Assessing Habitat Suitability for Hippophae rhamnoides subsp. turkestanica Amid Climate Change Using the MaxEnt Model
Abstract
1. Introduction
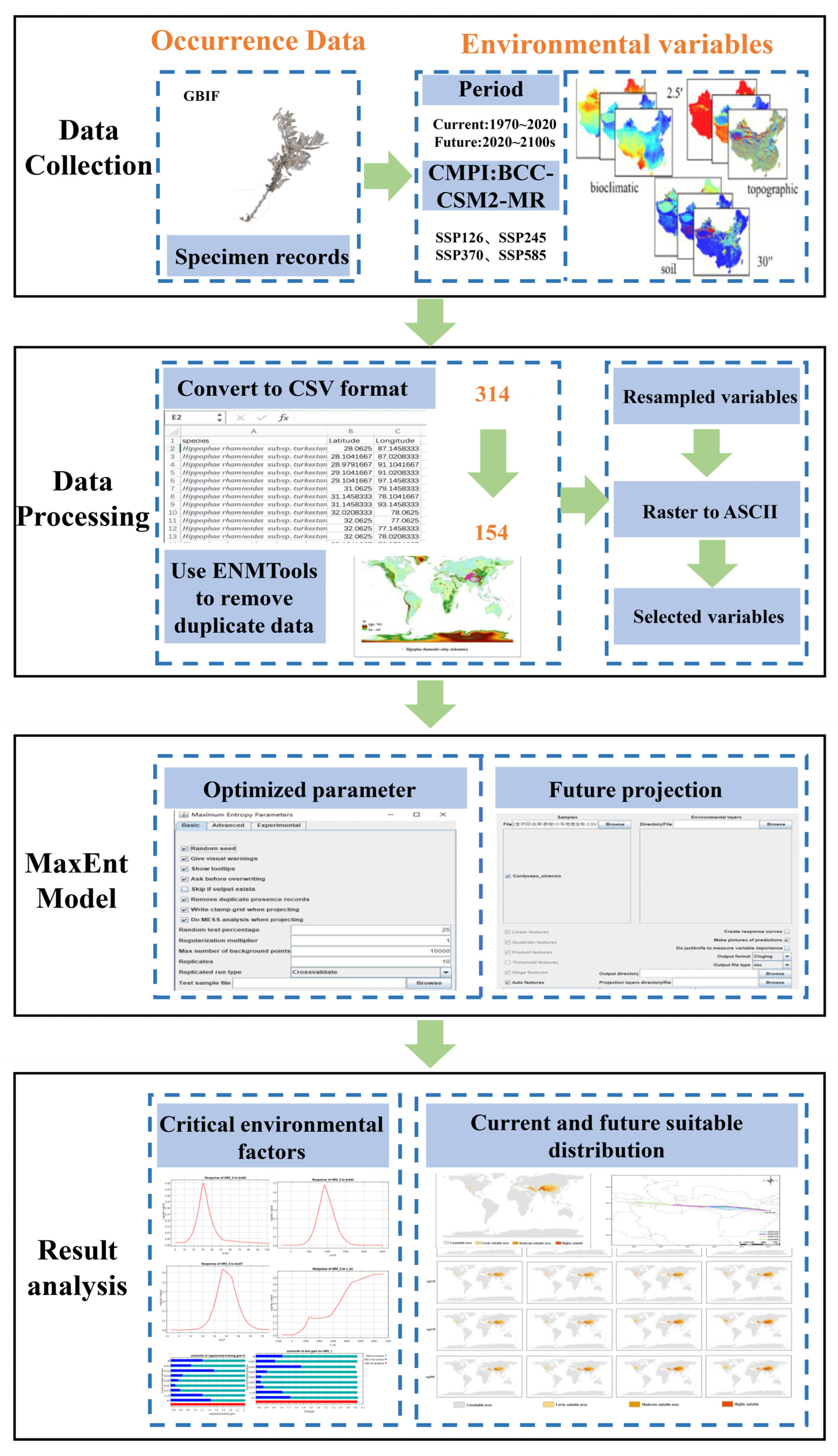
2. Materials and Methods
2.1. Species Distribution Data
2.2. Environmental Variables
3. Results
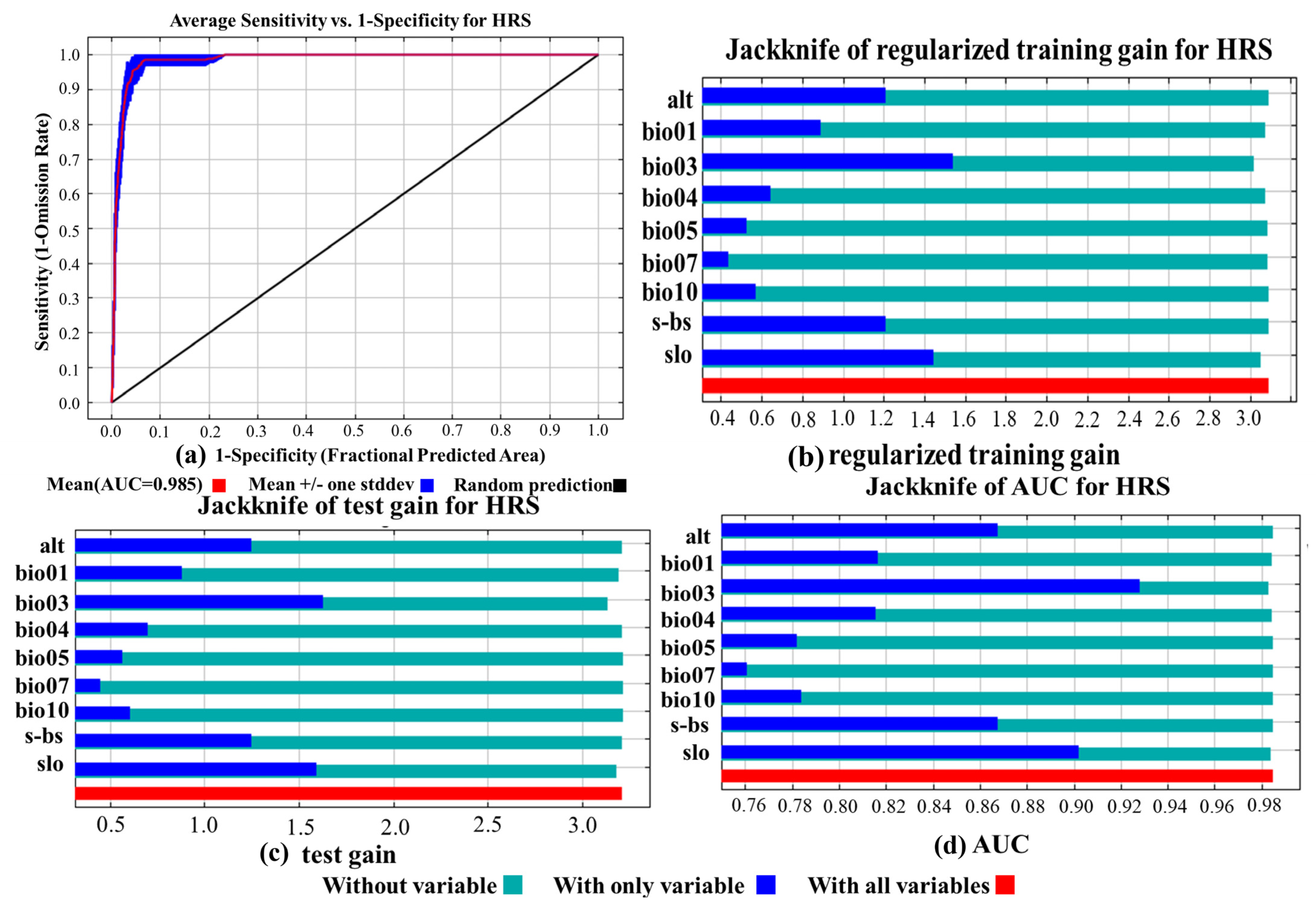
3.1. Model Accuracy Assessment and the Importance of Environmental Variables
3.2. Potential Distribution of Hippophae rhamnoides in Central Asia Under Current Climate Conditions
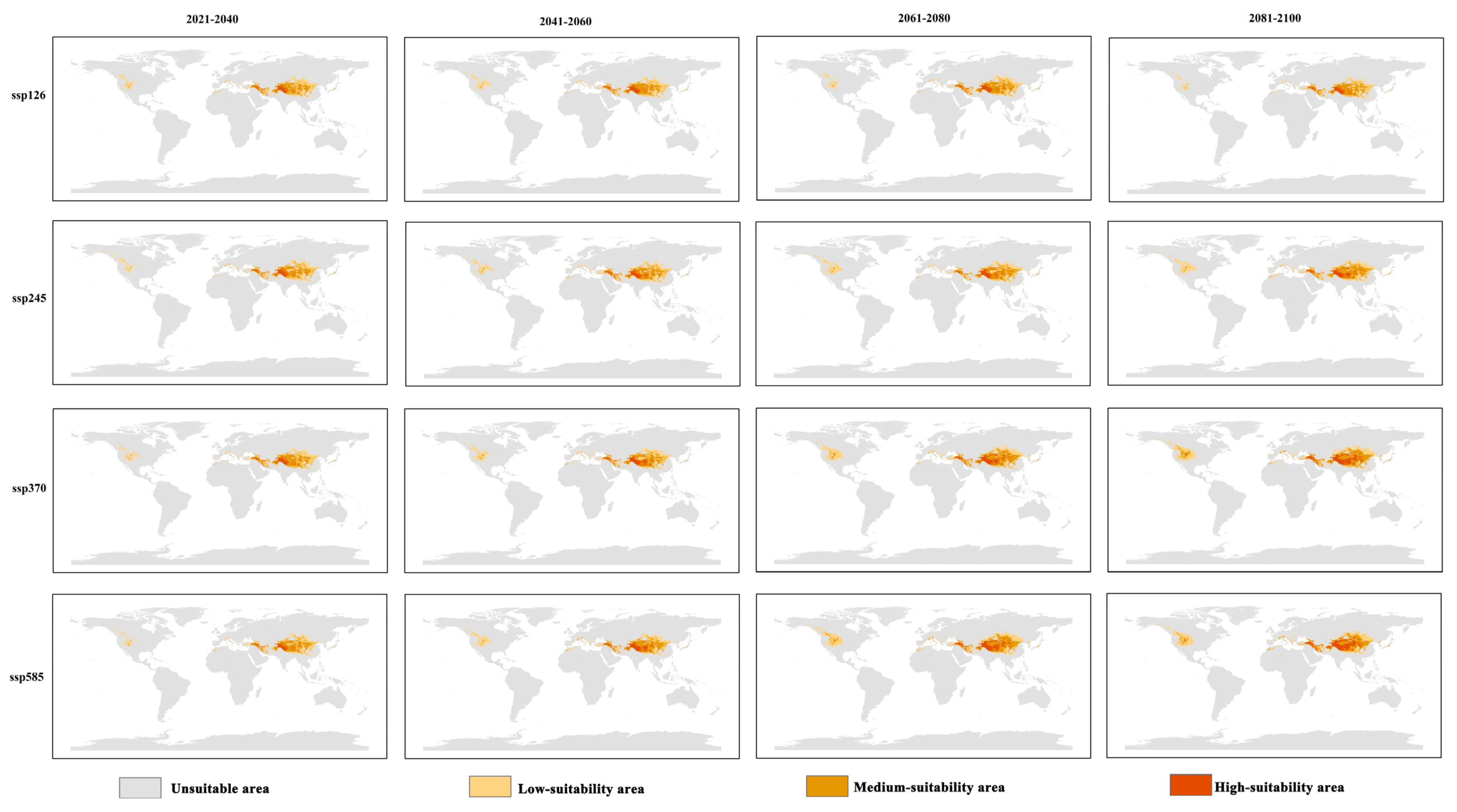
3.3. Global Distribution of Hippophae rhamnoides Under Future Climate Change
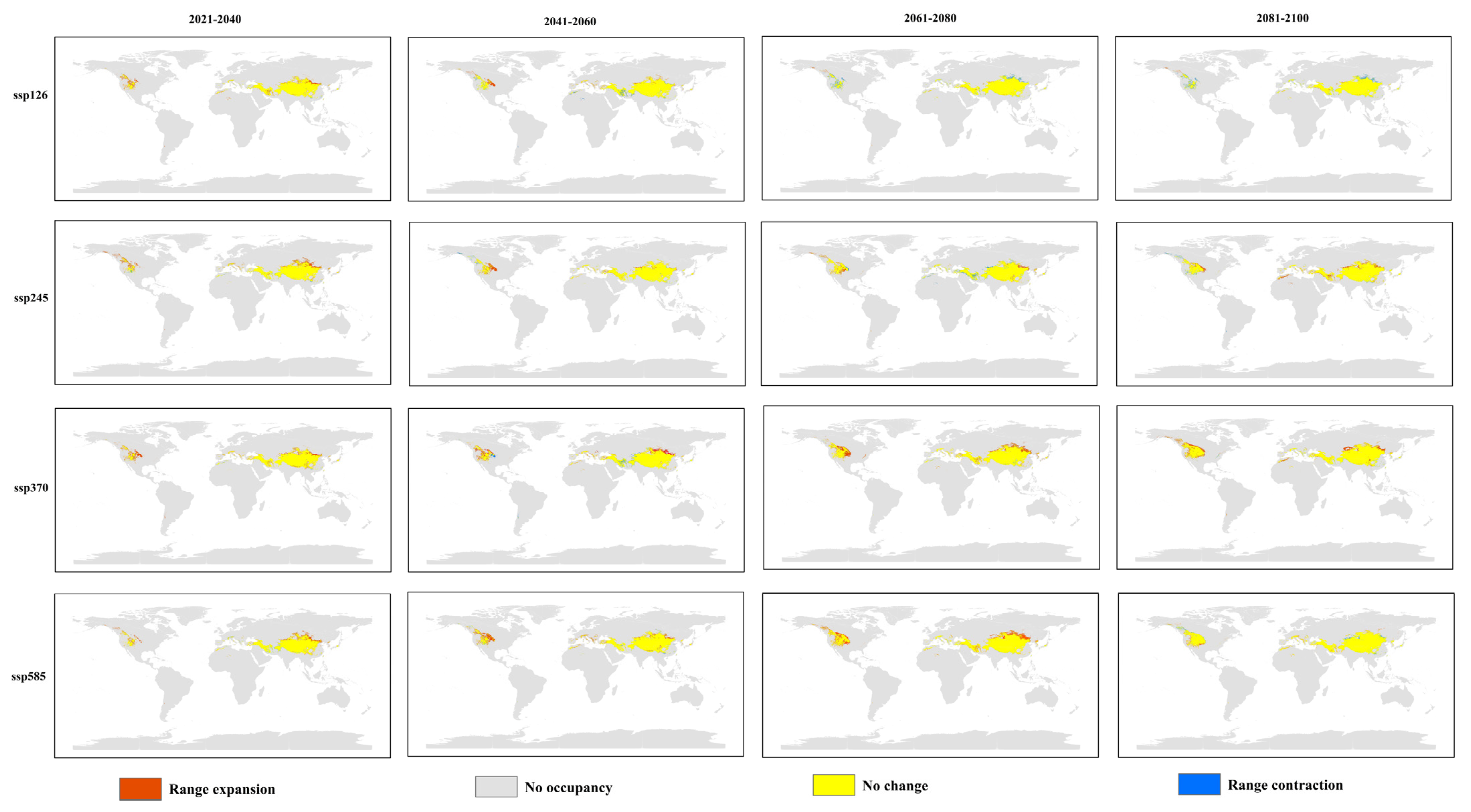
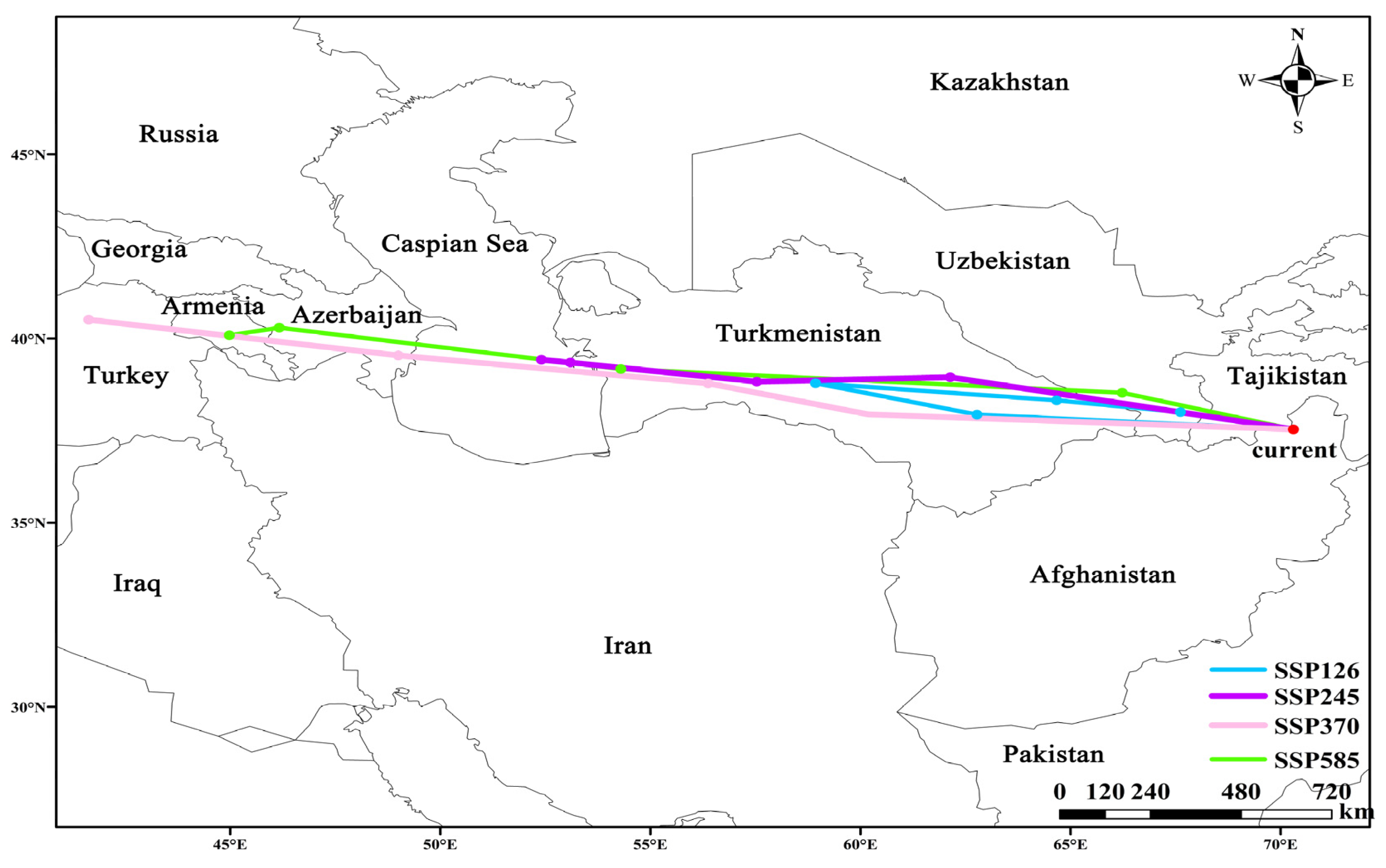
3.4. Centroid Shift of Hippophae rhamnoides Under Future Climate Scenarios
4. Discussion
4.1. Impact of Environmental Variables on the Distribution of H. rhamnoides subsp. turkestanica
4.2. Changes in Suitable Habitats for H. rhamnoides subsp. turkestanica Under Current and Future Climate Change Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Wang, Y.; Xiu, C.; Xiao, X.; Xia, J.; Jin, C. Optimizing local climate zones to mitigate urban heat island effect in human settlements. J. Clean. Prod. 2020, 275, 123767. [Google Scholar] [CrossRef]
- Lv, J.; Wu, J. Advances in the effects of climate change on the distribution of plant species and vegetation in China. Environ. Sci. Technol. 2009, 32, 85–95. [Google Scholar]
- Yuan, J.; Ni, J. Plant signals and ecological evidences of climate change in China. Arid. Land Geogr. 2007, 30, 465–473. [Google Scholar] [CrossRef]
- Ma, Q.-G.; He, N.-X.; Huang, H.-L.; Fu, X.-M.; Zhang, Z.-L.; Shu, J.-C.; Wang, Q.-Y.; Chen, J.; Wu, G.; Zhu, M.-N.; et al. Hippophae rhamnoides L.: A comprehensive review on the botany, traditional uses, phytonutrients, health benefits, quality markers, and applications. J. Agric. Food Chem. 2023, 71, 4769–4788. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Ferreres, F.; Moreno, D.A.; Nowicka, P. UPLC-PDA-Q/TOF-MS profiling of phenolic and carotenoid compounds and their influence on anticholinergic potential for AChE and BuChE inhibition and on-line antioxidant activity of selected Hippophaë rhamnoides L. cultivars. Food Chem. 2020, 309, 125766. [Google Scholar] [CrossRef]
- Baek, S.C.; Lee, D.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Kang, K.S.; Yamabe, N.; Kim, K.H. Inhibitory effect of 1,5-dimethyl citrate from sea buckthorn (Hippophae rhamnoides) on lipopolysaccharide-induced inflammatory response in RAW 264.7 mouse macrophages. Foods 2020, 9, 269. [Google Scholar] [CrossRef]
- Chen, A.; Feng, X.; Dorjsuren, B.; Chimedtseren, C.; Damda, T.-A.; Zhang, C. Traditional food, modern food, and nutritional value of sea buckthorn (Hippophae rhamnoides L.): A review. J. Future Foods 2023, 3, 191–205. [Google Scholar] [CrossRef]
- Su, T.; Zhao, J.; Zhu, Y.; Oyom, W.; Li, S.; Xie, P.; Bi, Y.; Wei, J.; George, G. Comparison of nutrient composition, phytochemicals, and antioxidant activities of two large fruit cultivars of sea buckthorn in Xinjiang of China. Sci. Hortic. 2024, 324, 112602. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Hong, C.; Xie, S.; Zha, X. Extraction, purification, structural characterization, and pharmacological activities of polysaccharides from sea buckthorn (Hippophae rhamnoides L.): A review. J. Ethnopharmacol. 2024, 324, 117809. [Google Scholar] [CrossRef]
- Mei, D.; Ma, X.; Fu, F.; Cao, F. Research status and development prospects of sea buckthorn (Hippophae rhamnoides L.) resources in China. Forests. 2023, 14, 2461. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Tian, X.; Yuan, N. Research progress on comprehensive value of sea-buckthorn. Cereals Oils 2020, 33, 15–16. [Google Scholar]
- Zhang, C.; He, L.; Dong, T.; Deng, D.; Liu, J. Responses of soil water carrying capacity for vegetation in different sandy land types in Zoigê to climate change based on the Biome-BGC model. Chin. J. Ecol. 2024, 43, 1833–1840. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.; Dormann, C.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, B.; Xu, Y.; Han, Z. CMIP6 evaluation and projection of temperature and precipitation over China. Adv. Atmos. Sci. 2021, 38, 817–830. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.; Zellweger, F.; Aalto, J.; Ashcroft, M.; Christiansen, D.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Change Biol. 2021, 27, 2279. [Google Scholar] [CrossRef]
- Ma, L.; Pan, J. Spatial identification and priority conservation areas determination of wilderness in China. J. Clean. Prod. 2024, 451, 142069. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Zhang, D.; Yu, W.; Chen, G.; Xie, T.; Liu, Z.; Ma, Z.; Du, J.; Chao, B.; et al. Mapping the potential of mangrove forest restoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, R.J.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Xiaoqing, X.; Haoxiang, Z.; Rui, W.; Hongkun, H.; Baoxiong, C.; Guifen, Z.; Wanxue, L.; Fanghao, W. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2022, 859, 160252. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.J.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Guang-Zhen, W.; Li, W.; Ling, J.; Juan, C. Evaluation of environmental factors affecting the quality of Codonopsis pilosula based on chromatographic fingerprint and MaxEnt model. Ind. Crop. Prod. 2021, 170, 113783. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, B. Potential geographical distributions of Tugarinovia in China under climate change scenarios. Arid. Zone Res. 2023, 40, 949–957. [Google Scholar] [CrossRef]
- Ma, W.; Li, S. Development of maximum entropy model at home and abroad and its applications in different climatic backgrounds and regional scales. For. Constr. 2023, 2, 32–40. [Google Scholar]
- Saha, A.; Rahman, S.; Alam, S. Modeling current and future potential distributions of desert locust Schistocerca gregaria (Forskål) under climate change scenarios using MaxEnt. J. Asia-Pac. Biodivers. 2021, 14, 399–409. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, X.; Li, M.; Zhang, G.; Huang, L.F. Global climate change promotes aboveground growth and high-latitude species distribution in medicinal plants: Insights from the genus Panax. J. Clean. Prod. 2024, 478, 143863. [Google Scholar] [CrossRef]
- Chen, N.; Yuan, L.; Wu, H.; Xue, J. Climate change’s influence on Chelydra serpentina’s global and Chinese distribution and invasion: A MaxEnt model-based prediction. Glob. Ecol. Conserv. 2024, 54, e03137. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhao, C.; Du, X.; He, P.; Meng, F. Predicting the spatial distribution of three Ephedra species under climate change using the MaxEnt model. Heliyon 2024, 10, e32696. [Google Scholar] [CrossRef]
- Chen, S.; Dong, H.; Yue, Y.; Hao, Y.; Liu, X.; Cao, X.; Ma, J. Geographical distribution and dynamic change prediction of Hippophae rhamnoides subsp. sinensis under different climate scenarios. Arid Zone Res. 2024, 41, 1560–1571. [Google Scholar] [CrossRef]
- Zang, X.; Jiang, Y.; Bi, Y.; Liu, X.; Sun, T.; Chen, H.; Li, J. Identification of potential distribution area for Hippophae rhamnoides subsp. sinensis by the MaxEnt model. Acta Ecol. Sin. 2022, 42, 1420–1428. [Google Scholar]
- Xie, J.; He, X.; Zhu, L.; Hao, R. Potential geographical distribution of Hippophae rhamnoides subsp. yunnanensis in China under the context of climate change. Protect. Forest Sci. Technol. 2023, 1, 24–29. [Google Scholar] [CrossRef]
- Wei, L.B.; Yiwen, Z.; Hui, T.H.; Yeo, D.C.J. Predictor complexity and feature selection affect Maxent model transferability: Evidence from global freshwater invasive species. Divers. Distrib. 2020, 27, 497–511. [Google Scholar] [CrossRef]
- Tebaldi, C.; Debeire, K.; Eyring, V.; Fischer, E.; Fyfe, J.; Friedlingstein, P.; Knutti, R.; Lowe, J.; O’Neill, B.; Sanderson, B.; et al. Climate model projections from the Scenario Model Intercomparison Project (ScenarioMIP) of CMIP6. Earth Syst. Dyn. 2021, 12, 253–293. [Google Scholar] [CrossRef]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z.; et al. BCC-CSM2-HR: A high-resolution version of the Beijing Climate Center Climate System Model. Geosci. Model Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Su, B.; Huang, J.; Mondal, S.K.; Zhai, J.; Wang, Y.; Wen, S.; Gao, M.; Lv, Y.; Jiang, S.; Jiang, T.; et al. Insight from CMIP6 SSP-RCP scenarios for future drought characteristics in China. Atmos. Res. 2020, 250, 105375. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, C.; Wei, X.; Ma, S.; Liu, W.; Song, J.; Peng, R.; Li, J. Potential suitable area forecast of jujube in Xinjiang based on MaxEnt model. Non-Wood Forest Res. 2020, 38, 152–161. [Google Scholar] [CrossRef]
- Rong, W.; Huang, X.; Niu, P.; Liu, J.; Yan, R.; Su, J.; Hu, S.; Chu, G. Potentially suitable areas for traditional Chinese medicinal material Ephedra equisetina based on MaxEnt model. Acta Ecol. Sin. 2023, 43, 8631–8646. [Google Scholar] [CrossRef]
- Cha, S.; Qi, B.; Hu, H.; Latancunbuer, A.; Yong, X.; Ao, W.; Bao, J.; Cao, W. Prediction of the potential distribution area of the endangered medicinal plant Gymnadenia conopsea in China under the background of climate change. Chin. J. Appl. Ecol. 2024, 35, 69–73. [Google Scholar] [CrossRef]
- Liu, G.; Lu, H. Research progress of climatic change in Western China on the background of global warming. Meteorol. Environ. Sci. 2009, 32, 69–73. [Google Scholar] [CrossRef]
- Liu, R. Effects of photosynthetic and transpiration rate on Hippophae rhamnoides sinensis and Hippophae rhamnoides in different soil water conditions. Bull. Soil Water Conserv. 2006, 1, 1–5+15. [Google Scholar]
- Du, S.; Bai, G.; Li, D. Photosynthetic characteristics of Chinese sea buckthorn, Russian sea buckthorn, Russian sea buckthorn × Chinese sea buckthorn and their influence factors. Bull. Soil Water Conserv. 2008, 4, 26–32. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, C.; Xie, Y.; Jiang, X. Nature reserves and reforestation expand the potential habitats for endangered plants: A model study in Cangshan, China. J. Nat. Conserv. 2024, 77, 126533. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Xu, J.; Yu, S.; Huang, Y.; Zhang, Y.; He, X.; Chen, W. Prediction of suitable planting areas of Rubia cordifolia in China based on a species distribution model and analysis of specific secondary metabolites. Ind. Crop. Prod. 2023, 206, 117651. [Google Scholar] [CrossRef]
- Chen, X.-L.; Ma, R.-J.; Sun, K.; Lian, Y.-S. Germplasm resource and habitat types of sea buckthorn in China. Acta Bot. Boreali-Occident. Sin. 2003, 3, 451–455. [Google Scholar]
- Jin, T.; Fu, B.; Liu, G.; Hu, C.; Su, C.; Liu, Y. Diurnal changes of photosynthetic characteristics of Hippophae rhamnoides and the relevant environmental factors at different slope locations. Acta Ecol. Sin. 2011, 31, 1783–1793. [Google Scholar]
- Majid, A. Critical findings of the sixth assessment report (AR6) of Working Group I of the Intergovernmental Panel on Climate Change (IPCC) for global climate change policymaking: A summary for policymakers (SPM) analysis. Int. J. Clim. Change Strat. Manag. 2023, 15, 652–670. [Google Scholar] [CrossRef]
- Xiang, H.; Guangming, C.; Jun, W.; Honghai, L.; Zhen’an, Y.; Lupeng, S.; Wenwen, R.; Mei, W. Integrated metabolomic and transcriptomic analysis of specialized metabolites and isoflavonoid biosynthesis in Sophora alopecuroides L. under different degrees of drought stress. Ind. Crop. Prod. 2023, 197, 116595. [Google Scholar] [CrossRef]
- Tan, J.; Huang, A.; Shi, X.; Zhang, N.; Zhang, Y.; Cao, L.; Wu, Y. Evaluating the performance of the BCC-CSM2-MR model in simulating land surface processes in China. Plateau Meteorol. 2022, 41, 1335–1347. [Google Scholar] [CrossRef]


| Period | SSP126 (Training Data) | SSP245 (Training Data) | SSP370 (Training Data) | SSP585 (Training Data) |
|---|---|---|---|---|
| 2021–2040 | 0.986 | 0.986 | 0.985 | 0.983 |
| 2041–2060 | 0.981 | 0.984 | 0.982 | 0.984 |
| 2061–2080 | 0.982 | 0.979 | 0.983 | 0.979 |
| 2081–2100 | 0.983 | 0.981 | 0.982 | 0.981 |
| Variable | Percent Contribution (%) | Permutation Importance |
|---|---|---|
| Slo | 35.2 | 0.8 |
| Alt | 20.6 | 21.6 |
| bio04 | 10.7 | 14.5 |
| bio05 | 10.3 | 1.3 |
| bio03 | 10.2 | 8.1 |
| s_bs | 6.5 | 7.4 |
| bio10 | 2.4 | 0.3 |
| bio07 | 2.4 | 2.5 |
| bio01 | 1.6 | 43.6 |
| Suitability Class | Predicted Value | Distribution Area/104 km2 |
|---|---|---|
| High-suitability area | 0.6~1 | 65.55 |
| Moderate-suitability area | 0.3~0.6 | 293.60 |
| Low-suitability area | 0.1~0.3 | 578.87 |
| Scenario | Period | Low-Suitability | Moderate-Suitability | High-Suitability | Total Suitable |
|---|---|---|---|---|---|
| SSP126 | 2021–2040 | 578.18 | 374.50 | 96.10 | 1048.78 |
| 2041–2060 | 599.00 | 375.94 | 112.64 | 1087.58 | |
| 2061–2080 | 577.03 | 411.29 | 121.30 | 1109.61 | |
| 2081–2100 | 556.51 | 386.98 | 109.18 | 1052.68 | |
| SSP245 | 2021–2040 | 598.37 | 370.46 | 106.59 | 1075.42 |
| 2041–2060 | 628.07 | 390.00 | 126.68 | 1144.75 | |
| 2061–2080 | 631.13 | 423.74 | 128.70 | 1183.57 | |
| 2081–2100 | 686.20 | 453.80 | 153.02 | 1293.02 | |
| SSP370 | 2021–2040 | 582.96 | 398.57 | 109.26 | 1090.79 |
| 2041–2060 | 637.59 | 375.89 | 151.73 | 1165.21 | |
| 2061–2080 | 684.66 | 479.26 | 206.93 | 1370.85 | |
| 2081–2100 | 742.44 | 523.84 | 278.70 | 1544.99 | |
| SSP585 | 2021–2040 | 556.38 | 392.00 | 90.99 | 1039.38 |
| 2041–2060 | 605.13 | 413.56 | 165.48 | 1184.17 | |
| 2061–2080 | 702.70 | 490.60 | 235.03 | 1428.34 | |
| 2081–2100 | 687.47 | 458.45 | 304.56 | 1450.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; He, M.; Wang, M.; Chu, G.; Yang, Z.; Luo, C.; Zhou, M.; Hui, Y.; Ding, J. Assessing Habitat Suitability for Hippophae rhamnoides subsp. turkestanica Amid Climate Change Using the MaxEnt Model. Forests 2025, 16, 468. https://doi.org/10.3390/f16030468
Ma F, He M, Wang M, Chu G, Yang Z, Luo C, Zhou M, Hui Y, Ding J. Assessing Habitat Suitability for Hippophae rhamnoides subsp. turkestanica Amid Climate Change Using the MaxEnt Model. Forests. 2025; 16(3):468. https://doi.org/10.3390/f16030468
Chicago/Turabian StyleMa, Fanyan, Mengyao He, Mei Wang, Guangming Chu, Zhen’an Yang, Cunkai Luo, Mingwang Zhou, Ying Hui, and Junjie Ding. 2025. "Assessing Habitat Suitability for Hippophae rhamnoides subsp. turkestanica Amid Climate Change Using the MaxEnt Model" Forests 16, no. 3: 468. https://doi.org/10.3390/f16030468
APA StyleMa, F., He, M., Wang, M., Chu, G., Yang, Z., Luo, C., Zhou, M., Hui, Y., & Ding, J. (2025). Assessing Habitat Suitability for Hippophae rhamnoides subsp. turkestanica Amid Climate Change Using the MaxEnt Model. Forests, 16(3), 468. https://doi.org/10.3390/f16030468






