Warming Does Not Change Vertical Variations in Microbial Resource Limitation in Subtropical Forests at China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Soil Sampling and Physicochemical Analyses
2.3. Soil Extracellular Enzymes and Enzymatic Stoichiometry Analyses
2.4. Soil Microbial Analyses
2.5. Statistical Analysis
3. Results
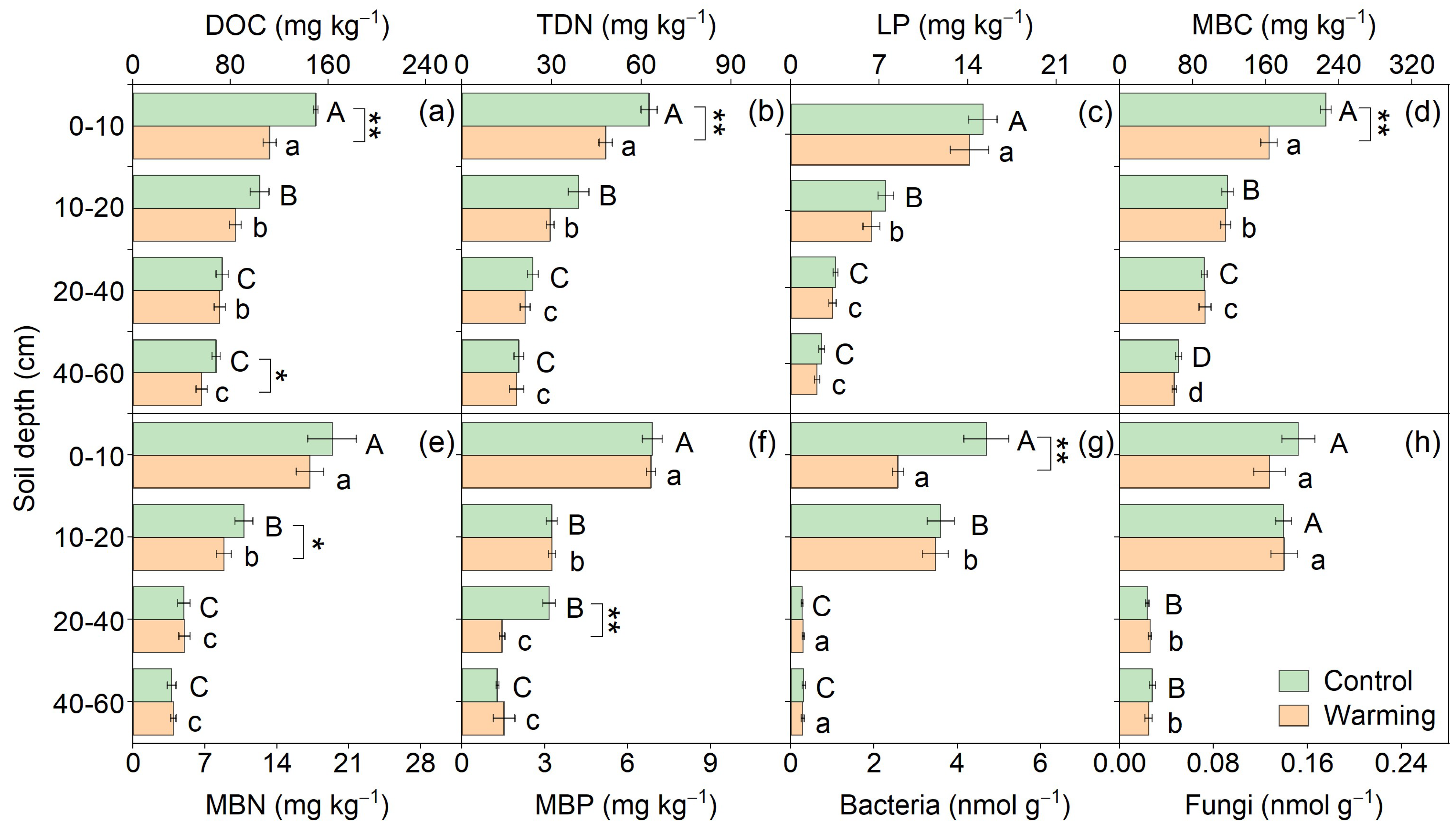
3.1. Soil Physiochemical and Microbial Properties
3.2. Soil Enzymatic Stoichiometry
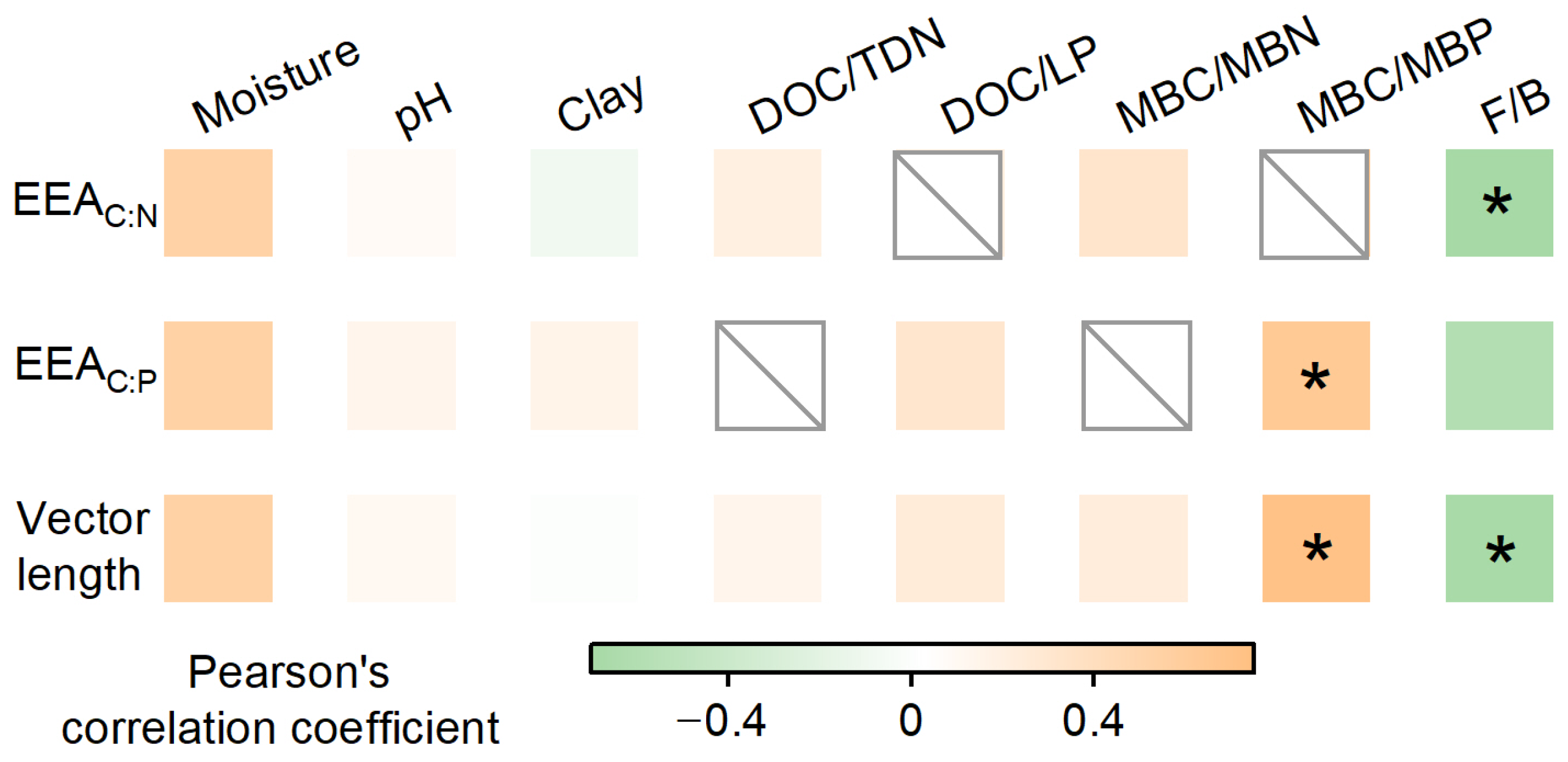
3.3. Drivers of Soil Enzymatic Stoichiometry
4. Discussion
4.1. Increased Microbial Nutrient Limitation but Decreased C Limitation Across Soil Depths
4.2. Limited Warming Effects on Microbial Resource Limitation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EEAC:N | enzymatic C:N ratio |
| EEAC:P | enzymatic C:P ratio |
| RC:N/TERC:N | ratio of soil C:N ratio to threshold elemental ratio model |
| RC:P/TERC:P | ratio of soil C:P ratio to threshold elemental ratio model |
| BG | β-1,4-glucosidase |
| CBH | β-D-cellobiosidase |
| NAG | β-1,4-N-acetylglucosaminidase |
| LAP | leucine aminopeptidase |
| AP | acid phosphatase |
| DOC/TDN | soil C:N ratio |
| DOC/LP | soil C:P ratio |
| MBC/MBN | microbial biomass C:N ratio |
| MBC/MBP | microbial biomass C:P ratio |
| F/B | fungal–bacterial ratio |
Appendix A
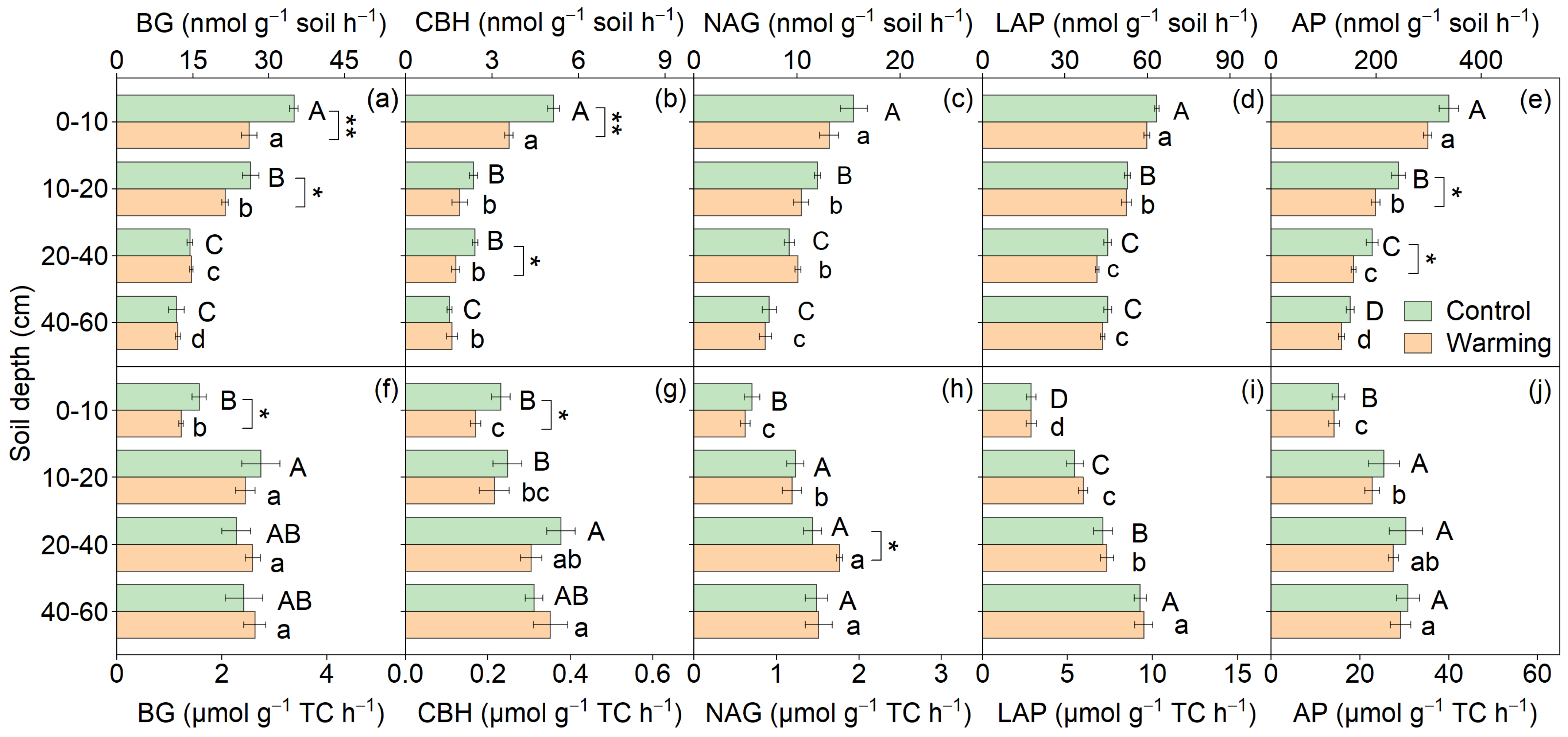
| Variables | Warming | Depth | Warming × Depth | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| BG (nmol g−1 soil h−1) | 41.36 | <0.01 | 61.25 | <0.01 | 7.75 | <0.01 |
| CBH (nmol g−1 soil h−1) | 11.71 | <0.01 | 43.68 | <0.01 | 1.95 | 0.13 |
| NAG (nmol g−1 soil h−1) | 17.51 | <0.01 | 52.39 | <0.01 | 1.27 | 0.29 |
| LAP (nmol g−1 soil h−1) | 3.14 | 0.08 | 19.76 | <0.01 | 0.01 | 1.00 |
| AP (nmol g−1 soil h−1) | 13.62 | <0.01 | 47.54 | <0.01 | 1.74 | 0.16 |
| BG (μmol g−1 soil h−1) | 1.25 | 0.27 | 28.14 | <0.01 | 0.25 | 0.86 |
| CBH (μmol g−1 soil h−1) | 1.13 | 0.29 | 10.29 | <0.01 | 2.87 | 0.04 |
| NAG (μmol g−1 soil h−1) | 0.04 | 0.84 | 40.40 | <0.01 | 0.11 | 0.95 |
| LAP (μmol g−1 soil h−1) | 0.90 | 0.34 | 34.66 | <0.01 | 0.30 | 0.83 |
| AP (μmol g−1 soil h−1) | 0.20 | 0.65 | 17.32 | <0.01 | 0.42 | 0.74 |
| Response Variables | Explanatory Variables | AICc | R2 |
|---|---|---|---|
| EEAC:N under control | Moisture + Fungal–bacterial ratio | −56.4 | 0.74 |
| EEAC:N under warming | Moisture + Fungal–bacterial ratio | −78.2 | 0.73 |
| EEAC:P under control | Moisture + Fungal–bacterial ratio | −71.6 | 0.72 |
| EEAC:P under warming | Soil C:P ratio + Fungal–bacterial ratio | −98.9 | 0.72 |
| Vector length under control | Moisture + Fungal–bacterial ratio | −49.7 | 0.74 |
| Vector length under warming | Soil C:P ratio + Fungal–bacterial ratio | −79.2 | 0.80 |
References
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Quan, Q.; Zhang, F.; Jiang, L.; Chen, H.Y.H.; Wang, J.; Ma, F.; Song, B.; Niu, S. High-level rather than low-level warming destabilizes plant community biomass production. J. Ecol. 2021, 109, 1607–1617. [Google Scholar] [CrossRef]
- Yu, J.; Bing, H.; Chang, R.; Cui, Y.; Shen, G.; Wang, X.; Zhang, S.; Fang, L. Microbial metabolic limitation response to experimental warming along an altitudinal gradient in alpine grasslands, eastern Tibetan Plateau. CATENA 2022, 214, 106243. [Google Scholar] [CrossRef]
- Dove, N.C.; Torn, M.S.; Hart, S.C.; Taş, N. Metabolic capabilities mute positive response to direct and indirect impacts of warming throughout the soil profile. Nat. Commun. 2021, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Marañón-Jiménez, S.; Peñuelas, J.; Richter, A.; Sigurdsson, B.D.; Fuchslueger, L.; Leblans, N.I.W.; Janssens, I.A. Coupled carbon and nitrogen losses in response to seven years of chronic warming in subarctic soils. Soil Biol. Biochem. 2019, 134, 152–161. [Google Scholar] [CrossRef]
- Lie, Z.; Huang, W.; Liu, X.; Zhou, G.; Yan, J.; Li, Y.; Huang, C.; Wu, T.; Fang, X.; Zhao, M.; et al. Warming leads to more closed nitrogen cycling in nitrogen-rich tropical forests. Glob. Change Biol. 2020, 27, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bing, H.; Moorhead, D.L.; Delgado-Baquerizo, M.; Ye, L.; Yu, J.; Zhang, S.; Wang, X.; Peng, S.; Guo, X.; et al. Ecoenzymatic stoichiometry reveals widespread soil phosphorus limitation to microbial metabolism across Chinese forests. Commun. Earth Environ. 2022, 3, 184. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, M.; You, C.; Tan, B.; Xu, L.; Li, H.; Zhang, L.; Wang, L.; Liu, S.; Hou, G.; et al. Warming effects on C:N:P stoichiometry and nutrient limitation in terrestrial ecosystems. Soil Till. Res. 2024, 235, 105896. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, Y.; Zhao, G.; Zhao, B.; Cong, N.; Zhu, J.; Zheng, Z.; Wu, W.; Duan, X. Excessive climate warming exacerbates nitrogen limitation on microbial metabolism in an alpine meadow of the Tibetan Plateau: Evidence from soil ecoenzymatic stoichiometry. Sci. Total Environ. 2024, 930, 172731. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, Y.; Chen, Y.; Zhang, J.; Li, H.; Wang, L.; Chen, Q. Short-term warming shifts microbial nutrient limitation without changing the bacterial community structure in an alpine timberline of the eastern Tibetan Plateau. Geoderma 2020, 360, 113985. [Google Scholar] [CrossRef]
- Li, H.; Tian, H.; Wang, Z.; Liu, C.; Nurzhan, A.; Megharaj, M.; He, W. Potential effect of warming on soil microbial nutrient limitations as determined by enzymatic stoichiometry in the farmland from different climate zones. Sci. Total Environ. 2022, 802, 149657. [Google Scholar] [CrossRef]
- Button, E.S.; Pett-Ridge, J.; Murphy, D.V.; Kuzyakov, Y.; Chadwick, D.R.; Jones, D.L. Deep-C storage: Biological, chemical and physical strategies to enhance carbon stocks in agricultural subsoils. Soil Biol. Biochem. 2022, 170, 108697. [Google Scholar] [CrossRef]
- Hicks Pries, C.E.; Sulman, B.N.; West, C.; O’Neill, C.; Poppleton, E.; Porras, R.C.; Castanha, C.; Zhu, B.; Wiedemeier, D.B.; Torn, M.S. Root litter decomposition slows with soil depth. Soil Biol. Biochem. 2018, 125, 103–114. [Google Scholar] [CrossRef]
- Li, J.; Ding, J.; Yang, S.; Zhao, L.; Li, J.; Huo, H.; Wang, M.; Tan, J.; Cao, Y.; Ren, S.; et al. Depth-dependent driver of global soil carbon turnover times. Soil Biol. Biochem. 2023, 185, 109149. [Google Scholar] [CrossRef]
- Qin, S.; Chen, L.; Fang, K.; Zhang, Q.; Wang, J.; Liu, F.; Yu, J.; Yang, Y. Temperature sensitivity of SOM decomposition governed by aggregate protection and microbial communities. Sci. Adv. 2019, 5, 03. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, X.; Li, S.; Zhou, W.; Chen, Z.; Bai, X. The vertical distribution and control factor of microbial biomass and bacterial community at macroecological scales. Sci. Total Environ. 2023, 869, 161754. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, J.; Ding, Z.; Tang, M.; Zhu, B. Changes in soil total, microbial and enzymatic C-N-P contents and stoichiometry with depth and latitude in forest ecosystems. Sci. Total Environ. 2022, 816, 151583. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, J.; Yuan, X.; Zhu, B. Effects of warming on carbon and nitrogen cycling in alpine grassland ecosystems on the Tibetan Plateau: A meta-analysis. Geoderma 2020, 370, 114363. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, J.; Zeng, H.; Wang, W. Vertical pattern and its driving factors in soil extracellular enzyme activity and stoichiometry along mountain grassland belts. Biogeochemistry 2018, 141, 23–39. [Google Scholar] [CrossRef]
- He, H.; Xu, M.; Li, W.; Chen, L.; Chen, Y.; Moorhead, D.L.; Brangarí, A.C.; Liu, J.; Cui, Y.; Zeng, Y.; et al. Linking soil depth to aridity effects on soil microbial community composition, diversity and resource limitation. CATENA 2023, 232, 107393. [Google Scholar] [CrossRef]
- Wu, L.; Zou, B.; Wang, S.; Zhou, L.; Zheng, Y.; Huang, Z.; He, J.-Z. Effects of multispecies restoration on soil extracellular enzyme activity stoichiometry in Pinus massoniana plantations of subtropical China. Soil Biol. Biochem. 2023, 178, 108967. [Google Scholar] [CrossRef]
- Zheng, W.; Lin, W.; Fan, Y.; Li, Y.; Zhou, J.; Zheng, Y.; Chen, S.; Liu, X.; Xiong, D.; Xu, C.; et al. Divergent effects of short-term warming on microbial resource limitation between topsoil and subsoil in a young subtropical Chinese fir forest. Biogeochemistry 2023, 163, 185–199. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, Q.; Sun, J.; Robinson, D.; Yang, Z.; Yao, X.; Wang, X.; Dai, X.; Chen, T.; Wu, D.; et al. Contrasting depth-related fine root plastic responses to soil warming in a subtropical Chinese fir plantation. J. Ecol. 2024, 112, 1058–1073. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Feng, J.; Li, X.; Zhang, Z.; He, J.-S.; Schimel, J.P.; Zhu, B. Whole-soil-profile warming does not change microbial carbon use efficiency in surface and deep soils. Proc. Natl. Acad. Sci. USA 2023, 120, e2302190120. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y. The carbon balance of tropical forest regions, 1990–2005. Curr. Opin. Environ. Sustain. 2010, 2, 237–244. [Google Scholar] [CrossRef]
- Melillo, J.M.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global climate change and terrestrial net primary production. Nature 1993, 363, 234–240. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Giardina, C.P.; Litton, C.M.; Crow, S.E.; Asner, G.P. Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nat. Clim. Change 2014, 4, 822–827. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, T.-C.; Wang, L.; Chen, S.; Liu, X.; Xiong, D.; Xu, C.; Arthur, M.; McCulley, R.; Shi, S.; et al. Recent photosynthates are the primary carbon source for soil microbial respiration in subtropical forests. Geophys. Res. Lett. 2022, 49, e2022GL101147. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Gloor, E.; Bååth, E.; Meir, P. Soil carbon and microbes in the warming tropics. Funct. Ecol. 2022, 36, 1338–1354. [Google Scholar] [CrossRef]
- Huang, J.; Lin, T.-C.; Xiong, D.; Yang, Z.; Liu, X.; Chen, G.; Xie, J.; Li, Y.; Yang, Y. Organic carbon mineralization in soils of a natural forest and a forest plantation of southeastern China. Geoderma 2019, 344, 119–126. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Li, X.; Yang, Z.; Xiong, D.; Xu, C.; Wanek, W.; Yang, Y. Soil warming delays leaf litter decomposition but exerts no effect on litter nutrient release in a subtropical natural forest over 450 days. Geoderma 2022, 427, 116139. [Google Scholar] [CrossRef]
- Mao, C.; Kou, D.; Chen, L.; Qin, S.; Zhang, D.; Peng, Y.; Yang, Y. Permafrost nitrogen status and its determinants on the Tibetan Plateau. Glob. Change Biol. 2020, 26, 5290–5302. [Google Scholar] [CrossRef] [PubMed]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Moorhead, D.L.; Guo, X.; Peng, S.; Wang, Y.; Zhang, X.; Fang, L. Stoichiometric models of microbial metabolic limitation in soil systems. Glob. Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Bates, D.; Mäechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. smatr 3- an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Mao, C.; Song, Y.; Peng, Y.; Kang, L.; Li, Z.; Zhou, W.; Liu, X.; Liu, F.; Zhu, G.; Yang, Y. Patterns and drivers of anaerobic nitrogen transformations in sediments of thermokarst lakes. Glob. Change Biol. 2023, 29, 2697–2713. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 0132. [Google Scholar] [CrossRef]
- Le Provost, G.; Badenhausser, I.; Le Bagousse-Pinguet, Y.; Clough, Y.; Henckel, L.; Violle, C.; Bretagnolle, V.; Roncoroni, M.; Manning, P.; Gross, N. Land-use history impacts functional diversity across multiple trophic groups. Proc. Natl. Acad. Sci. USA 2020, 117, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference, R package version 1.47.1; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Hou, E.; Wen, D.; Kuang, Y.; Cong, J.; Chen, C.; He, X.; Heenan, M.; Lu, H.; Zhang, Y. Soil pH predominantly controls the forms of organic phosphorus in topsoils under natural broadleaved forests along a 2500 km latitudinal gradient. Geoderma 2018, 315, 65–74. [Google Scholar] [CrossRef]
- Liu, L.; Wen, Z.; Liu, S.; Zhang, X.; Liu, X. Decline in atmospheric nitrogen deposition in China between 2010 and 2020. Nat. Geosci. 2024, 17, 733–736. [Google Scholar] [CrossRef]
- Mason, R.E.; Craine, J.M.; Lany, N.K.; Jonard, M.; Ollinger, S.V.; Groffman, P.M.; Fulweiler, R.W.; Angerer, J.; Read, Q.D.; Reich, P.B.; et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science 2022, 376, eabh3767. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Cen, X.; Groffman, P.M. Has nitrogen availability decreased over much of the land surface in the past century? A model-based analysis. Biogeochemistry 2024, 167, 793–806. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbágy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef]
- Hensgens, G.; Laudon, H.; Peichl, M.; Gil, I.A.; Zhou, Q.; Berggren, M. The role of the understory in litter DOC and nutrient leaching in boreal forests. Biogeochemistry 2020, 149, 87–103. [Google Scholar] [CrossRef]
- Scott, E.E.; Rothstein, D.E. Patterns of DON and DOC leaching losses across a natural N availability gradient in temperate hardwood forests. Ecosystems 2017, 20, 1250–1265. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, X.; Lu, S.; Cao, P.; Hui, D.; Chen, J.; Guo, J.; Yang, Y. Depth-dependent response of particulate and mineral-associated organic carbon to long-term throughfall reduction in a subtropical natural forest. CATENA 2023, 223, 106904. [Google Scholar] [CrossRef]
- Boer, W.d.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J. Plant responses to nutrient addition experiments conducted in tropical forests. Ecol. Monogr. 2019, 89, e01382. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Yang, Z.; Xu, C.; Xie, J.; Chen, G.; Lin, C.; Guo, J.; Liu, X.; Xiong, D.; et al. Large ecosystem service benefits of assisted natural regeneration. J. Geophys. Res.-Biogeosci. 2018, 123, 676–687. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Jayawardena, D.M.; Heckathorn, S.A.; Boldt, J.K. A meta-analysis of the combined effects of elevated carbon dioxide and chronic warming on plant %N, protein content and N-uptake rate. AoB PLANTS 2021, 13, plab031. [Google Scholar] [CrossRef]
- Noyce, G.L.; Kirwan, M.L.; Rich, R.L.; Megonigal, P. Asynchronous nitrogen supply and demand produce nonlinear plant allocation responses to warming and elevated CO2. Proc. Natl. Acad. Sci. USA 2019, 116, 21623–21628. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Chuckran, P.F.; Reibold, R.; Throop, H.L.; Reed, S.C. Multiple mechanisms determine the effect of warming on plant litter decomposition in a dryland. Soil Biol. Biochem. 2020, 145, 107799. [Google Scholar] [CrossRef]
- Wei, H.; Guenet, B.; Vicca, S.; Nunan, N.; AbdElgawad, H.; Pouteau, V.; Shen, W.; Janssens, I.A. Thermal acclimation of organic matter decomposition in an artificial forest soil is related to shifts in microbial community structure. Soil Biol. Biochem. 2014, 71, 1–12. [Google Scholar] [CrossRef]
- Guan, P.; Yang, J.; Yang, Y.; Wang, W.; Zhang, P.; Wu, D. Land conversion from cropland to grassland alleviates climate warming effects on nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Glob. Ecol. Conserv. 2020, 24, e01328. [Google Scholar] [CrossRef]
- Maslov, M.N.; Maslova, O.A. Nitrogen limitation of microbial activity in alpine tundra soils along an environmental gradient: Intra-seasonal variations and effect of rising temperature. Soil Biol. Biochem. 2021, 156, 108234. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Qu, C.; Huang, W.; Zhang, D.; Li, Y.; Yi, Z.; Liu, J. Translocating subtropical forest soils to a warmer region alters microbial communities and increases the decomposition of mineral-associated organic carbon. Soil Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Butler, S.M.; Melillo, J.M.; Johnson, J.E.; Mohan, J.; Steudler, P.A.; Lux, H.; Burrows, E.; Smith, R.M.; Vario, C.L.; Scott, L.; et al. Soil warming alters nitrogen cycling in a New England forest: Implications for ecosystem function and structure. Oecologia 2012, 168, 819–828. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.-G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Craine, J.M.; Fierer, N.; McLauchlan, K.K.; Elmore, A.J. Reduction of the temperature sensitivity of soil organic matter decomposition with sustained temperature increase. Biogeochemistry 2012, 113, 359–368. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.X.; Brookes, P.C.; Dahlgren, R.A.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Change Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef]

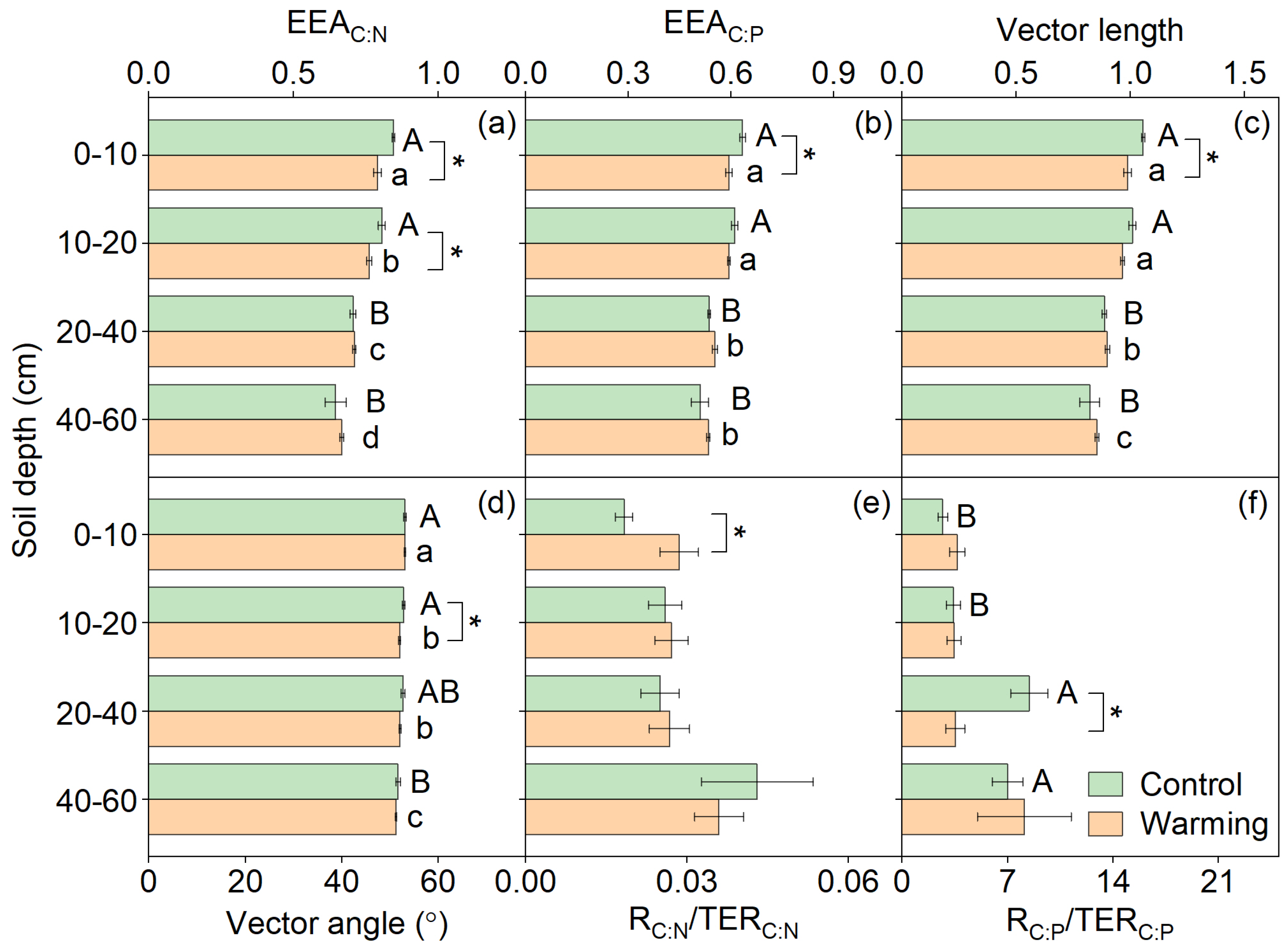

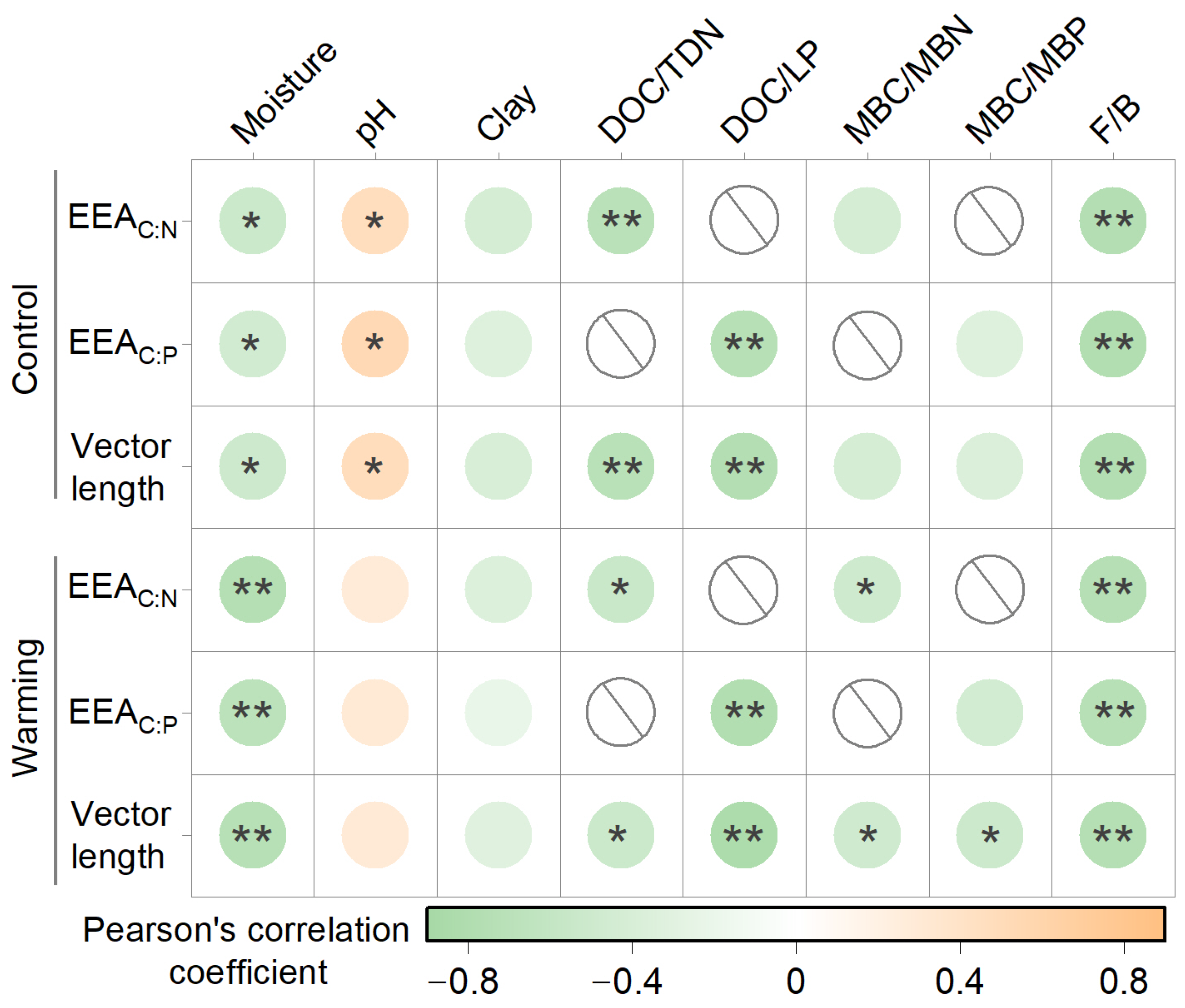

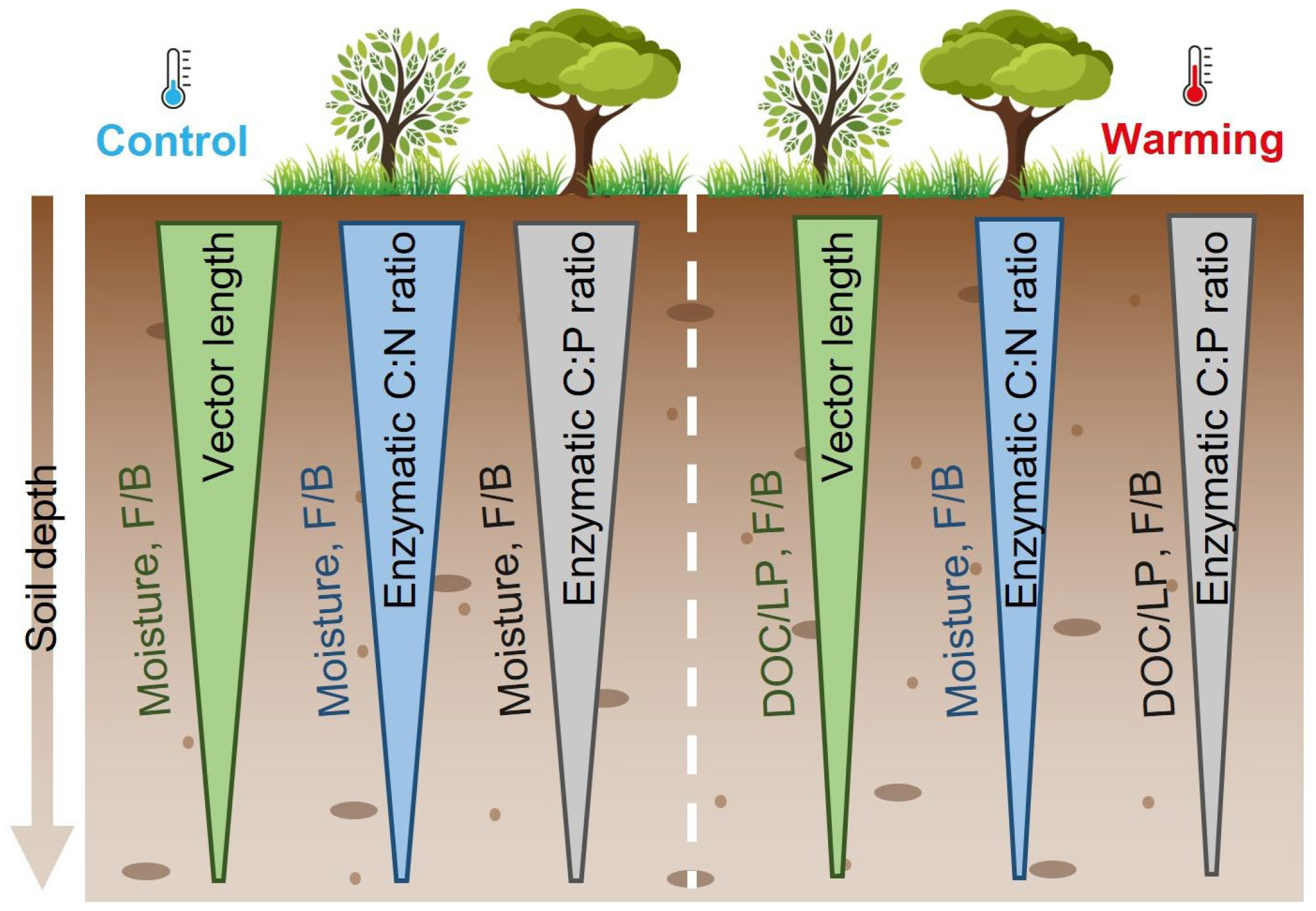
| Variables | Warming | Depth | Warming × Depth | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Soil moisture (%) | 14.32 | <0.01 | 16.64 | <0.01 | 2.43 | 0.07 |
| pH | 0.04 | 0.84 | 1.81 | 0.15 | 0.50 | 0.68 |
| Clay (%) | 0.90 | 0.34 | 11.57 | <0.01 | 0.11 | 0.96 |
| DOC/TDN | 3.16 | 0.08 | 17.16 | <0.01 | 1.24 | 0.30 |
| DOC/LP | 2.37 | 0.13 | 11.12 | <0.01 | 0.62 | 0.60 |
| MBC/MBN | 2.22 | 0.14 | 3.38 | 0.02 | 0.28 | 0.84 |
| MBC/MBP | 0.80 | 0.37 | 8.60 | <0.01 | 1.38 | 0.25 |
| F/B | 0.01 | 0.94 | 12.50 | <0.01 | 0.55 | 0.65 |
| EEAC:N | 6.99 | <0.01 | 9.31 | <0.01 | 1.56 | 0.20 |
| EEAC:P | 1.74 | 0.19 | 1.48 | 0.23 | 0.48 | 0.70 |
| Vector length | 4.48 | 0.04 | 4.96 | <0.01 | 1.03 | 0.38 |
| Vector angle (°) | 1.75 | 0.19 | 7.17 | <0.01 | 0.58 | 0.63 |
| RC:N/TERC:N | 2.82 | 0.10 | 0.21 | 0.89 | 0.12 | 0.95 |
| RC:P/TERC:P | 0.40 | 0.53 | 1.16 | 0.33 | 1.00 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, C.; Wang, Y.; Xiong, D.; Xu, C.; Chen, S.; Yang, Z.; Yang, Y. Warming Does Not Change Vertical Variations in Microbial Resource Limitation in Subtropical Forests at China. Forests 2025, 16, 402. https://doi.org/10.3390/f16030402
Mao C, Wang Y, Xiong D, Xu C, Chen S, Yang Z, Yang Y. Warming Does Not Change Vertical Variations in Microbial Resource Limitation in Subtropical Forests at China. Forests. 2025; 16(3):402. https://doi.org/10.3390/f16030402
Chicago/Turabian StyleMao, Chao, Yun Wang, Decheng Xiong, Chao Xu, Shidong Chen, Zhijie Yang, and Yusheng Yang. 2025. "Warming Does Not Change Vertical Variations in Microbial Resource Limitation in Subtropical Forests at China" Forests 16, no. 3: 402. https://doi.org/10.3390/f16030402
APA StyleMao, C., Wang, Y., Xiong, D., Xu, C., Chen, S., Yang, Z., & Yang, Y. (2025). Warming Does Not Change Vertical Variations in Microbial Resource Limitation in Subtropical Forests at China. Forests, 16(3), 402. https://doi.org/10.3390/f16030402





