in the prehistoric era, about 40% of that forest cover has been disappeared, and a large part of the surviving forest has been subjected to different types of disturbance, particularly in the past two centuries [120]. Forest conversion still accelerating in the 21st century [121], highlighting the importance of characterising the effects of forest disturbance on both terrestrial biodiversity and ecosystem functions. Such comprehension is essential biogeochemically, as forest ecosystems play a pivotal role in the elemental cycles and deliver valuable ecosystem services, such as carbon storage, nutrient cycle regulation, and the provision of clean drinking water [122]. Soil microorganisms are key drivers of these biogeochemical processes and associated ecosystem services, performing essential functions like litter decomposition and carbon and nitrogen cycling, highlighting an urgency to investigate the effects of forest perturbation on soil microbial communities [123].
The successions observed within forest ecosystems and the mechanisms that produce them represent an interconnected phenomenon. Disturbances produced especially by anthropic activities are visible in the occurrence of different successional patterns, which act as restoration stages. The changes in terrestrial microorganisms, plant biomass, species composition, and soil physicochemical properties are effects of the forest disturbances. Moreover, soil C and N stocks, which are known as drivers of microbial community structure, are affected by the forest disturbance processes [123], and the magnitude of change is proportional with the resilience of these communities. Several studies focus on the influence on soil microbial communities of timber harvest (e.g. Kohout et al. [124]), and prescribed fire (Shen et al. [125]), which can all modify bacterial and/or fungal diversity. Zhou et al. [126] found a connection between bacterial community variations in previously disturbed forests, as well as increased relative abundance of r-selected bacterial phyla (e.g., Proteobacteria) with previous disturbance. Thus, the selection within the native microflora assures a microbial pool that can persist in the restoration process. Moreover, changes in forest soil microbial communities are likely to occur due to disturbances. No studies fully investigate the long-term activity of bacterial communities, as well as fungi, as a result of past disturbances such as timber harvesting, agricultural conversion and timber plantation conversion [127]. Responses to perturbations of soil microbial communities have been found to be relevant in the forests of the Appalachian region (USA), where about 70% of the territory is covered by forests and almost all forest ecosystems in the region have suffered from past disturbances due to human activities, which includes the logging of commercial timber and/or switching to agriculture [128,129]. In this region, Keiser et al. [130] assessed long-term effects of disturbance on soil microbial N-cycle functions, finding elevated nitrification rates in previously disturbed forests. These results have been accentuated by Lin et al. [131], who found an elevated abundance of nitrifying microorganisms.
Hyphal networks developed in soil by mycorrhizas plays an important role in the success of forest restoration processes [132]. These networks act as a biological transfer system for nutrients, from areas apart from plant root activity up to their host root system. The restoration period is linked with the gradual increase in diversity of mycorrhizal species and the establishment of mycorrhizal links between different tree species [133]. This biological mechanism relies on the mycorrhizas’ capacity to form symbiosis with multiple hosts, but induces a series of negative interactions between native and inoculated mycoflora [134]. Multiple successional scenarios arise from these interactions: (a) Native mycorrhizal species act rapidly to colonize the new hots, completely eliminating the non-native (or inoculated) species; (b) Part of the non-native (or inoculated) species persist in the restored community, followed by a stratified successional process until a climax based on a dual origin of species in the community; (c) The non-native (or inoculated) mycoflora restrict the development of the native one and its access to plant hosts, which leads to a complete change in the long-term assemblage of the fungal community. The direction toward one successional scenario, or a combination of scenarios, is due to the mycorrhizal ability to adapt the harsh environmental conditions during the first stages of restoration [135,136,137]. Restoration is a process that targets forest function recovery, with the number of targeted functions positively correlated to the number of species used [138]. In this process, the feedback established between plants and their mycorrhizal partners leads to host’s survival, increased growth and development, and achievement of dominance in community [139]. The inoculum (both native and/or non-native mycorrhizas) used in restoration is responsible for the success of this process, with different efficiency levels between species [140].
The influence of harvesting, particularly in the context of forestry, on the diversity and structure of soil bacterial and fungal communities has a considerable ecological importance. The impact of harvesting on these microbial communities can have both short-term and long-term effects. Understanding the patterns of soil microbial community succession during forest ecosystem restoration is crucial for planning and executing successful restoration projects. By promoting the recovery of microbial diversity and function, restoration efforts can help rebuild resilient and healthy ecosystems with improved ecological services and long-term sustainability.
3.6. Impact of Global Change on Bacterial Communities Within Forest Ecosystems
Covering over 40 million km2 and accounting for 30% of the global land area, forests are among the most extensive and crucial ecosystems on earth [141]. Forests are present in most of the earth’s biomes and host a significant proportion of global diversity. The Northern Hemisphere’s temperate and boreal forests, for instance, occupy a significant portion of its land surface and house 46% of all trees on the planet—0.66 and 0.74 trillion, respectively [142]. Global change, which includes both climate change and other human-induced changes, has been identified as a primary threat to forests [143]. Human overexploitation has been the most relevant risk to forests over millennia, and the C balance of forests is mostly affected by human activities, comprising deforestation, productive forest management, reforestation, afforestation, and others [144]. Other perturbations related to climate change and atmospheric factors also impact the carbon balance of forests. As such, the impacts of temperature rise, drought persistence, fire recurrence, or the increase in native and invasive forest pests, combined with higher nitrogen and carbon dioxide loads, influence deeper nutrient cycles to such a degree that forests have the potential to become net sources rather than sinks of CO2 [145]. Consequently, a clear comprehension of the role of forests in C fluxes, which are strongly influenced by the bacterial and fungal activity, has been indicated as an essential requirement for making future predictions about the health of our planet [49]. If plants are primarily responsible for absorbing carbon from the atmosphere in forests, the microorganisms in forests play a major role in the carbon balance in these ecosystems.
From a global perspective, it is crucial to comprehend the diversity of fungi and bacteria within coniferous forest biomes. Fungi are dominant in decomposing litter material, whereas bacteria become increasingly important as soil depth increases [146]. Coniferous forests play a significant role in the global carbon cycle, and understanding the microbial processes in the soil is essential for predicting global carbon fluxes and their potential changes in the future.
Global change, which includes factors such as climate change, increased atmospheric carbon dioxide (CO2) levels, altered precipitation patterns, and human activities, can have a significant impact on bacterial communities within forest ecosystems. These changes can disrupt the composition, diversity, and functioning of soil and litter bacterial communities, leading to various ecological consequences. Understanding the impact of global change on bacterial communities within forest ecosystems is critical for predicting the responses of these ecosystems to ongoing environmental changes. Scientists continue to study these interactions to better inform forest management and conservation efforts, including strategies for mitigating the negative effects of global change on forest bacterial communities and their associated ecosystem functions.
Different studies show that climate change causes significant changes in the microbial populations, although not significantly to cause a change in vegetation, so it is possible to develop a microbial indicator to assess the disturbance of accumulated pressure on the forest ecosystem. The unpredicted vegetation change can be a serious problem caused by climate change, and the soil microbial community should imitate the pressure on forest ecosystems due to climate change [147,148]. Classen et al. [149] highlighted also that the global climate change is altering species distributions, and thus interactions among organisms. J Jansson and Hofmockel [150] studied current findings about the climate change impact on the microbiome of soil and the possible ways in which soil microorganisms can be utilized to mitigate the negative consequences of climate change.
Although it is known that climate changes infer changes in soil microbial community composition, species abundance, diversity, survival and resilience, changes in enzyme production, and changes in interactions between microbes and plant roots, etc. Singhal et al. [43] found that there is still limited knowledge about the potential impacts of climate change-induced disturbances on soil microbial communities. Limitations in understanding these changes are caused by the bi-directional nature of climate change effects on microbial communities and the continuous modification of both microbial communities and their associated biogeochemical cycles. This knowledge gap is significant, as the stability of microbial communities, which refers to their ability to withstand and recover from disturbances, is likely to affect ecosystem function. Given the essential roles of forest ecosystems in climate stability, biodiversity, and economic development, microbial community structure can serve as an indicator of forest ecosystem status. Changes in microbial community structure can reveal alterations in nutrient and energy flow patterns before they cause irreversible effects on long-term soil productivity. Therefore, understanding the dynamics of soil microbial communities is vital for effective conservation and management of forest ecosystems [8]. This context imposes a continuous monitoring of both climate and microbial changes, a continuous update of forecasting models, and improvement of indicator sensitivity.
Even more than a decade following tree harvesting, the diversity and structure of soil bacterial and fungal communities remained significantly altered by the disturbances caused by harvesting [151,152,153,154,155]. Moreover, individual taxonomic groups exhibited different responses to varying levels of disturbances. Some taxonomic groups, such as plant symbionts like ectomycorrhizal fungi, and saprobic taxa like Ascomycetes and Actinomycetes, were highly sensitive to harvesting disturbances. As these taxa play crucial ecological roles in forest development, their fate is essential for the sustainability of forest ecosystems. While abundant bacterial populations were found throughout the study area, abundant fungal populations often exhibited patchy distributions, which is consistent with their higher sensitivity to the soil disturbances examined.
Extensive research has been conducted on the impact of climate change on soil biota abundance, diversity, and activity. However, the literature reports inconsistent effects that are highly dependent on environmental context. Elevated atmospheric CO2 has been shown to increase microbial biomass, fungal abundance, and the abundance and body size of most faunal groups, resulting in changes in the structure of the soil food web. Bacterial diversity, on the other hand, is minimally affected by elevated CO2. Warming, however, increases the abundance of bacteria and fungi, as well as most soil faunal groups, resulting in changes in the soil food web structure [156,157]. Drought conditions reduce the biomass and abundance of the majority of soil microbial and faunal groups, but increase the abundance of fungi compared to bacteria. However, the impacts of increased rainfall and flooding on soil biota are still poorly understood and highly context-dependent. Changes in soil organism abundance and soil food web structure caused by climate change will have impacts on soil functioning and the provision of soil-based ecosystem services [158]. As such, the relative importance of these changes in relation to the direct impacts of climate change on soil functioning is not clear, and the relationships between soil biota and soil functioning are context-dependent. Further research is therefore needed to establish the effect of climate change-induced changes in soil biota on the suppression of diseases, and also the effect of invasive microbes on soil functioning [39,159]. The effects of global change on soil food webs and their functions is a growing concern. Soil communities address several global change factors, which include land-use change, contamination, habitat fragmentation and modification, invasive species, soil sealing, and climate change [160,161,162]. Although significant research has been conducted on the impacts of these drivers on soil food webs, most studies have concentrated on aboveground organisms.
Climate change is causing increased temperatures and extreme events, such as droughts, heat waves, and excessive rainfall, on a global scale, and these changes are already affecting the functioning of terrestrial ecosystems.
4. Conclusions
Studying the factors that drive forest soil microbiome is a complex and dynamic field. Forest soil microbiomes are extremely diverse and complex. The multiplicity of microbial species and their interactions can make it challenging to identify the specific factors that drive their composition and functions.
This review highlights that the forest soil microbiome is influenced by various environmental, ecological, and biotic factors. The main key drivers of the soil microbiome in forest ecosystems, addressed in the various sections, are soil composition and nutrient availability, plant composition, soil microbial interactions, disturbances, succesion, and temporal dynamics. Overall, these factors interact in complex ways, shaping the diversity, function, and resilience of the soil microbiome in forest ecosystems.
Microbial communities in forest soils can vary significantly over small spatial scales due to factors such as tree distribution, topography, and microclimate. This spatial variation can further hamper efforts to generalize results to larger forest ecosystems. Microbial communities can change over time due to seasonal variations, disturbances (e.g., forest fires or logging), and long-term environmental shifts (e.g., climate change). Capturing these dynamics requires long-term and comprehensive monitoring, which can be resource-intensive.
To understand the forest soil microbiome comprehensively, combinations of field studies and advanced techniques are often used. The key approaches and techniques for measuring the changes of the forest soil microbiome due to various factors are soil sampling, metagenomic sequencing, quantitative PCR, enzyme activity assays, Biolog plates, respiration rate measurements, microbial diversity indices, and long-term monitoring. Studying these factors and their interplay, insights into how the forest soil microbiome responds to environmental changes and contributes to the overall health and functioning of forest ecosystems can be provided. While advances in high-throughput sequencing technologies have enabled more in-depth studies of microbial communities, these methods not lack limitations. They may not capture all microbial taxa, and issues such as contamination and PCR bias can affect the data quality. While DNA sequencing can provide information about microbial composition, it does not directly reveal the functional roles of these microbes. Moreover, it is fundamental to know the microbial abundance related to different abiotic and biotic factors, which must be related to the soil microbial diversity. Determining how specific microbial taxa contribute to ecosystem functions is a challenging and complex issue. Microbial communities in forest soils do not exist alone. They interact with other microorganisms and are influenced by factors like nutrient availability, soil chemistry, and plant–microbe interactions. Understanding these complex interactions is a crucial challenge. Studying the factors that drive the forest soil microbiome is fundamental for understanding ecosystem health, nutrient cycling, and resilience when faced with environmental changes. Researchers continue to develop innovative techniques, collaborate across disciplines, and employ long-term studies to address these challenges and gain deeper insights into the complex relationships within forest ecosystems. A combination of molecular, chemical, and ecological tools is necessary to comprehensively measure the changes in the forest soil microbiome. These approaches allow for the identification of shifts in microbial communities in response to various environmental factors, such as soil properties, vegetation, disturbances, and human activities. By integrating these methods, we can better understand the dynamics of forest soil microbiomes and their role in ecosystem processes.
Author Contributions
Conceptualization, P.G., C.O., V.S., V.C. and A.O.; methodology, C.O. and A.O.; data curation, P.G., C.O., V.S., V.C. and A.O.; writing—original draft preparation, P.G., C.O., V.S., V.C. and A.O.; writing—review and editing, P.G., C.O., V.S., V.C. and A.O.; supervision, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Ministry University and Research, “Next Generation EU–Piano Nazionale Resistenza e Resilienza (PNRR)—Missione 4 Componente 2 Investimento 1.4—Notice No. 3138 16 December 2021 rectified by D.D. n. 3175 18 December 2021”. Award Number: CN_00000033, Decree MUR n. 1034 17 June 2022, CUP B83C22002930006, Project title “National Biodiversity Future Center—NBFC, Microbial diversity and functioning of freshwater and terrestrial ecosystems”.
Data Availability Statement
Data can be requested from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.; Kapos, V. Reducing greenhouse gas emissions from deforestation and forest degradation: Global land-use implications. Science 2008, 320, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, I.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.R.; Beter, H.L.; Schuster, R.; Walston, J.; Ray, J.C.; Robinson, J.G.; Callow, M.; et al. Anthropogenic modification of forests means only 40% of remaining forests have high ecosystem integrity. Nat. Commun. 2020, 11, 5978. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Laudon, H.; Sponseller, R.A.; Lucas, R.W.; Futter, M.N.; Egnell, G.; Bishop, K.; Ågren, A.; Ring, E.; Högberg, P. Consequences of more intensive forestry for the sustainable management of forest soils and waters. Forests 2011, 2, 243–260. [Google Scholar] [CrossRef]
- Titus, B.D.; Brown, K.; Helmisaari, H.S.; Vanguelova, E.; Stupak, I.; Evans, A.; Clarke, N.; Guidi, C.; Bruckman, V.J.; Varnagiryte-Kabasinskiene, I.; et al. Sustainable forest biomass: A review of current residue harvesting guidelines. Energ. Sustain. Soc. 2021, 11, 10. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Belowground microbiota and the health of tree crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Faure, D.; Simon, J.-C.; Heulin, T. Holobiont: A conceptual framework to explore the eco-evolutionary and functional implications of host–microbiota interactions in all ecosystems. New Phytol. 2018, 218, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.Z.; Heulin, T.; Guyonnet, J.; Achouak, W. Stable isotope probing of carbon flow in plant-holobionte. Curr. Opinion Biotechnol. 2016, 41, 9–13. [Google Scholar] [CrossRef]
- Puga-Freitas, R.; Barot, S.; Taconnat, L.; Renou, J.P.; Blouin, M. Signal molecules mediate the impact of the earthworm Aporrectodea caliginosa on growth, development and defence of the plant Arabidopsis thaliana. PLoS ONE 2015, 7, e49504. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Fromin, N.; Milcu, A.; Buatois, B.; Pontoizeau, C.; Hättenschwiler, S. Higher tree diversity increases soil microbial resistance to drought. Commun. Biol. 2020, 3, 377. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Mol. Biol. Rev. 2021, 85, e00026-20. [Google Scholar] [CrossRef]
- Dumonceaux, T. Composition and dynamics of plant- and soil-associated microbial communities in forest and agricultural ecosystems. Microorganisms 2023, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Osburn, E.D.; Aylward, F.O.; Barret, J.E. Historical land use has long-term effects on microbial community assembly processes in forest soils. ISME Comm. 2021, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.-M.; Lee, H.; Kwon, S.-L.; Yoo, Y.; Kim, D.; Han, S.-I.; Lee, A.-H.; Kim, C.; Kim, G.-H.; Kim, J.-J. Influence of tree vegetation on soil microbial communities in temperate forests and their potential as a proactive indicator of vegetation shift due to climate change. Sustainability 2020, 12, 10591. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Osburn, E.D.; McBride, S.G.; Aylward, F.O.; Badgley, B.D.; Strahm, B.D.; Knoepp, J.D.; Barrett, J.E. Soil bacterial and fungal communities exhibit distinct long-term responses to disturbance in temperate forests. Front. Microbiol. 2019, 10, 2872. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Yajun, L.; Wanjin, H.; Guobing, W.; Zhaoyu, K.; Lan, W.; Gang, G. Soil bacterial and fungal communities and the associated nutrient cycling responses to forest conversion after selective logging in a subtropical forest of China. Forest. Ecol. Manag. 2019, 444, 308–317. [Google Scholar] [CrossRef]
- Maltz, M.R.; Treseder, K.K. Sources of inocula influence mycorrhizal colonization of plants in restoration projects: A meta-analysis. Restor. Ecol. 2015, 23, 625–634. [Google Scholar] [CrossRef]
- Rodrigo, A.G. Modelling the evolution of holobionts: An incomplete review. New Zealand J. Zool. 2023, 52, 87–102. [Google Scholar] [CrossRef]
- Youle, M.; Knowlton, N.; Rohwer, F.; Gordon, J.; Relman, D. Superorganisms and holobionts. Microbe Mag. 2013, 8, 152–153. [Google Scholar] [CrossRef]
- Singh, B.K.; Liu, H.; Trivedi, P. Eco-holobiont: A new concept to identify drivers of host-associated microorganisms. Environ. Microbiol. 2020, 22, 564–567. [Google Scholar] [CrossRef]
- Mishra, S.; Hättenschwiler, S.; Yang, X. The Plant microbiome: A missing link for the understanding of community dynamics and multifunctionality in forest ecosystems. Appl. Soil. Ecol. 2020, 145, 103345. [Google Scholar] [CrossRef]
- Dessaux, Y.; Grandclément, C.; Faure, D. Engineering the rhizosphere. Trends Plant Sci. 2016, 21, 266–278. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes drive evolution of animals and plants: The hologenome concept. mBio 2016, 7, e01395-15. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.; Triviño, V. What is a hologenomic adaptation? Emergent individuality and inter-identity in multispecies systems. Front. Psychol. 2020, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Ahmad, J.; Musarrat, J.; Ehtesham, N.Z.; Hasnain, S.E. Emerging importance of holobionts in evolution and in probiotics. Gut Pathog. 2013, 5, 12. [Google Scholar] [CrossRef]
- Schneider, T. The holobiont self: Understanding immunity in context. Hist. Philos. Life Sci. 2021, 43, 99. [Google Scholar] [CrossRef] [PubMed]
- Mesny, F.; Hacquard, S.; Thomma, B.P. Co-evolution within the Plant Holobiont Drives Host Performance. EMBO Rep. 2023, 24, e57455. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.S.; Voolstra, C.R.; Sweet, M.; Duarte, C.M.; Carvalho, S.; Villela, H.; Lunshof, J.E.; Gram, L.; Woodhams, D.C.; Walter, J.; et al. Harnessing the microbiome to prevent global biodiversity loss. Nat. Microbiol. 2022, 7, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Dorador, C.; Egamberdieva, D.; Kostka, J.E.; Ryu, C.-M.; Wassermann, B. Shared governance in the plant holobiont and implications for one health. FEMS Microbiol. Ecol. 2024, 100, fiae004. [Google Scholar] [CrossRef] [PubMed]
- Huitzil, S.; Huepe, C.; Aldana, M.; Frank, A. The missing link: How the holobiont concept provides a genetic framework for rapid evolution and the inheritance of acquired characteristics. Front. Ecol. Evol. 2023, 11, 1279938. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [PubMed]
- Fengqiao, L.; Haiyun, Z.; Christian, S.; Xiaogang, L. Microbiome sustains forest ecosystem functions across hierarchical scales. Eco-Environ. Health 2023, 2, 24–31. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in microbial community composition and function by soil depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Pandey, S.; Kumari, N.; Chauhan, D.K.; Jha, P.K. Impact of climate change on soil microbes involved in biogeochemical cycling. In Climate Change and the Microbiome; Choudhary, D.K., Mishra, A., Varma, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 63–94. [Google Scholar] [CrossRef]
- Crowther, T.W.; Van Den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Fatichi, S.; Manzoni, S.; Or, D.; Paschalis, A. A mechanistic model of microbially mediated soil biogeochemical processes: A reality check. Glob. Biogeochem. Cycles 2019, 33, 620–648. [Google Scholar] [CrossRef]
- Hartmann, M.; Howes, C.; VanInsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Nilsson, R.H.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Brabcová, V.; Štursová, M.; Davidová, A.; Jansa, J.; Cajthaml, T.; Baldrian, P. Decomposer food web in a deciduous forest shows high share of generalist microorganisms and importance of microbial biomass recycling. ISME J. 2018, 12, 1768–1778. [Google Scholar] [CrossRef]
- Abdul Rahman, N.S.N.; Abdul Hamid, N.W.; Nadarajah, K. Effects of abiotic stress on soil microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, K.M.; Pold, G.; Topcuoglu, B.D.; van Diepen, L.T.A.; Varney, R.M.; Blanchard, J.L.; Melillo, J.; Frey, S.D. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2015, 6, 104. [Google Scholar] [CrossRef]
- Lauber, C.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil ph as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–56. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–22. [Google Scholar] [CrossRef]
- Chemidlin Prevost-Boure, N.; Maron, P.A.; Ranjard, L.; Nowak, V.; Dufrene, E.; Damesin, C.; Soudani, K.; Lata, J.C. Seasonal dynamics of the bacterial community in forest soils under different quantities of leaf litter. Appl. Soil. Ecol. 2011, 47, 14–23. [Google Scholar] [CrossRef]
- Baldrian, P.; Merhautová, V.; Cajthaml, T.; Petránková, M.; Šnajdr, J. Small-scale distribution of extracellular enzymes, fungal, and bacterial biomass in Quercus petraea forest topsoil. Biol. Fertil. Soils 2010, 46, 717–726. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, 35, 2190–2204. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.S.; Tripathi, B.M.; Adams, J. Distinct bacterial communities dominate tropical and temperate zone leaf litter. Microb. Ecol. 2014, 67, 837–848. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Purahong, W.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Daumlich, V.; Mital, S.; Buscot, F.; Hofrichter, M.; Gutknecht, J.L.; Kruger, D. Uncoupling of microbial community structure and function in decomposing litter across beech forest ecosystems in Central Europe. Sci. Rep. 2014, 4, 7014. [Google Scholar] [CrossRef] [PubMed]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hattenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Xia, Z.; Bai, E.; Wang, Q.; Gao, D.; Zhou, J.; Jiang, P.; Wu, J. Biogeographic distribution patterns of bacteria in typical chinese forest soils. Front. Microbiol. 2016, 7, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kardol, P.; Cregger, M.; Campany, C.; Classen, A. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Franciska, T.d.V.; Robert, I.G.; Chapter, F. Impacts of Climate Change on Soil Microbial Communities and Their Functioning. In Developments in Soil Science; Horwath, W.R., Kuzyakov, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–129. [Google Scholar] [CrossRef]
- Hussnain, M.; Rainer, F.W.; Adnan, M.; Andrianto, A.; Oleg, V.; Shipin, T.N.-D.C.; Yu-Pin, L. Soil microbiome feedback to climate change and options for mitigation. Sci. Total Env. 2023, 882, 163412. [Google Scholar] [CrossRef]
- Yilun, H.; Hasbagan, G.; Guozheng, H.; Xuexia, W.; Zhiqiang, W.; Qingzhu, G. Seasonal patterns of soil microbial community response to warming and increased precipitation in a semiarid steppe. Appl. Soil. Ecol. 2023, 182, 104712. [Google Scholar] [CrossRef]
- Wei, H.; Peng, C.; Yang, B.; Song, H.; Li, Q.; Jiang, L.; Wei, G.; Wang, K.; Wang, H.; Liu, S.; et al. Contrasting soil bacterial community, diversity, and function in two forests in China. Front. Microbiol. 2018, 9, 1693. [Google Scholar] [CrossRef]
- Lili, F.; Muhammad, W.K.T.; Yangyang, Z.; Yongzhen, H.; Jundong, R.; Xinhang, C.; Liguang, C.; Chengkun, S.; Yushan, Z. Patterns of soil microorganisms and enzymatic activities of various forest types in coastal sandy land. Glob. Ecol. Conserv. 2021, 28, e01625. [Google Scholar] [CrossRef]
- Jin, Z.-Z.; Lei, J.-Q.; Xu, X.-W.; Li, S.; Zhao, S.-F. Microbial diversities of shelter ’forest soils in the extreme arid area. Acta Ecolc. Sinica. 2009, 29, 4549–4559. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Y.; Zhao, Y. Diversity and functional potential of soil bacterial communities in different types of farmland shelterbelts in Mid-Western Heilongjiang, China. Forests 2019, 10, 1115. [Google Scholar] [CrossRef]
- Prada-Salcedo, L.D.; Prada-Salcedo, J.P.; Heintz-Buschart, A.; Buscot, F.; Goldmann, K. Effects of tree composition and soil depth on structure and functionality of belowground microbial communities in temperate European forests. Front. Microbiol. 2022, 13, 920618. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Andersen, K.; Morton, J.B. Arbuscular mycorrhizal communities in tropical forests are affected by host tree species and environment. Oecologia 2003, 135, 268–279. [Google Scholar] [CrossRef]
- Rożek, K.; Rola, K.; Błaszkowski, J.; Leski, T.; Zubek, S. How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil? For. Ecol. Manag. 2020, 465, 118091. [Google Scholar] [CrossRef]
- Ibáñez, B.; Gómez-Aparicio, L.; Ávila, J.M.; Pérez-Ramos, I.M.; García, L.V.; Marañón, T. Impact of tree decline on spatial patterns of seedling-mycorrhiza interactions: Implications for regeneration dynamics in Mediterranean forests. For. Ecol. Manag. 2015, 353, 1–9. [Google Scholar] [CrossRef]
- Carteron, A.; Vellend, M.; Laliberté, E. Mycorrhizal dominance reduces local tree species diversity across US forests. Nat. Ecol. Evol. 2022, 6, 370–374. [Google Scholar] [CrossRef]
- Barceló, M.; van Bodegom, P.M.; Tedersoo, L.; Olsson, P.A.; Soudzilovskaia, N.A. Mycorrhizal Tree impacts on topsoil biogeochemical properties in tropical forests. J. Ecol. 2022, 110, 1271–1282. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal Types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. 2022, 94, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Churchland, C.; Grayston, S.J. Specificity of Plant-Microbe Interactions in the Tree Mycorrhizosphere Biome and Consequences for Soil C Cycling. Front. Microbiol. 2014, 5, 261. [Google Scholar] [CrossRef] [PubMed]
- Boeraeve, M.; Leroux, O.; De Lange, R.; Verbeken, A.; Jacquemyn, H. The effect of surrounding vegetation on the mycorrhizal fungal communities of the temperate tree Crataegus monogyna Jacq. Front. Fungal Biol. 2021, 2, 741813. [Google Scholar] [CrossRef]
- Bainard, L.D.; Klironomos, J.N.; Gordon, A.M. The mycorrhizal status and colonization of 26 tree species growing in urban and rural environments. Mycorrhiza 2011, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Singavarapu, B.; Beugnon, R.; Bruelheide, H.; Cesarz, S.; Du, J.; Eisenhauer, N.; Guo, L.-D.; Nawaz, A.; Wang, Y.; Xue, K.; et al. Tree mycorrhizal type and tree diversity shape the forest soil microbiota. Environ. Microbiol. 2022, 24, 4236–4255. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Garbini, G.L.; Rolando, L.; Campanale, C.; Aimola, G.; Fernandez-Lopez, M.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Ancona, V. Characterization of the belowground microbial community in a poplar-phytoremediation strategy of a multi-contaminated Soil. Front. Microbiol. 2020, 11, 2073. [Google Scholar] [CrossRef]
- Almonacid-Muñoz, L.; Herrera, H.; Fuentes-Ramírez, A.; Vargas-Gaete, R.; Larama, G.; Jara, R.; Fernández-Urrutia, C.; da Silva Valadares, R.B. Tree cover species modify the diversity of rhizosphere-associated microorganisms in Nothofagus obliqua (Mirb.) Oerst temperate forests in South-Central Chile. Forests 2022, 13, 756. [Google Scholar] [CrossRef]
- Yurong, Y.; Miao, D.; Yaping, C.; Jinlong, W.; Ming, T.; Yihui, B. Comparisons of soil properties, enzyme activities and microbial communities in heavy metal contaminated bulk and rhizosphere soils of Robinia pseudoacacia L. in the Northern Foot of Qinling Mountain. Forests 2017, 8, 430. [Google Scholar] [CrossRef]
- Sapsford, S.J.; Paap, T.; Hardy, G.E.S.J.; Burgess, T.I. Anthropogenic disturbance impacts mycorrhizal communities and abiotic soil properties: Implications for an endemic forest disease. Front. For. Glob. Change 2021, 3, 593243. [Google Scholar] [CrossRef]
- Gerz, M.; Bueno, C.G.; Ozinga, W.A.; Zobel, M.; Moora, M. Responses of plant community mycorrhization to anthropogenic influence depend on the habitat and mycorrhizal type. Oikos 2019, 128, 1565–1575. [Google Scholar] [CrossRef]
- Sapsford, S.J.; Paap, T.; Hopkins, A.J.M.; Hardy, G.E.S.J.; Burgess, T.I. Habitat Fragmentation in a Mediterranean-type forest alters resident and propagule mycorrhizal fungal communities. Pedobiologia 2020, 78, 150611. [Google Scholar] [CrossRef]
- Jo, I.; Fei, S.; Oswalt, C.M.; Domke, G.M.; Phillips, R.P. Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci. Adv. 2019, 5, eaav6358. [Google Scholar] [CrossRef] [PubMed]
- Egli, S. Mycorrhizal mushroom diversity and productivity—An indicator of forest health? Ann. Forest Sci. 2011, 68, 81–88. [Google Scholar] [CrossRef]
- Sapsford, S.J.; Paap, T.; Hardy, G.E.S.J.; Burgess, T.I. The ‘chicken or the egg’: Which comes first, forest tree decline or loss of mycorrhizae? Plant Ecol. 2017, 218, 1093–1106. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Tan, S.; Yu, L.; Tang, C.; You, Y. Overview of vegetation factors related to the diversity of arbuscular mycorrhizal fungi and their interactions in karst areas. Appl. Soil Ecol. 2024, 198, 105387. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Meharg, A.A. Influences of anthropogenic pollution on mycorrhizal fungal communities. Environ. Poll. 1999, 106, 169–182. [Google Scholar] [CrossRef]
- Op De Beeck, M.; Lievens, B.; Busschaert, P.; Rineau, F.; Smits, M.; Vangronsveld, J.; Colpaert, J.V. Impact of metal pollution on fungal diversity and community structures. Environ. Microbiol. 2015, 17, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, T.; Van Geel, M.; Jacquemyn, H.; Boeraeve, M.; Plue, J.; Saar, L.; Kasari, L.; Peeters, G.; van Acker, K.; Crauwels, S.; et al. Arbuscular mycorrhizal fungi in european grasslands under nutrient pollution. Glob. Ecol. Biogeogr. 2019, 28, 1796–1805. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Kuyper, T.W.; Bidartondo, M.I.; Hobbie, E.A. Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: A review. Environ. Poll. 2019, 246, 148–162. [Google Scholar] [CrossRef]
- Egerton-Warburton, L.M.; Allen, E.B. Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol. Appl. 2000, 10, 484–496. [Google Scholar] [CrossRef]
- Pereira, S.; Leal, I.R.; Tabarelli, M.; Santos, M.G. Intense mycorrhizal root colonization in a human-modified landscape of the Caatinga dry forest. For. Ecol. Manag. 2020, 462, 117970. [Google Scholar] [CrossRef]
- Rusterholz, H.-P.; Studer, M.; Zwahlen, V.; Baur, B. Plant-mycorrhiza association in urban forests: Effects of the degree of urbanisation and forest size on the performance of Sycamore (Acer pseudoplatanus) saplings. Urban For. Urban Green. 2020, 56, 126872. [Google Scholar] [CrossRef]
- Chatterjee, A.; Vance, G.; Pendall, E.; Stahl, P.; Chatterjee, A.; Vance, G.F.; Pendall, E.; Stahl, P.D. Timber harvesting alters soil carbon mineralization and microbial community structure in coniferous forests. Soil Biol. Biochem. 2008, 40, 1901–1907. [Google Scholar] [CrossRef]
- Smith, N.R.; Kishchuk, B.E.; Mohn, W.W. Effects of wildfire and harvest disturbances on forest soil bacterial communities. Appl. Environ. Microbiol. 2008, 74, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Dighton, J.; White, J.; Oudemans, P. The Fungal Community: Its Organization and Role in the Ecosystem, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 960. [Google Scholar] [CrossRef]
- Farrar, J.; Hawes, M.; Jones, D.; Lindow, S. How roots control the flux of carbon to the rhizosphere. Ecology 2003, 84, 827–837. [Google Scholar] [CrossRef]
- Outerbridge, R.A.; Trofymow, J.A. Forest management and maintenance of ectomycorrhizae: A case study of green tree retention in south-coastal British Columbia. J. Ecosyst. Manag. 2009, 10, 59–80. [Google Scholar] [CrossRef]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Wagner, G. Carbon transformation and soil organic matter formation. In Principles and Applications of Soil Microbiology; Sylvia, D., Fuhrmann, J., Hartel, P., Zuberer, D., Eds.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2005; pp. 285–332. [Google Scholar]
- Nakatsu, C. Microbial genetics. In Principles and Applications of Soil Microbiology; Sylvia, D., Fuhrmann, J., Hartel, P., Zuberer, D., Eds.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2005; pp. 85–100. [Google Scholar]
- Mariani, L.; Chang, S.X.; Kabzems, R. Effects of tree harvesting, forest floor removal, and compaction on soil microbial biomass, microbial respiration, and N availability in a boreal aspen forest in British Columbia. Soil Biol. Biochem. 2006, 38, 1734–1744. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.X.; Kabzems, R. Effects of soil compaction and forest floor removal on soil microbial properties and N transformations in a boreal forest long-term soil productivity study. For. Ecol. Manag. 2005, 217, 158–170. [Google Scholar] [CrossRef]
- Shestak, C.J.; Busse, M.D. Compaction alters physical but not biological indices of soil health. Soil Sci. Soc. Am. J. 2005, 69, 236–246. [Google Scholar] [CrossRef]
- Jordán, A.; Martínez-Zavala, L. Soil loss and runoff rates on unpaved forest roads in southern Spain after simulated rainfall. For. Eco. Manag. 2008, 255, 913–919. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Dong, Y.; Wei, Y.; Jiao, R. Unravelling the functional diversity of the soil microbial community of Chinese fir plantations of different densities. Forest 2018, 9, 532. [Google Scholar] [CrossRef]
- Wilhelm, R.C.; Cardenas, E.; Maas, K.R.; Leung, H.; McNeil, L.; Berch, S.; Chapman, W.; Hope, G.; Kranabetter, J.M.; Dubé, S.; et al. Biogeography and organic matter removal shape long-term effects of timber harvesting on forest soil microbial communities. ISME J. 2017, 11, 2552–2568. [Google Scholar] [CrossRef] [PubMed]
- Dickie, I.A.; Richardson, S.J.; Wiser, S.K. Ectomycorrhizal fungal communities and soil chemistry in harvested and unharvested temperate nothofagus rainforests. Can. J. For. Res. 2009, 39, 1069–1079. [Google Scholar] [CrossRef]
- Parladé, J.; Martínez-Peña, F.; Pera, J. Effects of forest management and climatic variables on the mycelium dynamics and sporocarp production of the ectomycorrhizal fungus Boletus edulis. For. Ecol. Manag. 2017, 390, 73–79. [Google Scholar] [CrossRef]
- Wurzburger, N.; Elliott, K.J.; Miniat, C.F. Forest mycorrhizal dominance depends on historical land use and nitrogen-fixing trees. J. Appl. Ecol. 2023, 60, 1551–1561. [Google Scholar] [CrossRef]
- Tomao, A.; Antonio Bonet, J.; Castaño, C.; de-Miguel, S. How does forest management affect fungal diversity and community composition? Current knowledge and future perspectives for the conservation of forest fungi. For. Ecol. Manag. 2020, 457, 117678. [Google Scholar] [CrossRef]
- Marshall, V.G. Impacts of forest harvesting on biological processes in northern forest soils. For. Ecol. Manag. 2000, 133, 43–60. [Google Scholar] [CrossRef]
- Jurgensen, M.F.; Harvey, A.E.; Graham, R.T.; Page-Dumroese, D.S.; Tonn, J.R.; Larsen, M.J.; Jain, T.B. Impacts of timber harvesting on soil organic matter, nitrogen, productivity, and health of inland northwest forests. For. Sci. 1997, 43, 234–251. [Google Scholar] [CrossRef]
- Varenius, K.; Kårén, O.; Lindahl, B.; Dahlberg, A. Long-term effects of tree harvesting on ectomycorrhizal fungal communities in boreal Scots Pine forests. For. Ecol. Manag. 2016, 380, 41–49. [Google Scholar] [CrossRef]
- Simard, S.W.; Roach, W.J.; Beauregard, J.; Burkart, J.; Cook, D.; Law, D.; Murphy-Steed, A.; Schacter, T.; Zickmantel, A.; Armstrong, G.; et al. Partial retention of legacy trees protect mycorrhizal inoculum potential, biodiversity, and soil resources while promoting natural regeneration of interior douglas-fir. Front. For. Glob. Change 2021, 3, 620436. [Google Scholar] [CrossRef]
- Ellis, E.C. Anthropogenic transformation of the terrestrial biosphere. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 1010–1035. [Google Scholar] [CrossRef] [PubMed]
- Millenium Ecosystem Assessment 2005. Ecosystems and Human Well Being: Wetlands and Water Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Watson, S.J.; Luck, G.W.; Spooner, P.G.; Watson, D.M. Land-use change: Incorporating the frequency, sequence, time span, and magnitude of changes into ecological research. Front. Ecol. Environ. 2014, 12, 241–249. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- James, J.; Harrison, R. The effect of harvest on forest soil carbon: A meta-analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Kohout, P.; Charvátová, M.; Štursová, M.; Mašínová, T.; Tomšovský, M.; Baldrian, P. Clearcutting alters decomposition processes and initiates complex restructuring of fungal communities in soil and tree roots. ISME J. 2018, 12, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, C.R.; Lewis, T. Long term repeated fire disturbance alters soil bacterial diversity but not the abundance in an Australian wet sclerophyll forest. Sci. Rep. 2016, 6, 19639. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Effects of forest degradation on microbial communities and soil carbon cycling: A global meta-analysis. Glob. Ecol. Biogeogr. 2018, 27, 110–124. [Google Scholar] [CrossRef]
- Osburn, E.D.; Elliottt, K.J.; Knoepp, J.D.; Miniat, C.F.; Barrett, J.E. Soil microbial response to Rhododendron understory removal in southern appalachian forests: Effects on extracellular enzymes. Soil Biol. Biochem. 2018, 127, 50–59. [Google Scholar] [CrossRef]
- Simon, S.A.; Collins, T.K.; Kauffman, G.L.; McNab, W.H.; Ulrey, C.J. Ecological Zones in the Southern Appalachians: First Approximation; USDA Forest Service: Asheville, NC, USA, 2005. [CrossRef]
- Gragson, T.L.; Bolstad, P.V. Land use legacies and the future of Southern Appalachia. Soc. Nat. Resour. 2006, 19, 175–190. [Google Scholar] [CrossRef]
- Keiser, A.D.; Knoepp, J.D.; Bradford, M.A. Disturbance decouples biogeochemical cycles across forests of the southeastern US. Ecosystems 2016, 19, 50–61. [Google Scholar] [CrossRef]
- Lin, W.R.; Chen, W.C.; Wang, P.H. Soil microbial community is resilient to thinning disturbance. Trop. Ecol. 2023, 64, 62–71. [Google Scholar] [CrossRef]
- Teste, F.P.; Simard, S.W.; Durall, D.M.; Guy, R.D.; Jones, M.D.; Schoonmaker, A.L. Access to mycorrhizal networks and roots of trees: Importance for seedling survival and resource transfer. Ecology 2009, 90, 2808–2822. [Google Scholar] [CrossRef]
- Bonfim, J.A.; Vasconcellos, R.L.F.; Stürmer, S.L.; Cardoso, E.J.B.N. Arbuscular mycorrhizal fungi in the Brazilian Atlantic forest: A gradient of environmental restoration. Appl. Soil. Ecol. 2013, 71, 7–14. [Google Scholar] [CrossRef]
- Policelli, N.; Horton, T.R.; Hudon, A.T.; Patterson, T.R.; Bhatnagar, J.M. Back to Roots: The role of ectomycorrhizal fungi in boreal and temperate forest restoration. Front. For. Glob. Change 2020, 3, 97. [Google Scholar] [CrossRef]
- Becerra, A.G.; Diván, A.; Renison, D. Bare soil cover and arbuscular mycorrhizal community in the first montane forest restoration in Central Argentina. Restor. Ecol. 2019, 27, 804–812. [Google Scholar] [CrossRef]
- Neuenkamp, L.; Prober, S.M.; Price, J.N.; Zobel, M.; Standish, R.J. Benefits of mycorrhizal inoculation to ecological restoration depend on plant functional type, restoration context and time. Fungal Ecol. 2019, 40, 140–149. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Goto, B.T.; Ganade, G. Ecological restoration methods influence the structure of arbuscular mycorrhizal fungal communities in degraded drylands. Pedobiologia 2021, 84, 150690. [Google Scholar] [CrossRef]
- Wall, C.B.; Egan, C.P.; Swift, S.I.O.; Hynson, N.A. Three decades post-reforestation has not led to the reassembly of arbuscular mycorrhizal fungal communities associated with remnant primary forests. Mol. Ecol. 2020, 29, 4234–4247. [Google Scholar] [CrossRef]
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Lance, A.C.; Carrino-Kyker, S.R.; Burke, D.J.; Burns, J.H. Individual plant-soil feedback effects influence tree growth and rhizosphere fungal communities in a temperate forest restoration experiment. Front Ecol. Evol. 2020, 7, 500. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G.; et al. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Alkama, R.; Cescatti, A. Biophysical climate impacts of recent changes in global forest cover. Science 2016, 351, 600–604. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Gardi, C.; Jeffery, S.; Saltelli, A. An estimate of potential threats levels to soil biodiversity in EU. Glob. Change Biol. 2013, 19, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, L.M.; Aerts, R.; Brovkin, V.; Cavender-Bares, J.; Cornelissen, J.; Kattge, J.; Bodegom, P.M. Inclusion of ecologically based trait variation in plant functional types reduces the projected land carbon sink in an earth system model. Glob. Change Biol. 2015, 21, 3074–3086. [Google Scholar] [CrossRef]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 2015, 6, 130. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.I.; Putten, W.H.V.D.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Wenjing, C.; Huakun, Z.; Yang, W.; Jie, W.; Ziwen, Z.; Yuanze, L.; Leilei, Q.; Bing, Y.; Kelu, C.; Guobin, L.; et al. Loss of plant functional groups impacts soil carbon flow by changing multitrophic interactions within soil micro-food webs. Appl. Soil. Ecol. 2022, 178, 104566. [Google Scholar] [CrossRef]
- Uroz, S.; Buée, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the forest microbiome: Highlights of temperate and boreal ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Voříšková, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Treseder, K.K. Climate change feedbacks to microbial decomposition in boreal soils. Fungal Ecol. 2021, 4, 362–374. [Google Scholar] [CrossRef]
- Qiu, S.L.; Wang, L.M.; Huang, D.F.; Lin, X.J. Effects of fertilization regimes on tea yields, soil fertility, and soil microbial diversity. Chile J. Agric. Res. 2014, 74, 333–339. [Google Scholar] [CrossRef]
- Naether, A.; Foesel, B.U.; Naegele, V.; Wüst, P.K.; Weinert, J.; Bonkowski, M.; Alt, F.; Oelmann, Y.; Polle, A.; Lohaus, G.; et al. Environmental factors affect Acidobacterial communities below the subgroup level in grassland and forest soils. Appl. Environ. Microbiol. 2012, 78, 7398–7406. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A thready affair: Linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valaskova, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, C.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.B.; Knelman, J.E.; Schindlbacher, A.; Siciliano, S.; Breulmann, M.; Yannarell, A.; Beman, J.M.; Abell, G.; Philippot, L.; Prosser, J.; et al. Microbes as engines of ecosystem function: When does community structure enhance predictions of ecosystem processes? Front. Microbiol. 2016, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.K.; Cohen, H.; Higgins, L.M.; Kennedy, P.G. Testing the link between community structure and function for ectomycorrhizal fungi involved in a global tripartite symbiosis. New Phytol. 2014, 202, 287–296. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Brandstrom-Durling, M.; Clemmensen, K.E.; Lindahl, B.D. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).