Potential Distribution and Response of Camphora longepaniculata Gamble (Lauraceae) to Climate Change in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Occurrence Data
2.2. Environmental Variables for Analysis
2.3. Model Establishment and Performance Evaluation
2.4. Analysis of Potential Suitable Area
2.5. Analysis of Distribution Change in Suitable Area
3. Results
3.1. Accuracy Evaluation of the MaxEnt Model
3.2. Key Environmental Variables for Potential Distribution
3.3. Evaluation of Environmental Variables and Analysis of Response Curve
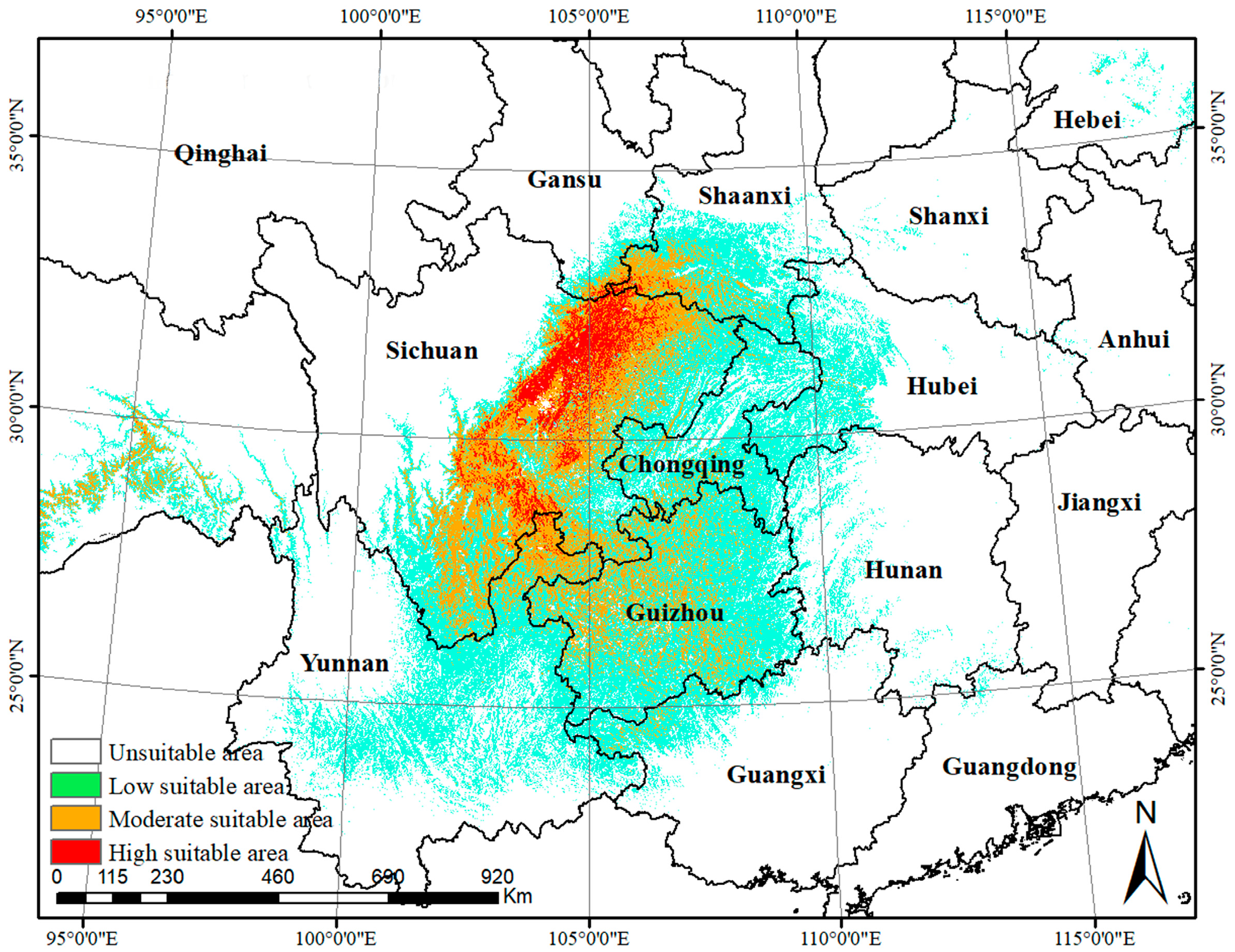
3.4. Current Potential Suitable Distribution
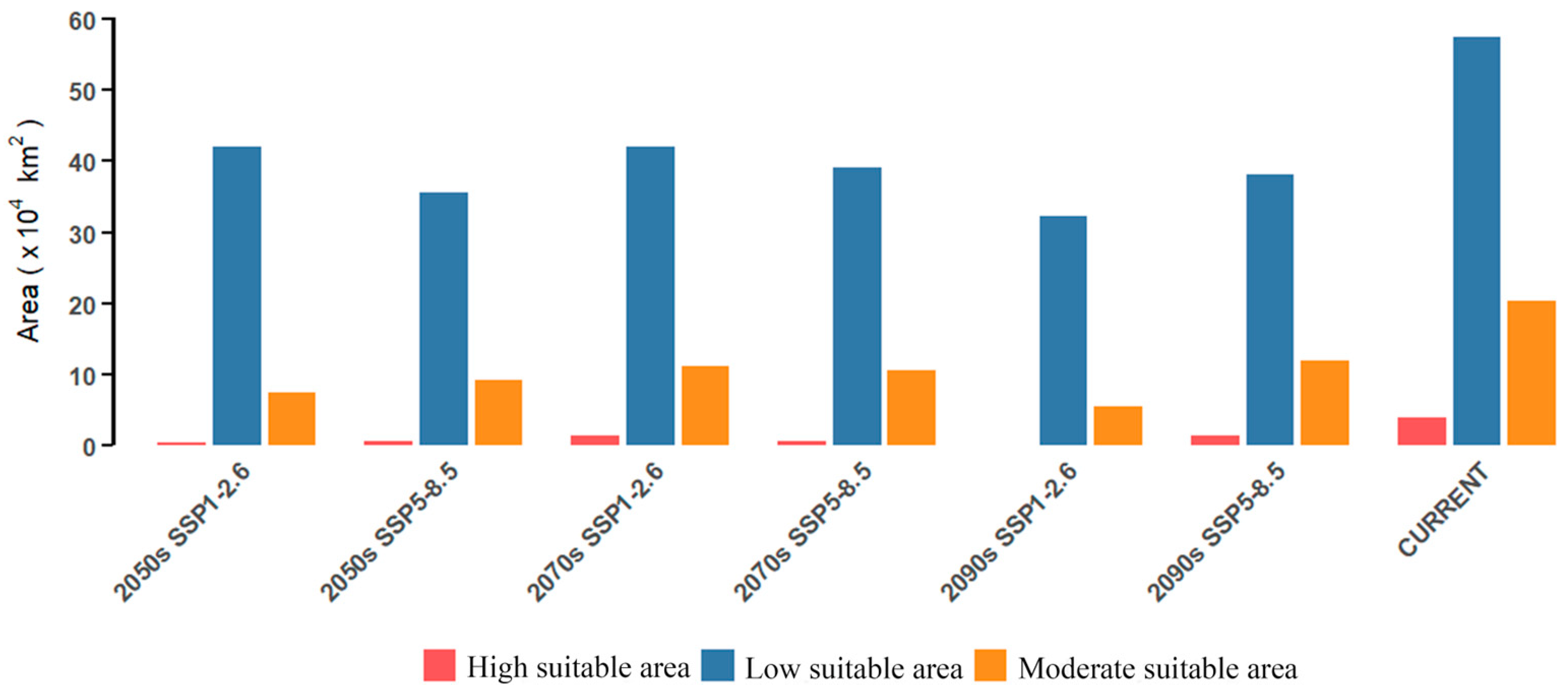
3.5. Potential Distribution Under Future Climate Conditions
3.6. Expansion and Shrinkage of the Potential Habitat in the Future
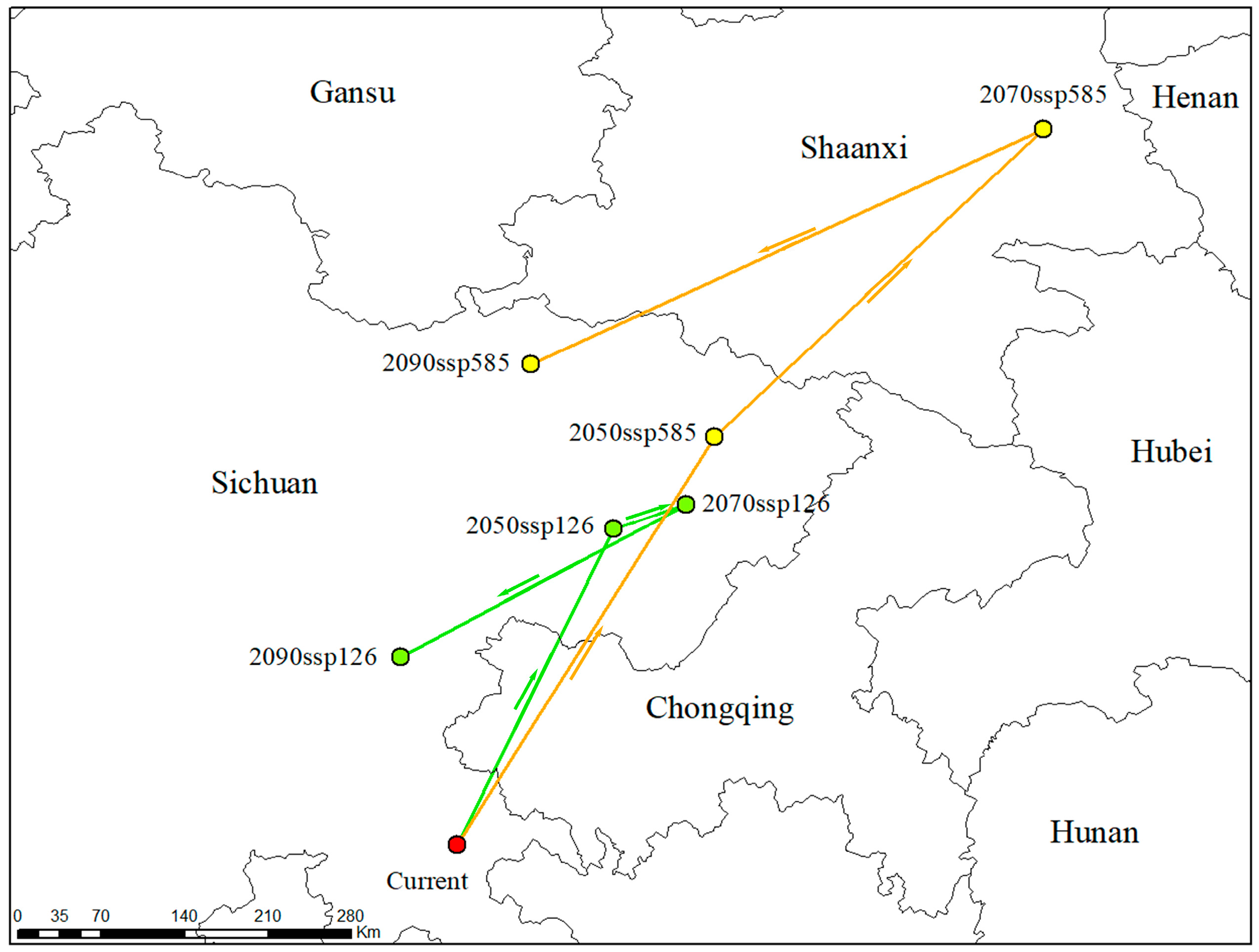
3.7. Centroid Shifts in the Predicted Potential Suitable Habitat of C. longepaniculata
4. Discussion
4.1. Restricting Environmental Factors and Current Potentially Suitable Areas for C. longepaniculata
4.2. Potential Distribution of C. longepaniculata in the Future and Response to Climate Change
4.3. Conservation Strategies for C. longepaniculata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Işik, K. Rare and endemic species: Why are they prone to extinction? Turk. J. Bot. 2011, 35, 11. [Google Scholar] [CrossRef]
- Klein, J.A.; Harte, J.; Zhao, X.Q. Decline in medicinal and forage species with warming is mediated by plant traits on the Tibetan plateau. Ecosystems 2008, 11, 775–789. [Google Scholar] [CrossRef]
- Okigbo, R.N.; Eme, U.E.; Ogbogu, S. Biodiversity and conservation of medicinal and aromatic 37 plants in Africa. Biotechnol. Mol. Biol. Rev. 2008, 3, 127–134. [Google Scholar]
- Gao, X.; Liu, J.; Huang, Z. The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol. Evol. 2022, 12, e9165. [Google Scholar] [CrossRef]
- Wu, D.J.; Zhu, P.T.; Wu, H.; Dai, H.F. Industry development status and prospect of Cinnamomum longepaniculatum. Open Access Libr. J. 2022, 9, e8616. [Google Scholar] [CrossRef]
- Urban, M.C.; Zarnetske, P.L.; Skelly, D.K. Moving forward: Dispersal and species interactions determine biotic responses to climate change. Ann. N. Y. Acad. Sci. 2013, 1297, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Mantyka-pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Change Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Zhang, X.F.; Nizamani, M.M.; Jiang, C.; Fang, F.Z.; Zhao, K.K. Potential planting regions of Pterocarpus santalinus (Fabaceae) under current and future climate in China based on MaxEnt modeling. Ecol. Evol. 2024, 14, e11409. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ostad-Ali-Askari, K.; Ghorbanizadeh, K.H.; Shayannejad, M. Effect of climate change on precipitation patterns in an arid region using GCM models: Case study of Isfahan-Borkhar Plain. Nat. Hazards Rev. 2020, 21, 04020006. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Hao, R.Z.; Yu, T.; Zhao, H.; Zhang, S.K.; Jing, Y.; Li, J.Q. Prediction of suitable distribution area of the endangered plant Acer catalpifolium under the background of climate change in China. J. Beijing For. Univ. 2021, 43, 33–43. [Google Scholar]
- Ostad-Ali-Askar, K.; Su, R.; Liu, L. Water resources and climate change. J. Water Clim. Change 2018, 9, 239. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Rodríguez-Sánchez, F.; Dobrowski, S.; Hu, F.S.; Gavin, D.G. Climate refugia: From the Last Glacial Maximum to the twenty-first century. New Phytol. 2013, 197, 16–18. [Google Scholar] [CrossRef]
- Wang, Q.; Abbott, R.J.; Yu, Q.S.; Lin, K.; Liu, J.Q. Pleistocene climate change and the origin of two desert plant species, Pugionium cornutum and Pugionium dolabratum (Brassicaceae), in northwest China. New Phytol. 2013, 199, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mi, Z.Y.; Lu, C.; Zhang, X.F.; Chen, L.J.; Wang, S.Q.; Niu, J.F.; Wang, Z.Z. Predicting potential distribution of Ziziphus spinosa (Bunge) HH Hu ex FH Chen in China under climate change scenarios. Ecol. Evol. 2022, 12, e8629. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wang, R.H.; Ru, X.Y.; Jiang, T.C.; Wang, J.H. Based on the phenological model to study the possible changes of apple flowering dates under future climate scenarios in shaanxi province. J. Agrometeorol. 2021, 42, 729. [Google Scholar]
- Ye, X.Z.; Zhang, M.Z.; Lai, W.F.; Yang, M.; Fan, H.; Zhang, G.; Chen, S.; Liu, B. Prediction of potential suitable distribution of Phoebe bournei based on MaxEnt optimization model. Acta Ecol. Sin. 2021, 41, 8135–8144. [Google Scholar]
- Khanum, R.; Mumtaz, A.S.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecol. 2013, 49, 23–31. [Google Scholar] [CrossRef]
- Li, L.H.; Liu, H.Y.; Lin, Z.S.; Jia, J.H.; Liu, X. Identifying priority areas for monitoring the invasion of Solidago canadensis based on MAXENT and ZONATION. Acta Ecol. Sin. 2017, 37, 3124–3132. [Google Scholar]
- Lemke, D.; Hulme, P.E.; Brown, J.A.; Tadesse, W. Distribution modelling of Japanese honeysuckle (Lonicera japonica) invasion in the Cumberland Plateau and Mountain Region, USA. For. Ecol. Manag. 2011, 262, 139–149. [Google Scholar] [CrossRef]
- Liu, C.; Huo, H.L.; Tian, L.M.; Dong, X.G.; Qi, D.; Zhang, Y.; Xu, J.Y.; Cao, Y.F. Potential geographical distribution of pyrus calleryana under different climate change scena-rios based on the maxent model. J. Appl. Ecol. 2018, 29, 3696–3704. [Google Scholar]
- Gong, Z.N.; Su, S.; Du, B.; Guan, H.; Zhang, Q. Habitat selection and dispersal of red-crowned cranes during breeding period in Zhalong Wetland National Nature Reserve. J. Nat. Resour. Dev. 2021, 36, 1964–1975. [Google Scholar] [CrossRef]
- Yan, K.; Zhu, H.; Cao, G.; Meng, L.; Li, J.; Zhang, J.; Liu, S.; Wang, Y.; Feng, R.; Soaud, S.A.; et al. Chromosome genome assembly of the Camphora longepaniculata (Gamble) with PacBio and Hi-C sequencing data. Front. Plant Sci. 2024, 15, 1372127. [Google Scholar] [CrossRef] [PubMed]
- Boutanaev, A.M.; Moses, T.; Zi, J.; Nelson, D.R.; Mugford, S.T.; Peters, R.J.; Osbourn, A. Investigation of terpene diversification across multiple sequenced plant genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E81–E88. [Google Scholar] [CrossRef]
- Wei, Q.; Tan, Y.Y.; Li, Q.; You, L.; Wang, C.; Wang, Y.; Liao, L. Effects of fungal endophytes on cell suspension culture of Cinnamomum longepaniculatum. GuangxiZhiwu/Guihaia 2016, 36, 923–929. [Google Scholar]
- Yan, K.; Wei, Q.; Feng, R.Z.; Zhou, W.H.; Chen, F. Transcriptome analysis of Cinnamomum longepaniculatum by high-throughput sequencing. Electron. J. Biotechnol. 2017, 28, 58–66. [Google Scholar] [CrossRef]
- Yan, K.; Wei, Q.; Feng, R.Z.; Zhou, W.H. Transcriptome analysis of the effects of endophytic fungi on the biosynthesis of essential oils in Cinnamomum longepaniculatum. Int. J. Agric. Biol. 2019, 6, 1301–1308. [Google Scholar]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R. Spatstat: An R package for analyzing spatial point patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Wu, T.w.; Yu, R.C.; Lu, Y.X.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z.; et al. BCC-CSM2-HR: A high-resolution version of the Beijing Climate Center Climate System Model. Geosci. Model Dev. 2020, 2020, 2977–3006. [Google Scholar] [CrossRef]
- Debella-Gilo, M.; Etzelmüller, B. Spatial prediction of soil classes using digital terrain analysis and multinomial logistic regression modeling integrated in GIS: Examples from Vestfold County, Norway. Catena 2009, 77, 8–18. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A. The Jackknife estimation method. arXiv 2016, arXiv:1606.00497. [Google Scholar]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, B.; Meng, Y.; Jia, Y.; Xu, Q.; Pan, Y. The Influence of Climate Change on the Distribution of Hibiscus mutabilis in China: MaxEnt Model-Based Prediction. Plants 2024, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, J.; Liu, Y.; Zeng, J.; Zeng, L.; He, R.; Guiang, M.M.; Li, Y.; Wu, H. Assessment of Chinese suitable habitats of Zanthoxylum nitidum in different climatic conditions by Maxent model, HPLC, and chemometric methods. Ind. Crops Prod. 2023, 196, 116515. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, F.Y.; Xia, P.G.; Zhao, G.H.; Wei, M.T.; Wei, F.G.; Han, R.L. Assessment of suitable cultivation region for Panax notoginseng under different climatic conditions using MaxEnt model and high-performance liquid chromatography in China. Ind. Crops Prod. 2022, 176, 114416. [Google Scholar] [CrossRef]
- Liu, L.; Qin, F.; Liu, Y.; Hu, Y.; Wang, W.; Duan, H.; Li, M. Forecast of potential suitable areas for forest resources in Inner Mongolia under the Shared Socioeconomic Pathway 245 scenario. Ecol. Indic. 2024, 167, 112694. [Google Scholar] [CrossRef]
- Yang, Q.; Xiang, Y.; Li, S.; Zhao, L.; Liu, Y.; Luo, Y.; Long, Y.; Yang, S.; Luo, X. Modeling the Impacts of Climate Change on Potential Distribution of Betula luminifera H. Winkler in China Using MaxEnt. Forests 2024, 15, 1624. [Google Scholar] [CrossRef]
- Cauwer, V.D.; Muys, B.; Revermann, R.; Trabucco, A. Potential, realised, future distribution and environmental suitability for Pterocarpus angolensis DC in southern Africa. For. Ecol. Manag. 2014, 315, 211–226. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.F.; Wu, M.L.; Guo, J.; Sun, C.Z.; Xie, C.X. Predicting the global areas for potential distribution of Gastrodia elata based on ecological niche models. Chin. J. Plant Ecol. 2017, 41, 770–778. [Google Scholar]
- Jia, X.; Wang, C.; Jin, H.; Zhao, Y.; Liu, L.-J.; Chen, Q.-H.; Li, B.-Y.; Xiao, Y.; Yin, H. Assessing the suitable distribution area of Pinus koraiensis based on an optimized MaxEnt model. Chin. J. Ecol. 2019, 38, 2570. [Google Scholar]
- Climate Resources. Available online: https://www.sc.gov.cn/10462/10464/10757/10868/2018/4/17/10449170.shtml (accessed on 5 October 2024).
- Liu, Y.; Shi, J. Predicting the potential global geographical distribution of two Icerya species under climate change. Forests 2020, 11, 684. [Google Scholar] [CrossRef]
- Homberger, J.M.; Lynch, A.; Riksen, M.; Limpens, J. Growth response of dune-building grasses to precipitation. Ecohydrology 2024, e2634. [Google Scholar] [CrossRef]
- Qi, D.M.; Li, Y.Q.; Zhou, C.Y.; Chen, D. Climatic change of summer rainstorms and the water vapor budget in the Sichuan Basin. J. Appl. Meteorol. Clim. 2022, 61, 537–557. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Tubiello, F.N.; Goldberg, R.; Mills, E.; Bloomfield, J. Increased crop damage in the US from excess precipitation under climate change. Glob. Environ. Change 2002, 12, 197–202. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, D.F.; Duan, H.L. Effects of drought and flooding on growth and physiology of Cinnamomum camphora seedlings. Forests 2023, 14, 1343. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.J.; He, X.Y.; Yan, Y.; Liu, M.J.; Liao, S.H.; Wei, Q. Effects of Different Temperatures on Physiology and Germination of Cinnamomum longepaniculatum Seed. Chin. Wild Pl. Resour. 2022, 41, 42–47. [Google Scholar]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Bi, B.; Shao, L.M.; Xu, T.; Du, H.; Li, D.Q. Transcriptomic analysis reveals calcium and ethylene signaling pathway genes in response to cold stress in Cinnamomum camphora. Horticulturae 2024, 10, 995. [Google Scholar] [CrossRef]
- Ouyang, X.H.; Lin, H.P.; Bai, S.H.; Jie, C.; Chen, A.L. Simulation the potential distribution of Dendrolimus houi and its hosts, Pinus yunnanensis and Cryptomeria fortunei, under climate change in China. Front. Plant Sci. 2022, 13, 1054710. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Zhaxi, D.Z.; Yuan, L.; Li, A.M.; Wang, J.H.; Liu, X.; Liu, Y.X. The Effects of Climate Change on the Distribution Pattern of Species Richness of Endemic Wetland Plants in the Qinghai-Tibet Plateau. Plants 2024, 13, 1886. [Google Scholar] [CrossRef]
- Xiao, Z.F.; Ai, Q.; Wang, Y.B.; Zhang, B.H.; Li, F.; Lv, X.W.; Jin, Z.N. Research Progress on Cinnamomum camphora. Jiangxi Sci. 2021, 39, 53–58. (In Chinese) [Google Scholar]
- Wang, P.; Luo, W.X.; Zhang, Q.Y.; Han, S.X.; Jin, Z.; Liu, J.C.; Li, Z.F.; Tao, J.P. Assessing the impact of climate change on three Populus species in China: Distribution patterns and implications. Glob. Ecol. Conserv. 2024, 50, e02853. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhao, J.; Weng, H.; Ye, X.; Liu, S.; Zhao, Z.; Ahmad, S.; Zhan, C. The Potential Habitat Response of Cyclobalanopsis gilva to Climate Change. Plants 2024, 13, 2336. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.L.; Xu, X.T.; Mao, K.S.; Wang, M.C.; Wang, K.; Xi, Z.X.; Liu, J.Q. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr. 2018, 45, 1334–1344. [Google Scholar] [CrossRef]
- Qiu, L.; Fu, Q.L.; Jacquemyn, H.; Burgess, K.S.; Cheng, J.J.; Mo, Z.Q.; Tang, X.D.; Yang, B.Y.; Tan, S.L. Contrasting range changes of Bergenia (Saxifragaceae) species under future climate change in the Himalaya and Hengduan Mountains Region. Theor. Appl. Climatol. 2023, 155, 1927–1939. [Google Scholar] [CrossRef]
- Waqar, Z.; Moraes, R.C.; Benchimol, M.; Morante-Filho, J.C.; Mariano-Neto, E.; Gaiotto, F.A. Gene flow and genetic structure reveal reduced diversity between generations of a tropical tree, Manilkara multifida Penn., in atlantic forest fragments. Genes 2021, 12, 2025. [Google Scholar] [CrossRef]
- Chase, J.M.; Blowes, S.A.; Knight, T.M.; Gerstner, K.; May, F. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 2020, 584, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H.L.; Chen, Y.K.; Nizamani, M.M.; Wu, T.T.; Liu, T.T.; Zhou, Q. Assessing genetic diversity in critically endangered Chieniodendron hainanense populations within fragmented habitats in Hainan. Sci. Rep. 2024, 14, 6988. [Google Scholar] [CrossRef] [PubMed]
- Baude, M.; Kunin, W.E.; Boatman, N.D.; Conyers, S.; Davies, N.; Gillespie, M.A.K.; Morton, R.D.; Smart, S.M.; Memmott, J. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 2016, 530, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.D.; Yin, Q.; Sang, Z.T.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Ma, Q.Q.; Li, X.Y.; Wu, S.X.; Zeng, F.J. Potential geographical distribution of Stipa purpurea across the Tibetan Plateau in China under climate change in the 21st century. Glob. Ecol. Conserv. 2022, 35, e02064. [Google Scholar] [CrossRef]
- Prevéy, J.S.; Parker, L.E.; Harrington, C.A.; Lamb, C.T.; Proctor, M.F. Climate change shifts in habitat suitability and phenology of huckleberry (Vaccinium membranaceum). Agric. Meteorol. 2020, 280, 107803. [Google Scholar] [CrossRef]
- Jinga, P.; Ashley, M.V. Climate change threatens some miombo tree species of sub-Saharan Africa. Flora 2019, 257, 151421. [Google Scholar] [CrossRef]
- Seale, M.; Nakayama, N. Seale. From passive to informed: Mechanical mechanisms of seed dispersal. New Phytol. 2019, 225, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Schurr, F.M.; Bond, W.J.; Midgley, G.F.; Higgins, S.I. A mechanistic model for secondary seed dispersal by wind and its experimental validation. J. Ecol. 2005, 93, 1017–1028. [Google Scholar] [CrossRef]
- Holzmann, K.L.; Walls, R.L.; Wiens, J.J. Accelerating local extinction associated with very recent climate change. Ecol. Lett. 2023, 26, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Burgess, K.S.; Gao, L.M.; Li, D.Z. Distributional responses to climate change for alpine species of Cyananthus and Primula endemic to the Himalaya-Hengduan Mountains. Plant Divers. 2019, 41, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Li, H.; Lu, S.F.; Yin, X.J.; Li, G.; Chen, Z.; Wang, Y. Responses of cold-temperature coniferous forest to climate change in southwestern China. J. Northwest For. Univ. 2023, 38, 33–44. [Google Scholar]
- Li, R. Protecting rare and endangered species under climate change on the Qinghai Plateau, China. Ecol. Evol. 2019, 9, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Meng, S.; Jiang, Q.; Hu, G.; Zhang, L.; Yao, X. Potential distribution under climate change and ecological niche differences between Actinidia chinensis complex. Sci. Hortic. 2024, 337, 113533. [Google Scholar] [CrossRef]








| Variable | Description | Unit | Contribution Rate% | Permutation Importance |
|---|---|---|---|---|
| bio12 | Annual precipitation | mm | 50 | 15.2 |
| bio6 | Min temperature of coldest month | °C | 12.4 | 20 |
| dem | Elevation | m | 11.7 | 10.5 |
| bio15 | Precipitation seasonality | / | 7 | 4.5 |
| bio7 | Variation range of annual average temperature | °C | 5.3 | 15.3 |
| bio2 | Mean diurnal range | °C | 3.6 | 9.3 |
| slope | Slope | ° | 3.1 | 5.5 |
| aspect | Aspect | rad | 2.8 | 2.5 |
| silt | Silt content | % | 2 | 1.2 |
| bio16 | Precipitation of the Wettest Month | mm | 1.3 | 15.2 |
| ph_water | Hydrogen ion concentration (acidity/alkalinity) | pH | 0.6 | 0.8 |
| Period | Longitude | Latitude | Migration Direction | Distance (Km) |
|---|---|---|---|---|
| Current | 105.39 | 28.71 | Current-2050s SSP126 | 289 |
| 2050s SSP126 | 106.58 | 31.10 | 2050s SSP126-2070s SSP126 | 56 |
| 2070s SSP126 | 107.13 | 31.28 | 2070s SSP126-2090s SSP126 | 243 |
| 2090s SSP126 | 104.96 | 30.13 | Current-2050s SSP585 | 390 |
| 2050s SSP585 | 107.34 | 31.79 | 2050s SSP585-2070s SSP585 | 346 |
| 2070s SSP585 | 109.82 | 34.11 | 2070s SSP585-2090s SSP585 | 410 |
| 2090s SSP585 | 105.95 | 32.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhao, H.; Liu, Y.; Zhu, M.; Wan, Z.; Yan, Y.; Wang, X.; Xiang, Y.; Gao, S.; Jiang, C.; et al. Potential Distribution and Response of Camphora longepaniculata Gamble (Lauraceae) to Climate Change in China. Forests 2025, 16, 338. https://doi.org/10.3390/f16020338
Zhu Y, Zhao H, Liu Y, Zhu M, Wan Z, Yan Y, Wang X, Xiang Y, Gao S, Jiang C, et al. Potential Distribution and Response of Camphora longepaniculata Gamble (Lauraceae) to Climate Change in China. Forests. 2025; 16(2):338. https://doi.org/10.3390/f16020338
Chicago/Turabian StyleZhu, Yanzhao, Hanzhi Zhao, Yidi Liu, Minghui Zhu, Zitong Wan, Yujie Yan, Xiaoying Wang, Ya Xiang, Shanshan Gao, Chenlong Jiang, and et al. 2025. "Potential Distribution and Response of Camphora longepaniculata Gamble (Lauraceae) to Climate Change in China" Forests 16, no. 2: 338. https://doi.org/10.3390/f16020338
APA StyleZhu, Y., Zhao, H., Liu, Y., Zhu, M., Wan, Z., Yan, Y., Wang, X., Xiang, Y., Gao, S., Jiang, C., Zhang, Y., & Zhao, G. (2025). Potential Distribution and Response of Camphora longepaniculata Gamble (Lauraceae) to Climate Change in China. Forests, 16(2), 338. https://doi.org/10.3390/f16020338






