Dendroclimatology of Cedrela fissilis Vell. and Copaifera langsdorffii Desf. in an Urban Forest Under Cerrado Domain
Abstract
1. Introduction
2. Materials and Methods
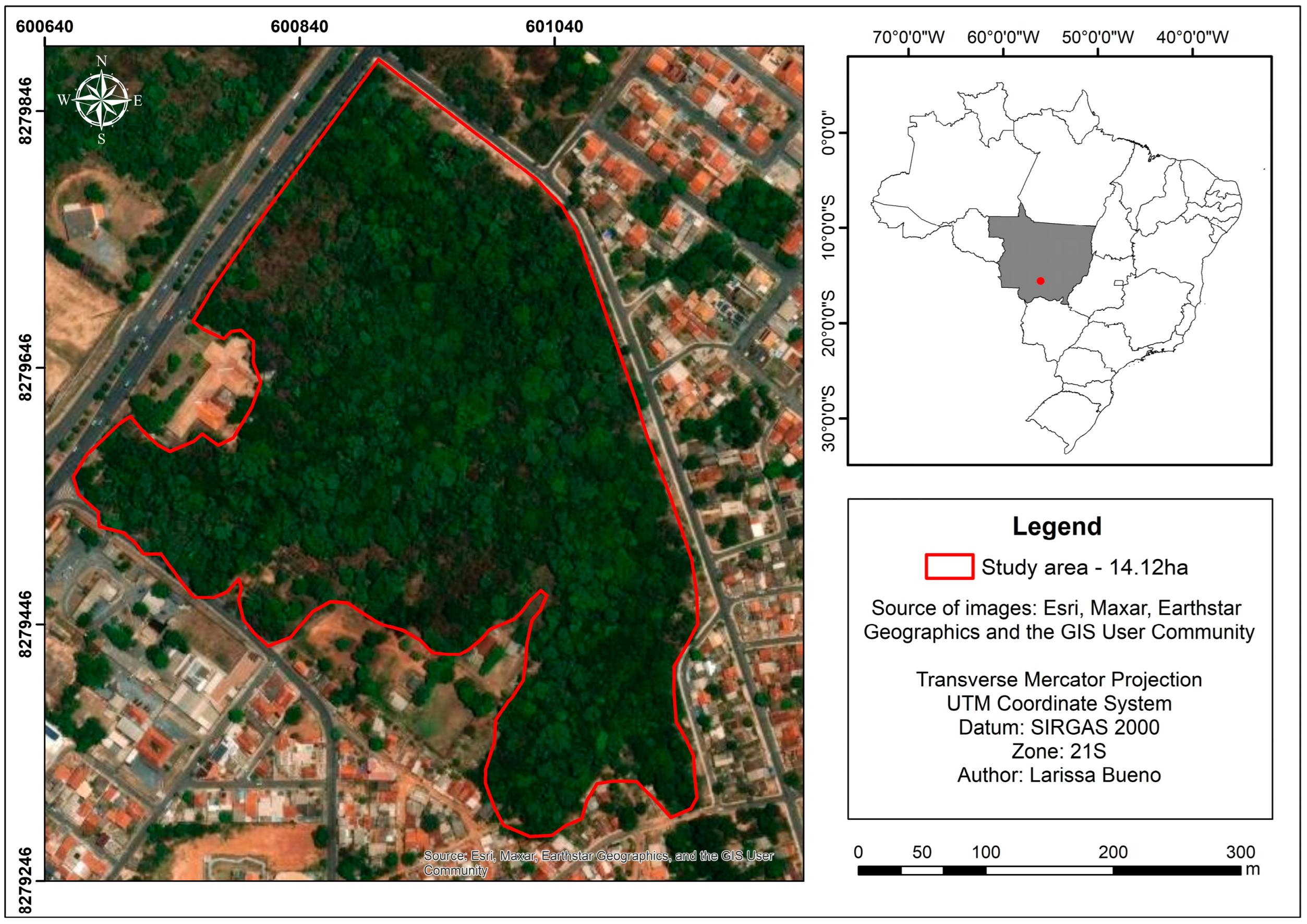
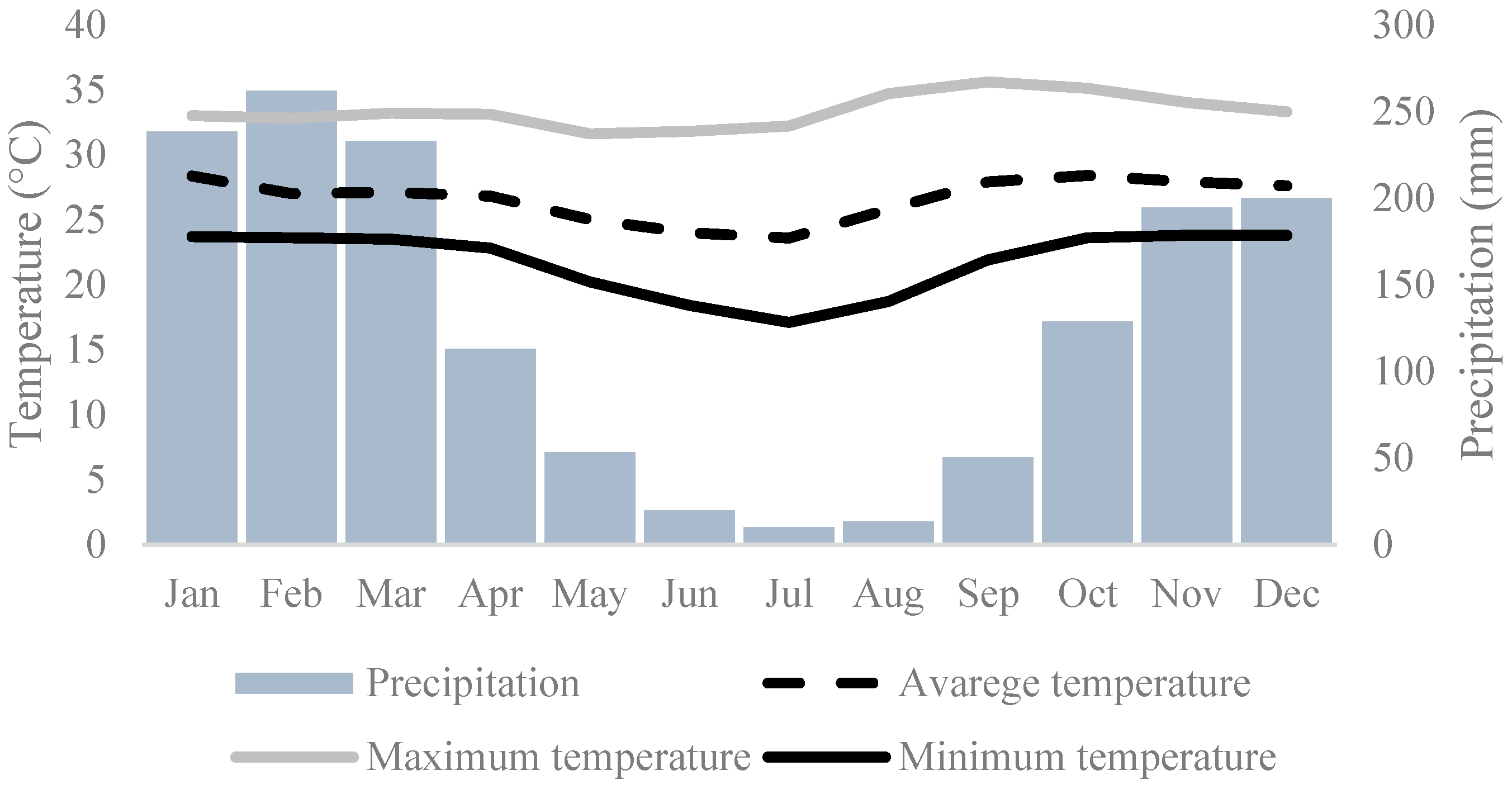
2.1. Description of the Study Area
2.2. Studied Species
2.3. Sample Collection and Preparation
2.4. Ring Marking
2.5. Data Analysis
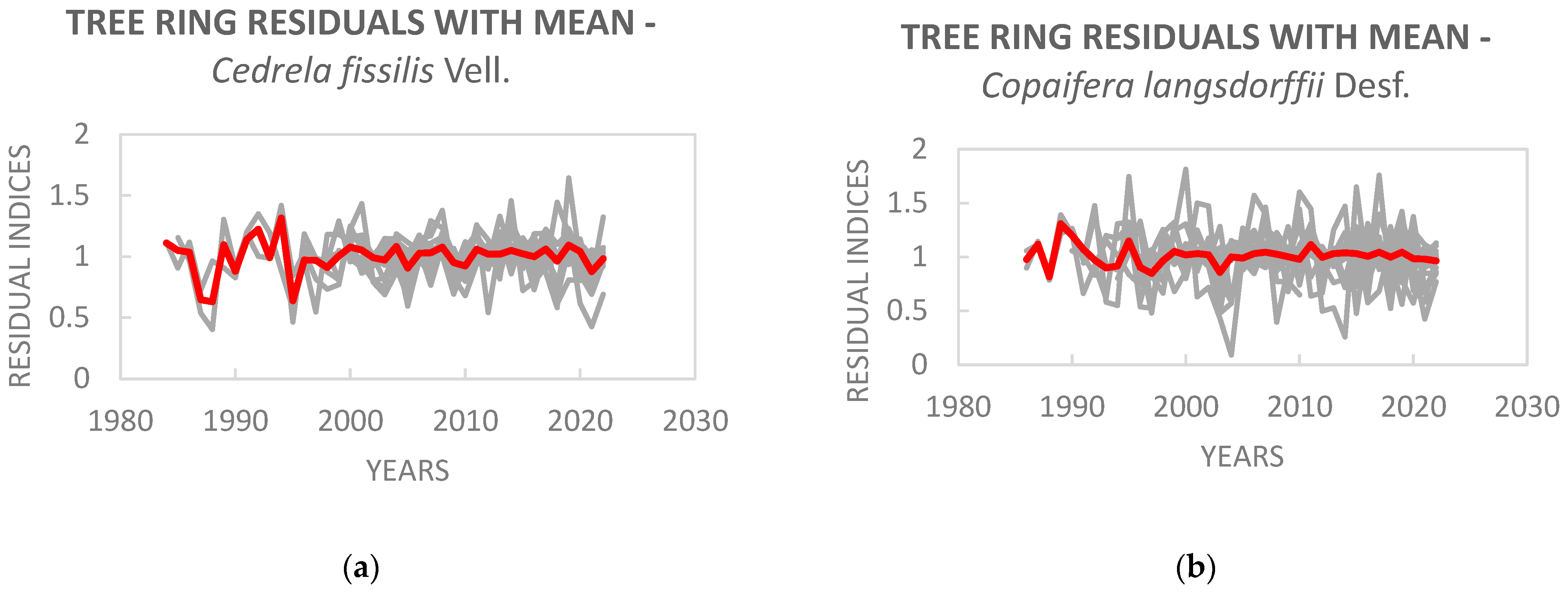
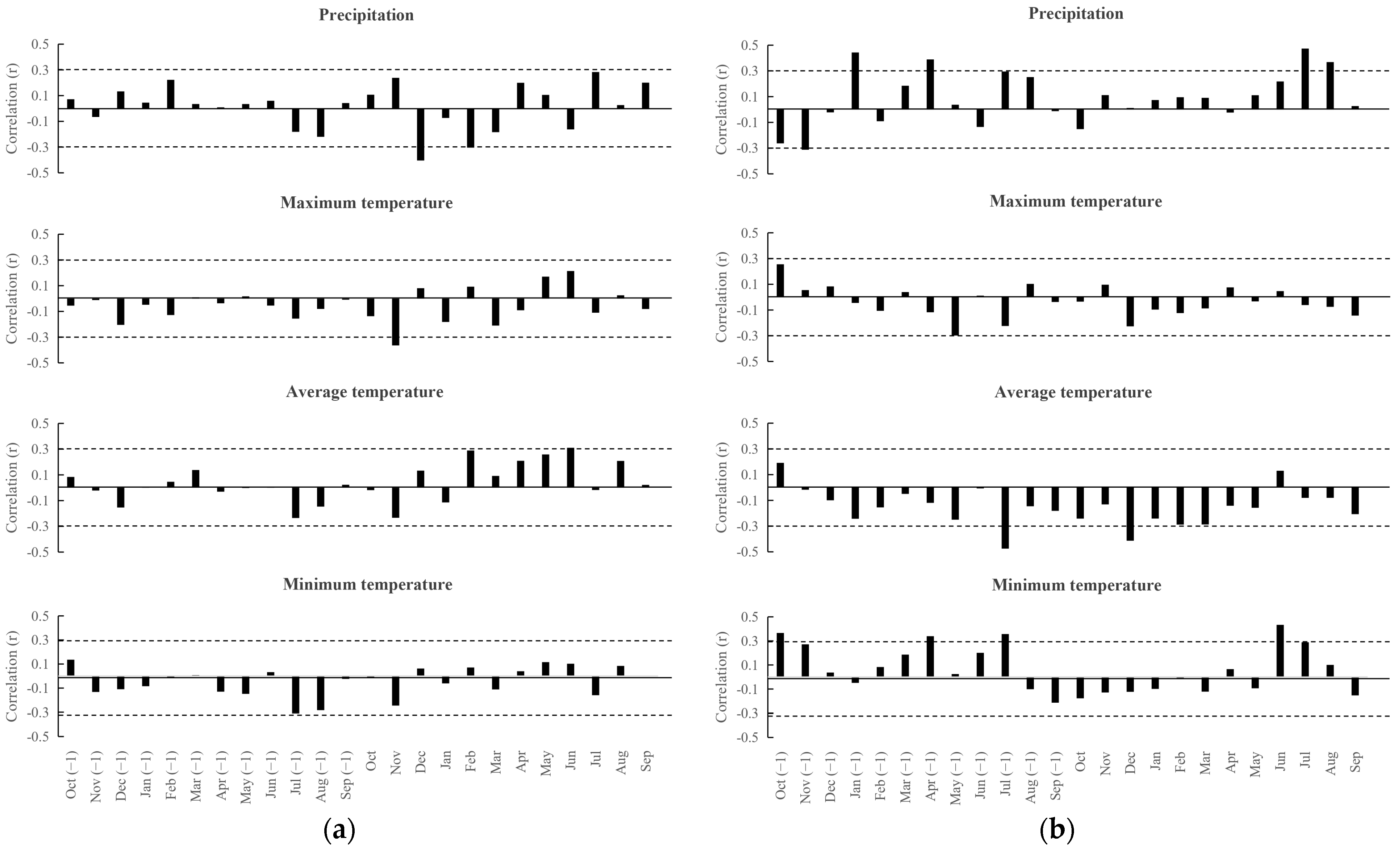
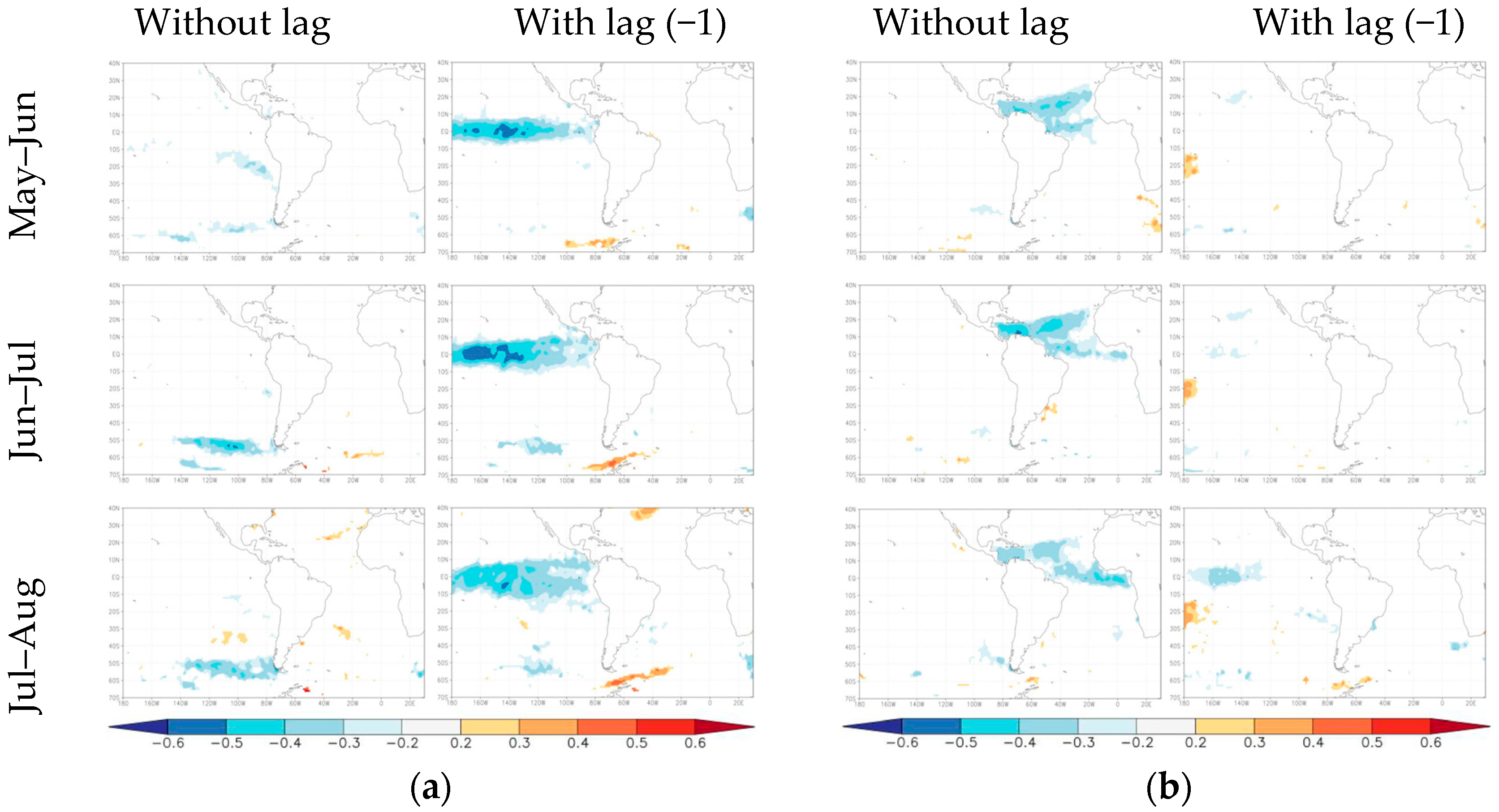
3. Results
4. Discussion
5. Conclusions
- Dendrochronological studies in urban areas of the Brazilian savannah show promise for detecting the influence of meteorological variables on the growth rings widht of the species in this study.
- In general, local precipitation and temperature variables correlate both positively and negatively with the width of the species growth rings, depending on the time of year, while only negative correlations were identified for ocean temperature and the Niño 3.4 index.
- This study is limited to the analysis of meteorological variables and climate anomalies, which partially affect the development of the species. However, more in-depth studies are needed to understand the role of eco-physiological variables.
- The results obtained here are promising for guiding new studies that consider urban forests and their importance in relation to climate change.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBAMA | Brazilian Institute of Environment and Renewable Natural Resources (in English)—Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (in Portuguese) |
| R-bar | Average intercorrelation |
| EPS | Express Population Signal |
| Res | residual |
| KNMI | Koninklijk Nederlands Meteorologisch Instituut |
| ITCZ | Intertropical Convergence Zone |
| SACZ | South Atlantic Convergence Zone |
| ENSO | El Niño–Southern Oscillation |
References
- Fleury, L.C.; Miguel, J.C.H.; Taddei, R. Mudanças climáticas, ciência e sociedade. Sociologias 2019, 21, 18–42. [Google Scholar] [CrossRef]
- Mendes, G.P.; Gomes, I.M.; Marques, R.F.d.P.V. Interferência industrial nas mudanças climáticas e seu impacto econômico no uso da água. Int. J. Environ. Resil. Res. Sci. 2020, 2, 133–143. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K.; et al. IPCC, 2023: Summary for Policymakers. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2021; ISBN 978-1-009-15789-6. [Google Scholar]
- Inouye, D.W. Climate Change and Phenology. WIREs Clim. Chang. 2022, 13, e764. [Google Scholar] [CrossRef]
- Yaning, C.; Yupeng, L.; Zhi, L.; Yongchang, L.I.U.; Wenjing, H.; Xigang, L.I.U.; Meiqing, F. Analysis of the Impact of Global Climate Change on Dryland Areas. Adv. Earth Sci. 2022, 37, 111. [Google Scholar]
- Li, X.; Zhou, Y.; Asrar, G.R.; Imhoff, M.; Li, X. The Surface Urban Heat Island Response to Urban Expansion: A Panel Analysis for the Conterminous United States. Sci. Total Environ. 2017, 605–606, 426–435. [Google Scholar] [CrossRef]
- Wang, J.; Tett, S.F.B.; Yan, Z. Correcting Urban Bias in Large-Scale Temperature Records in China, 1980–2009. Geophys. Res. Lett. 2017, 44, 401–408. [Google Scholar] [CrossRef]
- Liu, J.; Niyogi, D. Meta-Analysis of Urbanization Impact on Rainfall Modification. Sci. Rep. 2019, 9, 7301. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.-Y. Urbanization as a Major Driver of Urban Climate Change. Adv. Clim. Chang. Res. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Ceccantini, G.; Gamboa, C.S.; Schongart, J. Os Anéis de Crescimento Das Árvores: Desvendando as Mudanças Climáticas. Biol. Mudanças Clim. No Bras. 2008, 57, 5–23. [Google Scholar]
- Roquette, J.G.; Lobo, F.d.A.; Curado, L.F.A. Dendroclimatologia Na Amazônia: Aplicações e Potencialidades. Ciênci. Florest. 2019, 29, 451. [Google Scholar] [CrossRef]
- Bernert, M.R.; Jadoski, S.O.; Watzlawick, L.F.; Lima, V.A.d.; Zerbieli, L.C. Influência de variáveis ambientais no incremento radial dos anéis de crescimento de Pinus taeda L. Res. Soc. Dev. 2020, 9, e84932472. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1976; ISBN 0-12-268450-8. [Google Scholar]
- Hess, A.F.; Ricken, P.; Ciarnoschi, L.D. Dendrocronologia, Incremento E Manejo Florestal Em Floresta De Araucária-Sc. EBSCOhost 2018, 28, 1568–1582. [Google Scholar] [CrossRef]
- Evans, M.E.K.; DeRose, R.J.; Klesse, S.; Girardin, M.P.; Heilman, K.A.; Alexander, M.R.; Arsenault, A.; Babst, F.; Bouchard, M.; Cahoon, S.M.P.; et al. Adding Tree Rings to North America’s National Forest Inventories: An Essential Tool to Guide Drawdown of Atmospheric CO2. BioScience 2022, 72, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Rochner, M.L.; Moriarty, K.J.; Weatherbee, S. Dendrochronological Analyses of Tree Growth and Climate Response across an Urban-Rural Gradient, Louisville, Kentucky. Urban For. Urban Green. 2025, 104, 128592. [Google Scholar] [CrossRef]
- Miyahara, A.A.L.; Paixão, C.P.; dos Santos, D.R.; Pagin-Cláudio, F.; da Silva, G.J.; Bertoleti, I.A.F.; de Lima, J.S.; da Silva, J.L.; Candido, L.F.; Siqueira, M.C.; et al. Urban Dendrochronology Toolkit for Evidence-Based Decision-Making on Climate Risk, Cultural Heritage, Environmental Pollution, and Tree Management—A Systematic Review. Environ. Sci. Policy 2022, 137, 152–163. [Google Scholar] [CrossRef]
- Bard, E.; Miramont, C.; Capano, M.; Guibal, F.; Marschal, C.; Rostek, F.; Tuna, T.; Fagault, Y.; Heaton, T.J. A Radiocarbon Spike at 14 300 Cal Yr BP in Subfossil Trees Provides the Impulse Response Function of the Global Carbon Cycle during the Late Glacial. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2023, 381, 20220206. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Helle, G.; Pons, T.L.; Guyot, J.-L.; Gloor, M. Oxygen Isotopes in Tree Rings Are a Good Proxy for Amazon Precipitation and El Niño-Southern Oscillation Variability. Proc. Natl. Acad. Sci. USA 2012, 109, 16957–16962. [Google Scholar] [CrossRef]
- Vasconcellos, T.J.d.; Tomazello-Filho, M.; Callado, C.H. Dendrochronology and Dendroclimatology of Ceiba speciosa (A. St.-Hil.) Ravenna (Malvaceae) Exposed to Urban Pollution in Rio de Janeiro City, Brazil. Dendrochronologia 2019, 53, 104–113. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C.; Ibarra, M. Brooklyn’s Urban Forest; U.S. Department of Agriculture Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2002; p. NE-GTR-290. [Google Scholar]
- Raciti, S.M.; Hutyra, L.R.; Finzi, A.C. Depleted Soil Carbon and Nitrogen Pools beneath Impervious Surfaces. Environ. Pollut. 2012, 164, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Farmer, J.R.; Dickinson, S.L.; Robeson, S.M.; Fischer, B.C.; Reynolds, H.L. Climate Change Impacts and Urban Green Space Adaptation Efforts: Evidence from U.S. Municipal Parks and Recreation Departments. Urban Clim. 2021, 39, 100962. [Google Scholar] [CrossRef]
- Siqueira, S.d.F.; Higuchi, P.; Silva, A.C. da contemporary and future potential geographic distribution of Cedrela fissilis vell. under climate change scenarios. Rev. Árvore 2019, 43, e430306. [Google Scholar] [CrossRef]
- Muellner, A.N.; Pennington, T.D.; Koecke, A.V.; Renner, S.S. Biogeography of Cedrela (Meliaceae, Sapindales) in Central and South America. Am. J Bot. 2010, 97, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Lobão, M.S. Dendrocronologia, Fenologia, Atividade Cambial e Qualidade Do Lenho de Árvores de Cedrela odorata, L., Cedrela fissilis Vell. e Schizolobium parahyba Var. amazonicum Hub. ex Ducke, no Estado do Acre, Brasil. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2011. [Google Scholar]
- Aguiar, O.T.D.; Pastore, J.A.; Rocha, F.T.; Batista, J. Flora Fanerogâmica De Um Trecho Da Floresta Densa Secundária No Parque Estadual Da Serra Do Mar—Núcleo Cunha/Indaiá: Cunha (Sp). Rev. Inst. Florest. 2001, 13, 1–18. [Google Scholar] [CrossRef]
- Stefano, M.; Calazans, L.; Sakuragui, C. Meliaceae. In Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras; Embrapa Florestas: Colombo, Brasil, 2008; ISBN 978-85-7383-429-1. [Google Scholar]
- Rosa, D.B.C.J.; Scalon, S.d.P.Q.; Cremon, T.; Dresch, D.M. Gas Exchanges and Antioxidant Activity in Copaifera langsdorffii Desf. Seedlings after Flooding. Am. J. Plant Sci. 2018, 9, 979. [Google Scholar] [CrossRef][Green Version]
- Fagundes, M. Galling Insect Community Associated with Copaifera langsdorffii (Fabaceae): The Role of Inter- and Intra-Annual Host Plant Phenology. In Neotropical Insect Galls; Fernandes, G.W., Santos, J.C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2014; pp. 163–177. ISBN 978-94-017-8782-6. [Google Scholar]
- Costa, M.D.P.; Pereira, J.A.A.; Benicio, M.H.M.; De Sousa, H.; Fontes, M.A.L.; Garcia, P.O. Alometria e Arquitetura de Copaifera langsdorffii (Desf.) Kuntze (Fabaceae) Em Fitofisionomias Neotropicais No Sul de Minas Gerais. Ciênc. Florest. 2012, 22, 223–240. [Google Scholar] [CrossRef]
- Santos, M.D.O.; Almeida, B.V.D.; Campos, N.B.; Macedo, J.G.F.; Macêdo, M.J.F.; Ribeiro, D.A.; Silva, M.A.P.D.; Calixto Júnior, J.T.; Costa, J.G.M.D.; Souza, M.M.D.A. Vegetative and Reproductive Phenology of Copaifera langsdorffii Desf. in Different Phytophysiognomies. RSD 2022, 11, e41011427288. [Google Scholar] [CrossRef]
- Carvalho, D.C.d.; Pereira, M.G.G.; Carmo, J.F.d.; Pace, J.H.C.; Silva, L.D.S.A.B.d.; Latorraca, J.V.F. Dendrochronology and growth of Copaifera langsdorffii wood in the vegetation dynamics of the Pirapitinga Ecological Station, state of Minas Gerais, Brazil. FLORESTA 2018, 48, 49–58. [Google Scholar] [CrossRef]
- Fontana, C.; López, L.; Santos, G.M.; Villalba, R.; Hornink, B.; Assis-Pereira, G.; Roig, F.A.; Tomazello-Filho, M. A New Chronology of Cedrela fissilis (Meliaceae) for Southern Brazil: Combining Classical Dendrochronology and Radiocarbon Dating. Dendrochronologia 2024, 85, 126214. [Google Scholar] [CrossRef]
- Chiaranda, R.; Colpini, C.; Soares, T.S. Caracterização da Bacia Hidrográfica do Rio Cuiabá. Adv. For. Sci. 2016, 3, 13–20. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.d.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- INMET—Instituto Nacional de Meteorologia Banco de Dados Meteorológicos 2024. Informativo Meteorológico N°39/2024—Confira a Previsão do Tempo Entre os Dias 23 e 30 de Dezembro de 2024. Available online: https://portal.inmet.gov.br/noticias/informativo-meteorol%C3%B3gico-n-39-2024 (accessed on 30 December 2024).
- Souza, M.M.X.; Agostini, G.B.; Santos, G.A.; Favalessa, C.M.C.; Kanieski, M.R.; Milani, J.E.F. Floristic Diversity and Edaphic Filters in an Urban Forest under Cerrado Domain, in Cuiabá, Central Western Brazil. Braz. J. Biol. 2024, 84, e279583. [Google Scholar] [CrossRef]
- Marcati, C.R.; Angyalossy, V.; Evert, R.F. Seasonal Variation in Wood Formation of Cedrela fissilis (Meliaceae). Iawa J. 2006, 27, 199–211. [Google Scholar] [CrossRef]
- Andreacci, F.; Botosso, P.C.; Galvão, F. Climatic Signals in Growth Rings of Cedrela fissilis in Different Types of South Brazil Rainforests. Floresta 2014, 44, 323–332. [Google Scholar] [CrossRef]
- Hammerschlag, I.; Macario, K.D.; Barbosa, A.C.; Pereira, G.d.A.; Farrapo, C.L.; Cruz, F. Annually Verified Growth of Cedrela fissilis from Central Brazil. Radiocarbon 2019, 61, 927–937. [Google Scholar] [CrossRef]
- Rodriguez, D.R.O.; Sánchez-Salguero, R.; Hevia, A.; Granato-Souza, D.; Assis-Pereira, G.; Roig, F.A.; Tomazello-Filho, M. Long- and Short-Term Impacts of Climate and Dry-Season on Wood Traits of Cedrela fissilis Vell. in Southern Brazilian Amazon. Agric. For. Meteorol. 2023, 333, 109392. [Google Scholar] [CrossRef]
- Tomazello Filho, M.; Botosso, P.C.; Lisi, C.S. Potencialidade Da Família Meliaceae Para Dendrocronologia Em Regiões Tropicais e Subtropicais. In Dendrocronología en América Latina; Ediunc: Sarmiento, Argentina, 2000; pp. 381–431. [Google Scholar]
- Pereira, G.d.A.; Barbosa, A.C.M.C.; Torbenson, M.C.A.; Stahle, D.W.; Granato-Souza, D.; Santos, R.M.D.; Barbosa, J.P.D. The Climate Response of Cedrela fissilis Annual Ring Width in the Rio São Francisco Basin, Brazil. Tree-Ring Res. 2018, 74, 162–171. [Google Scholar] [CrossRef]
- Miranda, B.P.; Andrade, V.H.F.; Botosso, P.C.; Longhi-Santos -SANTOS, T.; Milani, J.d.F.; Roderjan, C.V. Potencial de Copaifera langsdorffii Desf.(Fabaceae) Como Indicadora de Mudanças Climáticas Por Meio Da Análise Dos Anéis de Crescimento, Paraná, Brasil. Ciênc. Agrovet. 2019, 18, 301–312. [Google Scholar] [CrossRef]
- Marcati, C.R.; Angyalossy-Alfonso, V.; Benetati, L. Anatomia comparada do lenho de Copaifera langsdorffii Desf. (Leguminosae–Caesalpinoideae) de floresta e cerradão. Braz. J. Bot. 2001, 24, 311–320. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Pinto, A.C. The Copaifera l. Genus. Quím. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Pieri, F.A.; Mussi, M.C.; Moreira, M.a.S. Óleo de copaíba (Copaifera sp.): Histórico, extração, aplicações industriais e propriedades medicinais. Rev. Bras. Plantas Med. 2009, 11, 465–472. [Google Scholar] [CrossRef]
- Bendito, B.P.C.; Souza, P.A.D.; Ferreira, R.Q.D.S.; Bonfim, E.; Cândido, J.; Souza, P.B.D. Espécies do cerrado com potencial para recuperação de áreas degradadas, Gurupi (TO). Rev. Agrogeoambient. 2018, 10, 99–106. [Google Scholar] [CrossRef]
- Deepak, M.; Sinha, S.; Rao, R. Tree-Ring Analysis of Teak (Tectona grandis L. f.) from Western Ghats of India as a Tool to Determine Drought Years. Emir. J. Food Agric 2010, 22, 388. [Google Scholar] [CrossRef]
- Larsson, L.A. CooRecorder & Cdendro, v7.8; Cybis Elektronik & Data AB: Saltsjöbaden, Sweden, 2013.
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Arstan: Guide for Computer Program; University of Arizona: Tucson, AZ, USA, 1986. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutorial for the Computer Program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Barbosa, A.C.M.; Pereira, G.A.; Granato-Souza, D.; Santos, R.M.; Leite Fontes, M.A. Tree Rings and Growth Trajectories of Tree Species from Seasonally Dry Tropical Forest. Aust. J. Bot. 2018, 66, 414–427. [Google Scholar] [CrossRef]
- Leclerc, N.; Halfar, J.; Porter, T.J.; Black, B.A.; Hetzinger, S.; Zulian, M.; Tsay, A. Utility of Dendrochronology Crossdating Methods in the Development of Arctic Coralline Red Algae Clathromorphum Compactum Growth Increment Chronology for Sea Ice Cover Reconstruction. Front. Mar. Sci. 2022, 9, 923088. [Google Scholar] [CrossRef]
- Buras, A. A Comment on the Expressed Population Signal. Dendrochronologia 2017, 44, 130–132. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tuscon, AZ, USA, 2010. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology; Springer Netherlands: Dordrecht, The Netherlands, 1990; ISBN 978-90-481-4060-2. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Users Manual for Program ARSTAN; University of Arizona: Tucson, AZ, USA, 1999. [Google Scholar]
- Roquette, J.G.; de Almeida Lobo, F.; Vourlitis, G.L.; Roig, F.A.; Ortíz, C.E.R.; Banga, N.M.; Portal-Cahuana, L.A.; Tomazello-Filho, M. Hymenaea Stignocarpa Mart. Ex Hayne Growth–Climate Relationships Are Regulated by Soil Water Saturation in Cerrado-Pantanal Ecotone. Dendrochronologia 2023, 81, 126130. [Google Scholar] [CrossRef]
- Rodriguez, D.R.O.; Sánchez-Salguero, R.; Hevia, A.; Granato-Souza, D.; Cintra, B.B.; Hornink, B.; Andreu-Hayles, L.; Assis-Pereira, G.; Roig, F.A.; Tomazello-Filho, M. Climate Variability of the Southern Amazon Inferred by a Multi-Proxy Tree-Ring Approach Using Cedrela fissilis Vell. Sci. Total Environ. 2023, 871, 162064. [Google Scholar] [CrossRef] [PubMed]
- Locosselli, G.M.; Buckeridge, M.S.; Moreira, M.Z.; Ceccantini, G. A Multi-Proxy Dendroecological Analysis of Two Tropical Species (Hymenaea spp., Leguminosae) Growing in a Vegetation Mosaic. Trees 2013, 27, 25–36. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS® OnDemand for Academics 2023; SAS Institute Inc.: Cary, NC, USA, 2023. [Google Scholar]
- López, L.; Rodríguez-Catón, M.; Villalba, R. Convergence in Growth Responses of Tropical Trees to Climate Driven by Water Stress. Ecography 2019, 42, 1899–1912. [Google Scholar] [CrossRef]
- Hughes, M.K. Dendrochronology in Climatology—The State of the Art. Dendrochronologia 2002, 20, 95–116. [Google Scholar] [CrossRef]
- Björklund, J.; Seftigen, K.; Fonti, P.; Nievergelt, D.; von Arx, G. Dendroclimatic Potential of Dendroanatomy in Temperature-Sensitive Pinus sylvestris. Dendrochronologia 2020, 60, 125673. [Google Scholar] [CrossRef]
- Pallardy, S.G. Physiology of Woody Plants; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The Dynamics of Cambial Stem Cell Activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Larcher, W. Ecofisiologia Vegetal; RIMA: São Carlos, Brazil, 2000. [Google Scholar]
- Sette, D.M. O Holorritmo e as Interações Trópico-Extratrópico na Gênese do Clima e as Paisagens do Mato Grosso. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2000. [Google Scholar]
- Mendonça, F.; Danni-Oliveira, I.M. Climatologia: Noções Básicas e Climas do Brasil; Oficina de Textos: Sao Paulo, Brazil, 2017. [Google Scholar]
- Sette, D.M. Os Climas Do Cerrado Do Centro-Oeste. Rev. Bras. Clim. 2005, 1, 29–42. [Google Scholar] [CrossRef]
- Leite, P.T.d.P. Dendroecologia de Tabebuia aurea (Manso) Benth. & Hook e Tabebuia heptaphylla (Vell.) Toledo (Bignoniaceae) no Pantanal de Mato Grosso, Brasil. Master’s Thesis, Universidade Federal de Mato Grosso, Cuiaba, Brazil, 2012. [Google Scholar]
- Macedo, M.d.O. Efeito das Variações Climáticas e Hidrológicas no Padrão de Crescimento Diamétrico da Espécie Arbórea Macrolobium acaciifolium (Benth.) Benth. (Fabaceae) em Florestas Alagáveis por Águas Claras na Amazônia Oriental. Master’s thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus, Amazonas, Brazil, 2018. [Google Scholar]
- Alves, L.M.; Coelho, C.A.S.; Melo, A.B.C.; Pesquero, J.F. Condições Climáticas Observadas No Brasil Em 2009. Bol. Soc. Bras. De Meteorol. 2009, 35. [Google Scholar]
- Diniz, F.R.; Miranda, G.S.B.; Piacenti-Silva, M.; Machado, J.P. The Impact of El Niño on Fire Outbreaks and Human Thermal Discomfort in Brazil in the Period Between Summer of 2014/2015 until the Autumn of 2016. Anu. IGEO UFRJ 2019, 42, 192–201. [Google Scholar] [CrossRef]
- Rodrigues, M.L.G.; Lima, M.d.; Martins, M.; Cruz, G. El Niño e suas lições. Agropecu. Catarin. 2023, 36, 7–11. [Google Scholar]
- Barbosa, E.L.S.Y. Variabilidade Climática Nas Regiões Sudeste e Centro-Oeste Do Brasil: Influência Dos Oceanos Atlântico e Pacífico. Ph.D. Thesis, Universidade de São Paulo, Sao Paulo, Brazil, 2017. [Google Scholar]
- Carvalho, L.M.V.; Jones, C.; Liebmann, B. The South Atlantic Convergence Zone: Intensity, Form, Persistence, and Relationships with Intraseasonal to Interannual Activity and Extreme Rainfall. J. Clim. 2004, 17, 88–108. [Google Scholar] [CrossRef]
- Cai, W.; McPhaden, M.J.; Grimm, A.M.; Rodrigues, R.R.; Taschetto, A.S.; Garreaud, R.D.; Dewitte, B.; Poveda, G.; Ham, Y.-G.; Santoso, A.; et al. Climate Impacts of the El Niño–Southern Oscillation on South America. Nat. Rev. Earth Environ. 2020, 1, 215–231. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Taschetto, A.S.; Sen Gupta, A.; Foltz, G.R. Common Cause for Severe Droughts in South America and Marine Heatwaves in the South Atlantic. Nat. Geosci. 2019, 12, 620–626. [Google Scholar] [CrossRef]
- Cavalcanti, I.F.D.A.; Marengo, J.A.; Alves, L.M.; Costa, D.F. On the Opposite Relation between Extreme Precipitation over West Amazon and Southeastern Brazil: Observations and Model Simulations. Int. J. Climatol. 2017, 37, 3606–3618. [Google Scholar] [CrossRef]
- Santos, L. Trends in Climate Extremes and Their Relationships with the Tropical Ocean Temperature in the Mid-West Region of Brazil. Master’s Thesis, Institute of Physics, Federal University of Mato Grosso, Cuiaba, Brazil, 2021. [Google Scholar]
- Dos Santos, L.O.F.; Machado, N.G.; Querino, C.A.S.; Biudes, M.S. Trends of Climate Extremes and Their Relationships with Tropical Ocean Temperatures in South America. Earth 2024, 5, 844–872. [Google Scholar] [CrossRef]
- Reboita, M.S.; Ambrizzi, T.; Crespo, N.M.; Dutra, L.M.M.; Ferreira, G.W.D.S.; Rehbein, A.; Drumond, A.; Da Rocha, R.P.; Souza, C.A.D. Impacts of Teleconnection Patterns on South America Climate. Ann. N. Y. Acad. Sci. 2021, 1504, 116–153. [Google Scholar] [CrossRef] [PubMed]
- Gebrekirstos, A.; Mitlöhner, R.; Teketay, D.; Worbes, M. Climate–Growth Relationships of the Dominant Tree Species from Semi-Arid Savanna Woodland in Ethiopia. Trees 2008, 22, 631–641. [Google Scholar] [CrossRef]
- Yeh, S.-W.; Kug, J.-S.; Dewitte, B.; Kwon, M.-H.; Kirtman, B.P.; Jin, F.-F. El Niño in a Changing Climate. Nature 2009, 461, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Lorensi, C.; Prestes, A. Dendroclimatologia com amostras de Araucaria angustifolia coletadas em Santa Catarina. Rev. Univap 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Kageyama, P.Y.; Castro, C. de A. Sucessão Secundária, Estrutura Genética e Plantações de Espécies Arbóreas Nativas. IPEF Piracicaba 1989, 41, 83–93. [Google Scholar]
- Sá, L.F.d.; Tambarussi, E.V. Melhoramento genético como estratégia de gestão da produtividade florestal. Rev. Inst. Florest. 2023, 35, 99–112. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Shah, S.K.; Roy, A.; Tripathi, S.K. Dendroclimatology of Teak Indicates Prevailing Climatic Conditions of Tropical Moist Forests in India. Ecol. Indic. 2021, 129, 107888. [Google Scholar] [CrossRef]
- Gaston, K.J. The Structure and Dynamics of Geographic Ranges; Oxford University Press: Manhattan, NY, USA, 2003. [Google Scholar]
- Hengeveld, R.; Giller, P.S.; Riddle, B.R. Species Ranges. In Foundations of Biogeography; Universityof Chicago Press: Chicago, IL, USA, 2004. [Google Scholar]
- Rapoport, E.H. Areography: Geographical Strategies of Species; Pergamon Press: Oxford, UK, 1982; Volume 1. [Google Scholar]


| Description | Species | |
|---|---|---|
| Cedrela fissilis | Copaifera langsdorffii | |
| Observed period | 1984–2022 | 1986–2022 |
| Number of dated series (from the total of) | 15 (20) | 18 (20) |
| Individuals | 10 | 10 |
| Total of rings in all the samples | 377 | 432 |
| Total of checked rings | 376 | 432 |
| Intercorrelation | 0.384 | 0.421 |
| Average sensitivity | 0.396 | 0.513 |
| Average correlation among the series (R-bar) ± standard deviation | 0.325 ± 0.227 1 | 0.216 ± 0.182 2 |
| Express populational signal (EPS) | 0.88 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.d.S.B.d.; Carvalho, L.S.d.; Roquette, J.G.; Souza, M.M.X.d.; Agostini, G.B.d.; Drescher, R.; Milani, J.E.d.F.; Favalessa, C.M.C. Dendroclimatology of Cedrela fissilis Vell. and Copaifera langsdorffii Desf. in an Urban Forest Under Cerrado Domain. Forests 2025, 16, 289. https://doi.org/10.3390/f16020289
Santos LdSBd, Carvalho LSd, Roquette JG, Souza MMXd, Agostini GBd, Drescher R, Milani JEdF, Favalessa CMC. Dendroclimatology of Cedrela fissilis Vell. and Copaifera langsdorffii Desf. in an Urban Forest Under Cerrado Domain. Forests. 2025; 16(2):289. https://doi.org/10.3390/f16020289
Chicago/Turabian StyleSantos, Larissa da Silva Bueno dos, Letícia Seles de Carvalho, José Guilherme Roquette, Matheus Marcos Xavier de Souza, Gabriel Bazanela de Agostini, Ronaldo Drescher, Jaçanan Eloisa de Freitas Milani, and Cyro Matheus Cometti Favalessa. 2025. "Dendroclimatology of Cedrela fissilis Vell. and Copaifera langsdorffii Desf. in an Urban Forest Under Cerrado Domain" Forests 16, no. 2: 289. https://doi.org/10.3390/f16020289
APA StyleSantos, L. d. S. B. d., Carvalho, L. S. d., Roquette, J. G., Souza, M. M. X. d., Agostini, G. B. d., Drescher, R., Milani, J. E. d. F., & Favalessa, C. M. C. (2025). Dendroclimatology of Cedrela fissilis Vell. and Copaifera langsdorffii Desf. in an Urban Forest Under Cerrado Domain. Forests, 16(2), 289. https://doi.org/10.3390/f16020289






