Hydrologic Perturbation Is a Key Driver of Tree Mortality in Bottomland Hardwood Wetland Forests of North Carolina, USA
Abstract
1. Introduction
2. Materials and Methods
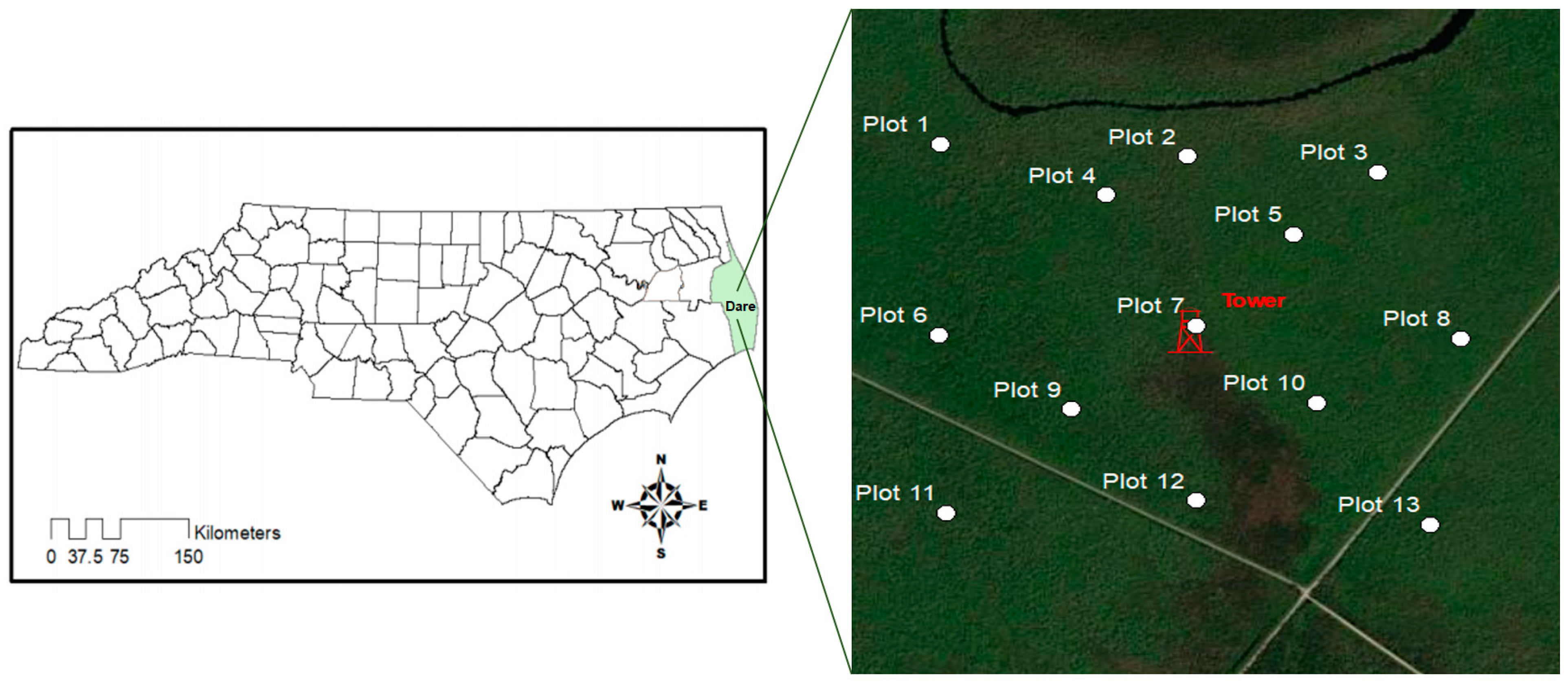
2.1. The Study Site
2.2. Mortality Rate Calculation
2.3. The Key Drivers of Tree Mortality
2.3.1. Hydrologic Variables
2.3.2. Biological Variables
2.3.3. Climatic Variables
2.4. Modeling the Effects of Key Drivers on Tree Mortality
2.5. Other Statistical Analysis and Visualization
3. Results
3.1. Species Composition and Tree Density
3.2. Inter-Annual Mortality Rates
3.3. Mortality Rate per Species
3.4. Variations of Predictor Variables
3.4.1. LAI—The Biological Driver
3.4.2. Hydrologic Drivers
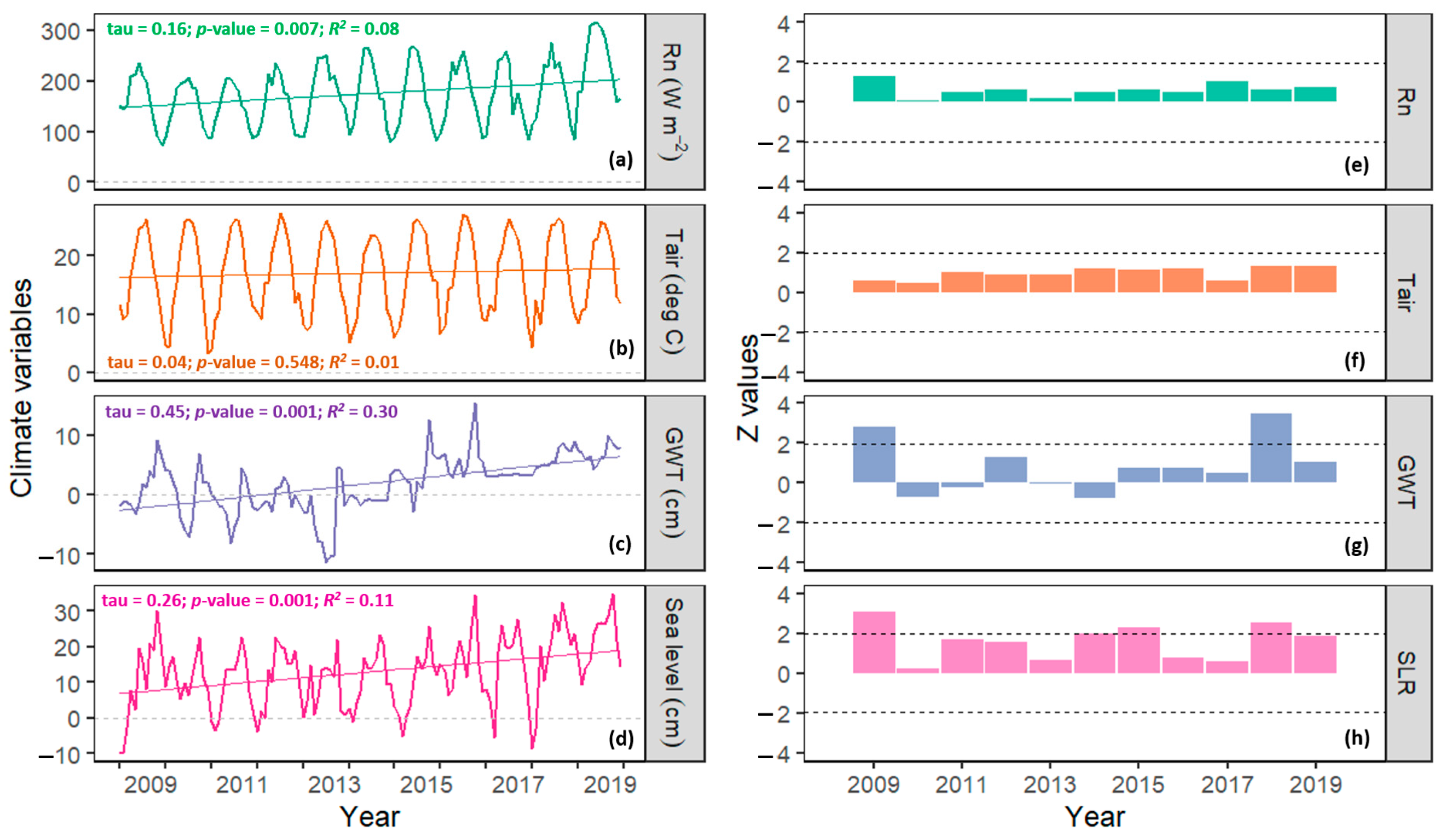
3.4.3. Climatic Drivers
3.5. Drivers of Tree Mortality
4. Discussion
4.1. Species Adaptation to Inundation
4.2. Tree Mortality
4.3. What Drives the Tree Mortality?
4.3.1. Impacts of Hydrologic Drivers
4.3.2. Impacts of Biological Drivers
4.3.3. Impacts of Climatic Drivers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Baiser, B.; James, R.T.; Reddy, K.R. An analysis of long-term Everglades Stormwater Treatment Areas performance using structural equation models. Ecol. Eng. 2024, 198, 107130. [Google Scholar] [CrossRef]
- White, E.; Kaplan, D. Restore or retreat? saltwater intrusion and water management in coastal wetlands. Ecosyst. Health Sustain. 2017, 3, e01258. [Google Scholar] [CrossRef]
- Osland, M.J.; Enwright, N.M.; Day, R.H.; Gabler, C.A.; Stagg, C.L.; Grace, J.B. Beyond just sea-level rise: Considering macroclimatic drivers within coastal wetland vulnerability assessments to climate change. Glob. Change Biol. 2016, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blankespoor, B.; Dasgupta, S.; Laplante, B. Sea-Level Rise and Coastal Wetlands. Ambio 2014, 43, 996–1005. [Google Scholar] [CrossRef]
- Spencer, T.; Schuerch, M.; Nicholls, R.J.; Hinkel, J.; Lincke, D.; Vafeidis, A.T.; Reef, R.; McFadden, L.; Brown, S. Global coastal wetland change under sea-level rise and related stresses: The DIVA Wetland Change Model. Glob. Planet. Change 2016, 139, 15–30. [Google Scholar] [CrossRef]
- Schuerch, M.; Spencer, T.; Temmerman, S.; Kirwan, M.L.; Wolff, C.; Lincke, D.; McOwen, C.J.; Pickering, M.D.; Reef, R.; Vafeidis, A.T.; et al. Future response of global coastal wetlands to sea-level rise. Nature 2018, 561, 231–234. [Google Scholar] [CrossRef]
- Fluet-Chouinard, E.; Stocker, B.D.; Zhang, Z.; Malhotra, A.; Melton, J.R.; Poulter, B.; Kaplan, J.O.; Goldewijk, K.K.; Siebert, S.; Minayeva, T.; et al. Extensive global wetland loss over the past three centuries. Nature 2023, 614, 281–286. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Reef, R. Variable Impacts of Climate Change on Blue Carbon. One Earth 2020, 3, 195–211. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Naidoo, G. The mangroves of Africa: A review. Mar. Pollut. Bull. 2023, 190, 114859. [Google Scholar] [CrossRef]
- Ilman, M.; Dargusch, P.; Dart, P.; Onrizal. A historical analysis of the drivers of loss and degradation of Indonesia’s mangroves. Land Use Policy 2016, 54, 448–459. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Basyuni, M.; Kridalaksana, A.; Saragi-Sasmito, M.F.; Lovelock, C.E.; Murdiyarso, D. Challenges and opportunities for achieving Sustainable Development Goals through restoration of Indonesia’s mangroves. Nat. Ecol. Evol. 2023, 7, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Naz, A.; Sharma, S.B.; Dasgupta, R. Changes in Salinity, Mangrove Community Ecology, and Organic Blue Carbon Stock in Response to Cyclones at Indian Sundarbans. Life 2023, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Lacerda, L.D. Degradation and conservation of Brazilian mangroves, status and perspectives. Ocean Coast. Manag. 2016, 125, 38–46. [Google Scholar] [CrossRef]
- Barletta, M.; Melo, R.C.B.; Whitfield, A.K. Past and present conservation of South American estuaries. Estuar. Coast. Shelf Sci. 2023, 295, 108542. [Google Scholar] [CrossRef]
- Fragal, E.H.; Silva, T.S.F.; de Moraes Novo, E.M.L. Reconstructing historical forest cover change in the lower amazon floodplains using the landtrendr algorithm. Acta Amaz. 2016, 46, 13–24. [Google Scholar] [CrossRef]
- McDowell, N.G.; Ball, M.; Bond-Lamberty, B.; Kirwan, M.L.; Krauss, K.W.; Megonigal, J.P.; Mencuccini, M.; Ward, N.D.; Weintraub, M.N.; Bailey, V. Processes and mechanisms of coastal woody-plant mortality. Glob. Change Biol. 2022, 28, 5881–5900. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Gedan, K.B. Sea-level driven land conversion and the formation of ghost forests. Nat. Clim. Change 2019, 9, 450–457. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Lovelock, C.E.; Santos, I.R.; Sanders, C.J.; Maher, D.T. Mangrove mortality in a changing climate: An overview. Estuar. Coast. Shelf Sci. 2018, 215, 241–249. [Google Scholar] [CrossRef]
- Chen, Y.; Kirwan, M.L. Climate-driven decoupling of wetland and upland biomass trends on the mid-Atlantic coast. Nat. Geosci. 2022, 15, 913–918. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Megonigal, J.P. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 2013, 504, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Fatoyinbo, L.; Smetanka, C.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Thomas, N.; Van der Stocken, T. Mangrove canopy height globally related to precipitation, temperature and cyclone frequency. Nat. Geosci. 2019, 12, 40–45. [Google Scholar] [CrossRef]
- Schepers, L.; Brennand, P.; Kirwan, M.L.; Guntenspergen, G.R.; Temmerman, S. Coastal Marsh Degradation Into Ponds Induces Irreversible Elevation Loss Relative to Sea Level in a Microtidal System. Geophys. Res. Lett. 2020, 47, e2020GL089121. [Google Scholar] [CrossRef]
- Carr, J.; Guntenspergen, G.; Kirwan, M. Modeling Marsh-Forest Boundary Transgression in Response to Storms and Sea-Level Rise. Geophys. Res. Lett. 2020, 47, e2020GL088998. [Google Scholar] [CrossRef]
- Celis-Hernandez, O.; Villoslada-Peciña, M.; Ward, R.D.; Bergamo, T.F.; Perez-Ceballos, R.; Girón-García, M.P. Impacts of environmental pollution on mangrove phenology: Combining remotely sensed data and generalized additive models. Sci. Total Environ. 2022, 810, 152309. [Google Scholar] [CrossRef]
- Holmquist, J.R.; Windham-Myers, L.; Bliss, N.; Crooks, S.; Morris, J.T.; Megonigal, J.P.; Troxler, T.; Weller, D.; Callaway, J.; Drexler, J.; et al. Accuracy and precision of tidal wetland soil carbon mapping in the conterminous United States. Sci. Rep. 2018, 8, 9478. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Temmerman, S.; Skeehan, E.E.; Guntenspergen, G.R.; Fagherazzi, S. Overestimation of marsh vulnerability to sea level rise. Nat. Clim. Change 2016, 6, 253–260. [Google Scholar] [CrossRef]
- Aguilos, M.; Warr, I.; Irving, M.; Gregg, O.; Grady, S.; Peele, T.; Noormets, A.; Sun, G.; Liu, N.; McNulty, S.; et al. The Unabated Atmospheric Carbon Losses in a Drowning Wetland Forest of North Carolina: A Point of No Return? Forests 2022, 13, 1264. [Google Scholar] [CrossRef]
- Mackay, D.S.; Ewers, B.E.; Cook, B.D.; Davis, K.J. Environmental drivers of evapotranspiration in a shrub wetland and an upland forest in northern Wisconsin. Water Resour. Res. 2007, 43, 1–14. [Google Scholar] [CrossRef]
- Aguilos, M.; Mitra, B.; Noormets, A.; Minick, K.; Prajapati, P.; Gavazzi, M.; Sun, G.; McNulty, S.; Li, X.; Domec, J.C.J.-C.; et al. Long-term carbon flux and balance in managed and natural coastal forested wetlands of the Southeastern USA. Agric. For. Meteorol. 2020, 288–289, 108022. [Google Scholar] [CrossRef]
- Ward, N.D.; Megonigal, J.P.; Bond-Lamberty, B.; Bailey, V.L.; Butman, D.; Canuel, E.A.; Diefenderfer, H.; Ganju, N.K.; Goñi, M.A.; Graham, E.B.; et al. Representing the function and sensitivity of coastal interfaces in Earth system models. Nat. Commun. 2020, 11, 2458. [Google Scholar] [CrossRef] [PubMed]
- Ury, E.A.; Yang, X.I.; Wright, J.P.; Bernhardt, E.S.; Ury, C.; Yang, X.I.; Wright, J.P.; Bernhardt, E.S. Rapid deforestation of a coastal landscape driven by sea-level rise and extreme events. Ecol. Appl. 2021, 31, 2021. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Rivera-Ocasio, E.; Heartsill-Scalley, T.; Davila-Casanova, D.; Rios-López, N.; Gao, Q. Landscape-Level Consequences of Rising Sea-Level on Coastal Wetlands: Saltwater Intrusion Drives Displacement and Mortality in the Twenty-First Century. Wetlands 2019, 39, 1343–1355. [Google Scholar] [CrossRef]
- Krauss, K.W.; Duberstein, J.A.; Doyle, T.W.; Conner, W.H.; Day, R.H.; Inabinette, L.W.; Whitbeck, J.L. Site condition, structure, and growth of baldcypress along tidal/non-tidal salinity gradients. Wetlands 2009, 29, 505–519. [Google Scholar] [CrossRef]
- Noormets, A.; King, J.; Mitra, B.; Miao, G.; Aguilos, M.; Minick, K.; Prajapati, P.; Domec, J.-C. AmeriFlux FLUXNET-1F US-NC4 NC_AlligatorRiver; Ver. 3-5, AmeriFlux AMP, (Dataset); Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA; Texas A&M University: College Station, TX, USA, 2022. [Google Scholar] [CrossRef]
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.C.; McNulty, S.; Gavazzi, M.; Minick, K.; Mitra, B.; Prajapati, P.; Yang, Y.; et al. Effects of land-use change and drought on decadal evapotranspiration and water balance of natural and managed forested wetlands along the southeastern US lower coastal plain. Agric. For. Meteorol. 2021, 303, 108381. [Google Scholar] [CrossRef]
- Lewis, R.R. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 2005, 24, 403–418. [Google Scholar] [CrossRef]
- Sheil, D.; May, R. Mortality and Recruitment Rate Evaluations in Heterogeneous Tropical Forests. J. Ecol. 1996, 84, 91–100. [Google Scholar] [CrossRef]
- Sun, G.; Alstad, K.; Chen, J.; Chen, S.; Ford, C.R.; Lin, G.; Liu, C.; Lu, N.; Mcnulty, S.G.; Miao, H.; et al. A general predictive model for estimating monthly ecosystem evapotranspiration. Ecohydrology 2011, 4, 245–255. [Google Scholar] [CrossRef]
- Gunston, H.; Batchelor, C.H. Defining crop evaporation A major problem in defining crop evaporation is the number of terms transpiration and reference crop evapotranspiration. To add confusion, each. Agric. Water Manag. 1983, 6, 65–77. [Google Scholar] [CrossRef]
- Ponraj, A.S.; Vigneswaran, T. Daily evapotranspiration prediction using gradient boost regression model for irrigation planning. J. Supercomput. 2020, 76, 5732–5744. [Google Scholar] [CrossRef]
- Priestley, C.H.B.; Taylor, R.J. On the Assessment of Surface Heat Flux and Evaporation Using Large-Scale Parameters. Mon. Weather Rev. 1972, 100, 81–92. [Google Scholar] [CrossRef]
- Yang, Y.; Anderson, M.; Gao, F.; Hain, C.; Noormets, A.; Sun, G.; Wynne, R.; Thomas, V.; Sun, L. Investigating impacts of drought and disturbance on evapotranspiration over a forested landscape in North Carolina, USA using high spatiotemporal resolution remotely sensed data. Remote Sens. Environ. 2020, 238, 111018. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–35. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Griffin, C., Ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- Rauf, A.U.; Rafi, M.S.; Ali, I.; Muhammad, U.W. Temperature trend detection in upper Indus basin by using Mann-Kendall test. Adv. Sci. Technol. Eng. Syst. 2016, 1, 5–13. [Google Scholar] [CrossRef]
- Yue, S.; Pilon, P.; Cavadias, G. Power of the Mann-Kendall and Spearman’s rho tests for detecting monotonic trends in hydrological series. J. Hydrol. 2002, 259, 254–271. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Langston, A.K.; Kaplan, D.A.; Putz, F.E. A casualty of climate change? Loss of freshwater forest islands on Florida’s Gulf Coast. Glob. Change Biol. 2017, 23, 5383–5397. [Google Scholar] [CrossRef]
- Hallett, R.; Johnson, M.L.; Sonti, N.F. Assessing the tree health impacts of salt water flooding in coastal cities: A case study in New York City. Landsc. Urban Plan. 2018, 177, 171–177. [Google Scholar] [CrossRef]
- Hook, D.D.; Debell, D.S.; Mckee, W.H.; Askew, J.L. Responses of loblolly pine (mesophyte) and swamp tupelo (hydrophyte) seedlings to soil flooding and phosphorus. Plant Soil 1983, 71, 387–394. [Google Scholar] [CrossRef]
- Mccarron, J.K.; Mcleod, K.W.; Conner, W.H. Flood and salinity stress of wetland woody species, Buttonbush (Cephalanthus occidentalis) and Swamp tupelo (Nyssa sylvatica vail. biflora). Wetlands 1998, 18, 165–175. [Google Scholar] [CrossRef]
- Conner, W.; Doyle, T.; Krauss, K. Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9781402050947. [Google Scholar]
- Burns, R.; Honkala, B. Silvics of North America: 1. Conifers; 2. Hardwoods. Ahriculture Handbook 654; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2.
- Anella, L.B.; Whitlow, T.H. Flood-tolerance ranking of red and freeman maple cultivars. J. Arboric. 1999, 25, 31–36. [Google Scholar] [CrossRef]
- Lauer, N.T. Physiological and Biochemical Responses of Bald Cypress to Salt Stress. Master’s Thesis, University of North Florida, Jacksonville, FL, USA, 2013. [Google Scholar]
- Allen, J.A.; Pezeshki, S.R.; Chambers, J.L. Interaction of flooding and salinity stress on baldcypress (Taxodium distichum). Tree Physiol. 1996, 16, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S. Implications of population phases on the integrated pest management of the southern pine beetle, Dendroctonus frontalis. J. Integr. Pest Manag. 2012, 3, F1–F7. [Google Scholar] [CrossRef]
- Clarke, S.R.; Riggins, J.J.; Stephen, F.M. Forest management and southern pine beetle outbreaks: A historical perspective. For. Sci. 2016, 62, 166–180. [Google Scholar] [CrossRef]
- Nowak, J.T.; Meeker, J.R.; Coyle, D.R.; Steiner, C.A.; Brownie, C. Southern pine beetle infestations in relation to forest stand conditions, previous thinning, and prescribed burning: Evaluation of the southern pine beetle prevention program. J. For. 2015, 113, 454–462. [Google Scholar] [CrossRef]
- Aoki, C.F.; Cook, M.; Dunn, J.; Finley, D.; Fleming, L.; Yoo, R.; Ayres, M.P. Old pests in new places: Effects of stand structure and forest type on susceptibility to a bark beetle on the edge of its native range. For. Ecol. Manag. 2018, 419–420, 206–219. [Google Scholar] [CrossRef]
- Sarma, V.; Paul, S.; Guanghua, W. Structural Transformation, Growth, and Inequality: Evidence from Viet Nam; ADB Working Paper No. 681; Asian Development Bank Institute (ADBI): Tokyo, Japan, 2017. [Google Scholar]
- Nanjo, Y.; Maruyama, K.; Yasue, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Komatsu, S. Transcriptional responses to flooding stress in roots including hypocotyl of soybean seedlings. Plant Mol. Biol. 2011, 77, 129–144. [Google Scholar] [CrossRef]
- Schieder, N.W.; Kirwan, M.L. Sea-level driven acceleration in coastal forest retreat. Geology 2019, 47, 1151–1155. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Conner, W.H.; Kroeger, S.; Sharitz, R.R. Aboveground production in Southeastern floodplain forests: A test of the subsidy-stress hypothesis. Ecology 1997, 78, 370–384. [Google Scholar] [CrossRef]
- Sánchez-Carrillo, S.; Angeler, D.G.; Sánchez-Andrés, R.; Alvarez-Cobelas, M.; Garatuza-Payán, J. Evapotranspiration in semi-arid wetlands: Relationships between inundation and the macrophyte-cover:open-water ratio. Adv. Water Resour. 2004, 27, 643–655. [Google Scholar] [CrossRef]
- Alvarez-Cobelas, M.; Cirujano, S.; Saâ Nchez-Carrillo, S. Hydrological and botanical man-made changes in the Spanish wetland of Las Tablas de Daimiel. Biol. Conserv. 2001, 97, 89–98. [Google Scholar] [CrossRef]
- Roznere, I.; Titus, J.E. Zonation of emergent freshwater macrophytes: Responses to small-scale variation in water depth. J. Torrey Bot. Soc. 2017, 144, 254–266. [Google Scholar] [CrossRef]
- Henman, J.; Poulter, B. Inundation of freshwater peatlands by sea level rise: Uncertainty and potential carbon cycle feedbacks. J. Geophys. Res. Biogeosci. 2008, 113, 1–11. [Google Scholar] [CrossRef]
- Ardón, M.; Morse, J.L.; Colman, B.P.; Bernhardt, E.S. Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob. Change Biol. 2013, 19, 2976–2985. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardon, M.; Hopfensperger, K.N.; Lamers, L.P.M.M.; Gell, P.; Langley, J.A. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 1–43. [Google Scholar] [CrossRef]
- Helton, A.M.; Bernhardt, E.S.; Fedders, A. Biogeochemical regime shifts in coastal landscapes: The contrasting effects of saltwater incursion and agricultural pollution on greenhouse gas emissions from a freshwater wetland. Biogeochemistry 2014, 120, 133–147. [Google Scholar] [CrossRef]
- Aguilos, M.; Brown, C.; Minick, K.; Fischer, M.; Ile, O.J.; Hardesty, D.; Kerrigan, M.; Noormets, A.; King, J. Millennial-scale carbon storage in natural pine forests of the north carolina lower coastal plain: Effects of artificial drainage in a time of rapid sea level rise. Land 2021, 10, 1294. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Yang, J.; Cao, M.; Swenson, N.G. Why Functional Traits Do Not Predict Tree Demographic Rates. Trends Ecol. Evol. 2018, 33, 326–336. [Google Scholar] [CrossRef]
- Visser, M.D.; Bruijning, M.; Wright, S.J.; Muller-Landau, H.C.; Jongejans, E.; Comita, L.S.; de Kroon, H. Functional traits as predictors of vital rates across the life cycle of tropical trees. Funct. Ecol. 2016, 30, 168–180. [Google Scholar] [CrossRef]
- Aleixo, I.; Norris, D.; Hemerik, L.; Barbosa, A.; Prata, E.; Costa, F.; Poorter, L. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Change 2019, 9, 384–388. [Google Scholar] [CrossRef]
- Mrad, A.; Johnson, D.M.; Love, D.M.; Domec, J.C. The roles of conduit redundancy and connectivity in xylem hydraulic functions. New Phytol. 2021, 231, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.N.; Huang, J.; Tay, A.H.T.; Sim, H.J.; Chan, P.J.; Lim, K.E.; Lei, M.; Aritsara, A.N.A.; Chong, R.; Ting, Y.Y.; et al. Leaf and twig traits predict habitat adaptation and demographic strategies in tropical freshwater swamp forest trees. New Phytol. 2024, 243, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Rivera-Monroy, V.H.; Li, C.; Vargas-Lopez, I.A.; Rohli, R.V.; Xue, Z.G.; Castañeda-Moya, E.; Coronado-Molina, C. Temperature Across Vegetation Canopy-Water-Soil Interfaces Is Modulated by Hydroperiod and Extreme Weather in Coastal Wetlands. Front. Mar. Sci. 2022, 9, 852901. [Google Scholar] [CrossRef]
- Jones, P.D.; Lister, D.H.; Osborn, T.J.; Harpham, C.; Salmon, M.; Morice, C.P. Hemispheric and large-scale land-surface air temperature variations: An extensive revision and an update to 2010. J. Geophys. Res. Atmos. 2012, 117, D05127. [Google Scholar] [CrossRef]
- Kathilankal, J.C.; Mozdzer, T.J.; Fuentes, J.D.; D’Odorico, P.; McGlathery, K.J.; Zieman, J.C. Tidal influences on carbon assimilation by a salt marsh. Environ. Res. Lett. 2008, 3, 044010. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, G. Characteristics and modeling of evapotranspiration over a temperate desert steppe in Inner Mongolia, China. J. Hydrol. 2011, 396, 139–147. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, K.; Lai, Q.; Zhang, C.; Zheng, L.; Wang, J. Carbon Dioxide Fluxes and Their Environmental Control in a Reclaimed Coastal Wetland in the Yangtze Estuary. Estuaries Coasts 2016, 39, 344–362. [Google Scholar] [CrossRef]



| A. Interannual variability | |||||||||||
| Mortality rate (%) | |||||||||||
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Standing dead | 1.09 | 1.10 | 1.10 | 2.87 | 5.23 | 5.97 | 10.69 | 11.31 | 14.95 | 18.30 | 19.01 |
| Downed dead | 0.55 | 0.82 | 1.38 | 4.55 | 4.95 | 7.41 | 24.54 | 26.92 | 28.57 | 29.81 | 31.61 |
| Total mortality | 1.64 | 1.91 | 2.46 | 7.29 | 9.94 | 12.97 | 33.00 | 35.67 | 40.01 | 43.70 | 45.82 |
| B. Mortality rate per species | |||||||||||
| General species composition | Scientific name | Total tree density (TPH) | Population percentage (%) | Tree mortality per species (TPH) | Live trees (TPH) | Mortality rate (%) | |||||
| Standing dead | Downed dead | Total dead | |||||||||
| American holly | Ilex opaca | 10 | 3 | 1 | 0 | 1 | 9 | 11 | |||
| Bald cypress | Taxodium distichum | 34 | 9 | 5 | 5 | 10 | 24 | 35 | |||
| Black gum | Nyssa sylvatica | 140 | 38 | 13 | 36 | 49 | 91 | 43 | |||
| Loblolly pine | Pinus taeda | 40 | 11 | 9 | 11 | 20 | 20 | 69 | |||
| Red maple | Acer rubrum | 50 | 14 | 8 | 8 | 16 | 34 | 39 | |||
| Swamp bay | Persea palustris | 57 | 15 | 8 | 20 | 28 | 29 | 68 | |||
| Sweet gum | Liquidambar styraciflua | 39 | 11 | 5 | 7 | 12 | 27 | 37 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilos, M.; Carter, C.; Middlebrough, B.; Bulluck, J.; Webb, J.; Brannum, K.; Watts, J.O.; Lobeira, M.; Sun, G.; McNulty, S.; et al. Hydrologic Perturbation Is a Key Driver of Tree Mortality in Bottomland Hardwood Wetland Forests of North Carolina, USA. Forests 2025, 16, 39. https://doi.org/10.3390/f16010039
Aguilos M, Carter C, Middlebrough B, Bulluck J, Webb J, Brannum K, Watts JO, Lobeira M, Sun G, McNulty S, et al. Hydrologic Perturbation Is a Key Driver of Tree Mortality in Bottomland Hardwood Wetland Forests of North Carolina, USA. Forests. 2025; 16(1):39. https://doi.org/10.3390/f16010039
Chicago/Turabian StyleAguilos, Maricar, Cameron Carter, Brandon Middlebrough, James Bulluck, Jackson Webb, Katie Brannum, John Oliver Watts, Margaux Lobeira, Ge Sun, Steve McNulty, and et al. 2025. "Hydrologic Perturbation Is a Key Driver of Tree Mortality in Bottomland Hardwood Wetland Forests of North Carolina, USA" Forests 16, no. 1: 39. https://doi.org/10.3390/f16010039
APA StyleAguilos, M., Carter, C., Middlebrough, B., Bulluck, J., Webb, J., Brannum, K., Watts, J. O., Lobeira, M., Sun, G., McNulty, S., & King, J. (2025). Hydrologic Perturbation Is a Key Driver of Tree Mortality in Bottomland Hardwood Wetland Forests of North Carolina, USA. Forests, 16(1), 39. https://doi.org/10.3390/f16010039











