The Impact of Microhabitat and Microtopography on the Photosynthetic Characteristics of Typical Karst Forest Plants in Guizhou, China
Abstract
:1. Introduction
2. Materials and Methods
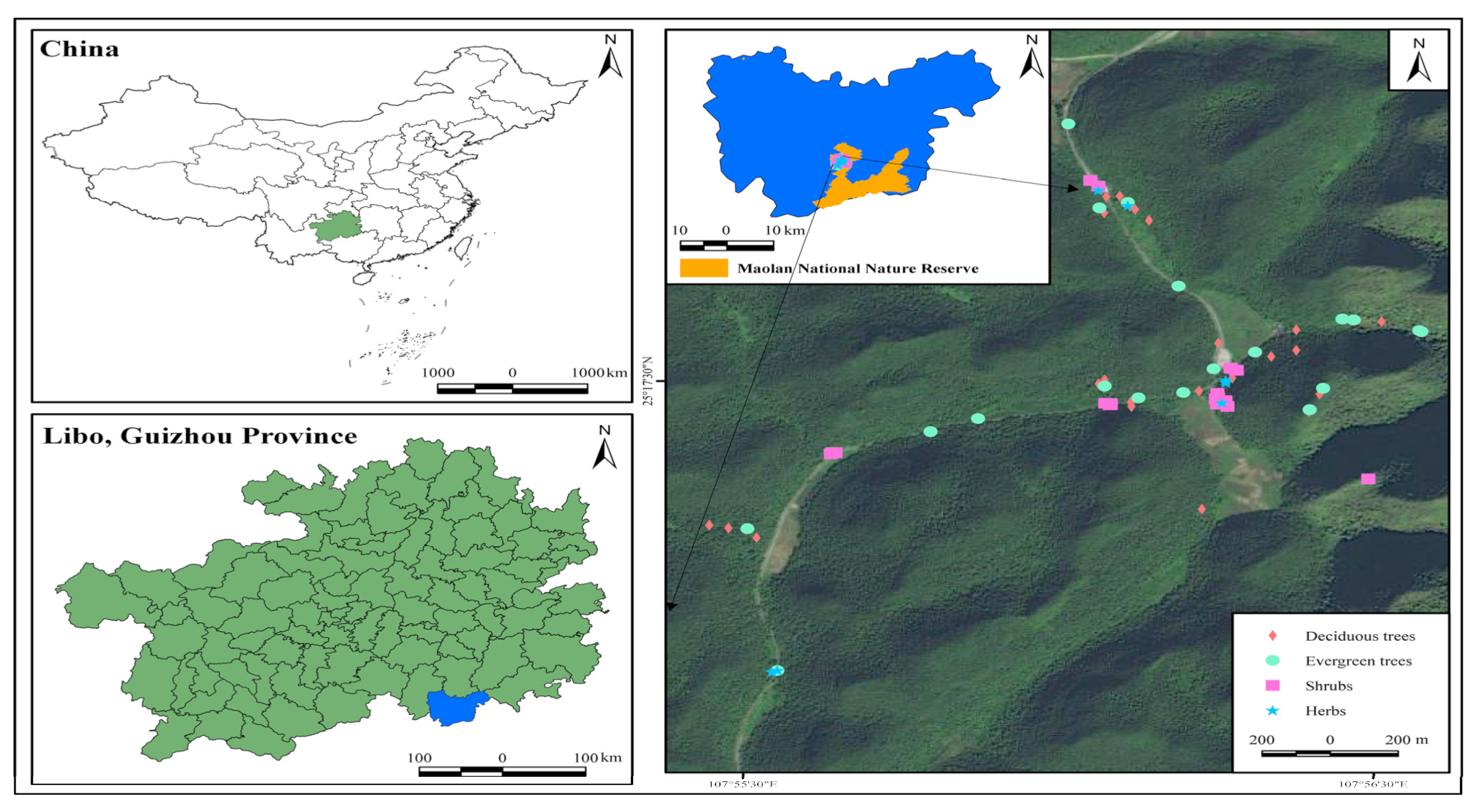
2.1. Study Region
2.2. Experimental Design
2.2.1. Microhabitat Type Classification
- Stone surface: The exposed rate of bedrock is more than 50%. The groove depth is less than 30 cm when irregular bare rocks such as shallow small gullies, shallow stone pits, small grooves, and narrow stone crevices are formed. These are usually soil-less or are only covered by less than 1 m2 of soil; soil thickness is less than 20 cm in stone surfaces. Although the ventilation conditions are favorable, the rapid water loss, weak water and fertilizer retention capacity, and susceptibility to temporary drought create a harsh environment. The soil water holdup is 45.06% [5]. The soil pH is 7.15, the contents of K, N, C, P of soil were 13.15 g·kg−1, 14.55 g·kg−1, 171.19 g·kg−1, and 1.15 g·kg−1, respectively.
- Stone gully: Under conditions in which the bare rock rate surpasses 50%, the groove depth is generally greater than 30 cm, with the soil coverage region being confined to below 1 m2. The soil thickness exceeds 20 cm when the groove depth is below 30 cm, or the soil layer is thicker than 20 cm when the groove depth is under 30 cm. Alternatively, the area is predominantly soil-covered with a groove depth exceeding 30 cm, provided the bare rock rate is below 50%. The soil layer is thicker, with improved water and fertilizer retention capabilities, making it less prone to short-term drought, subsequently resulting in relatively favorable conditions. The soil water holdup is 54.97% [5]. The soil pH is 7.04, and the contents of K, N, C, P of soil are 14.62 g·kg−1, 11.59 g·kg−1, 122.70 g·kg−1, and 0.97g·kg−1, respectively.
- Soil surface: The contiguous soil coverage area exceeds 1 m2. For areas smaller than 1 m2, the bare rock rate is less than 50%, and the groove depth is under 30 cm. The soil layer is thicker, with enhanced water and fertilizer retention capacity, better ventilation conditions, and slower water loss, making the soil conditions relatively optimal, though short-term drought remains a possibility. The soil water holdup is 49.11% [5]. The soil pH is 6.56, and the contents of K, N, C, P of soil are 15.61g·kg−1, 7.34 g·kg−1, 79.79 g·kg−1, and 0.85 g·kg−1, respectively.
2.2.2. Microtopography Classification
2.2.3. Sampling Methods
2.2.4. Measurement of Plant Photosynthetic Parameters
2.2.5. Calculation Method for Photosynthetic Parameters [25]
2.2.6. Data Processing and Analysis
3. Results
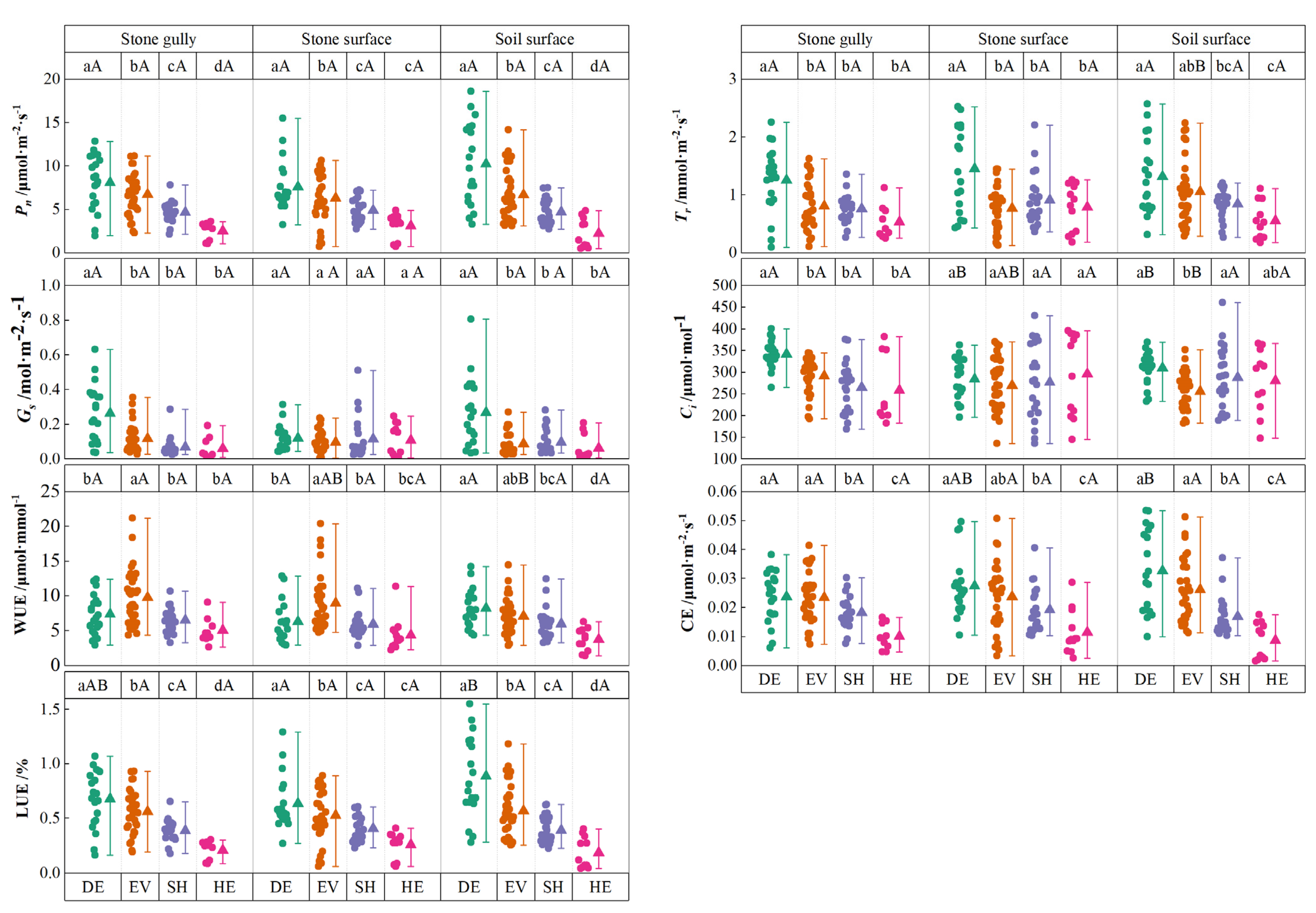
3.1. Plant Photosynthetic Characteristics in Various Microhabitats
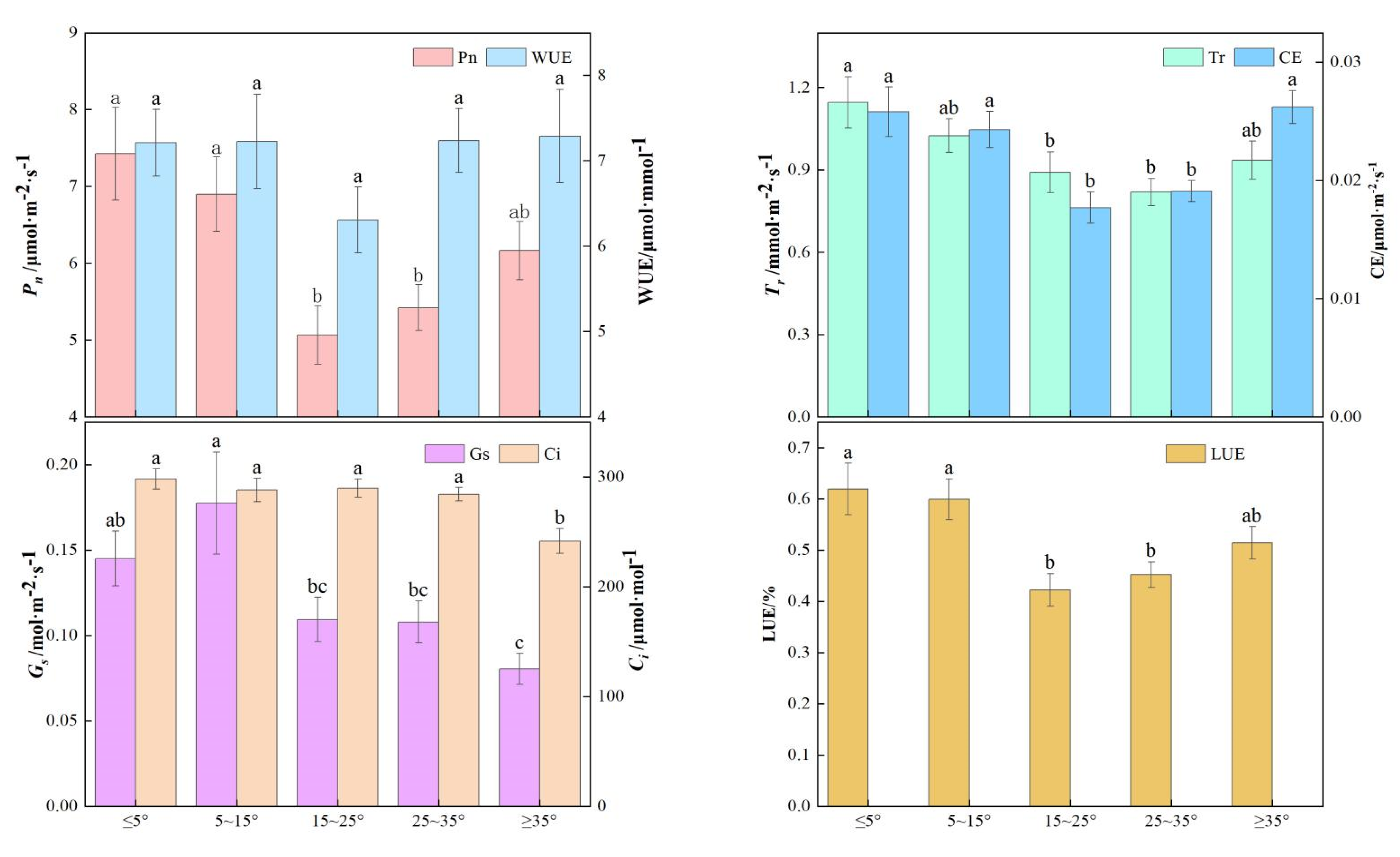
3.2. Plant Photosynthetic Characteristics in Various Microtopographies
3.2.1. Plant Photosynthetic Characteristics in Various Slope Aspects
3.2.2. Plant Photosynthetic Characteristics in Various Slope Positions
3.2.3. Plant Photosynthetic Characteristics in Various Slope Degrees
3.3. Relationship Between Plant Photosynthetic Characteristics and Microhabitat
3.4. Relationship Between Plant Photosynthetic Characteristics and Microtopographies
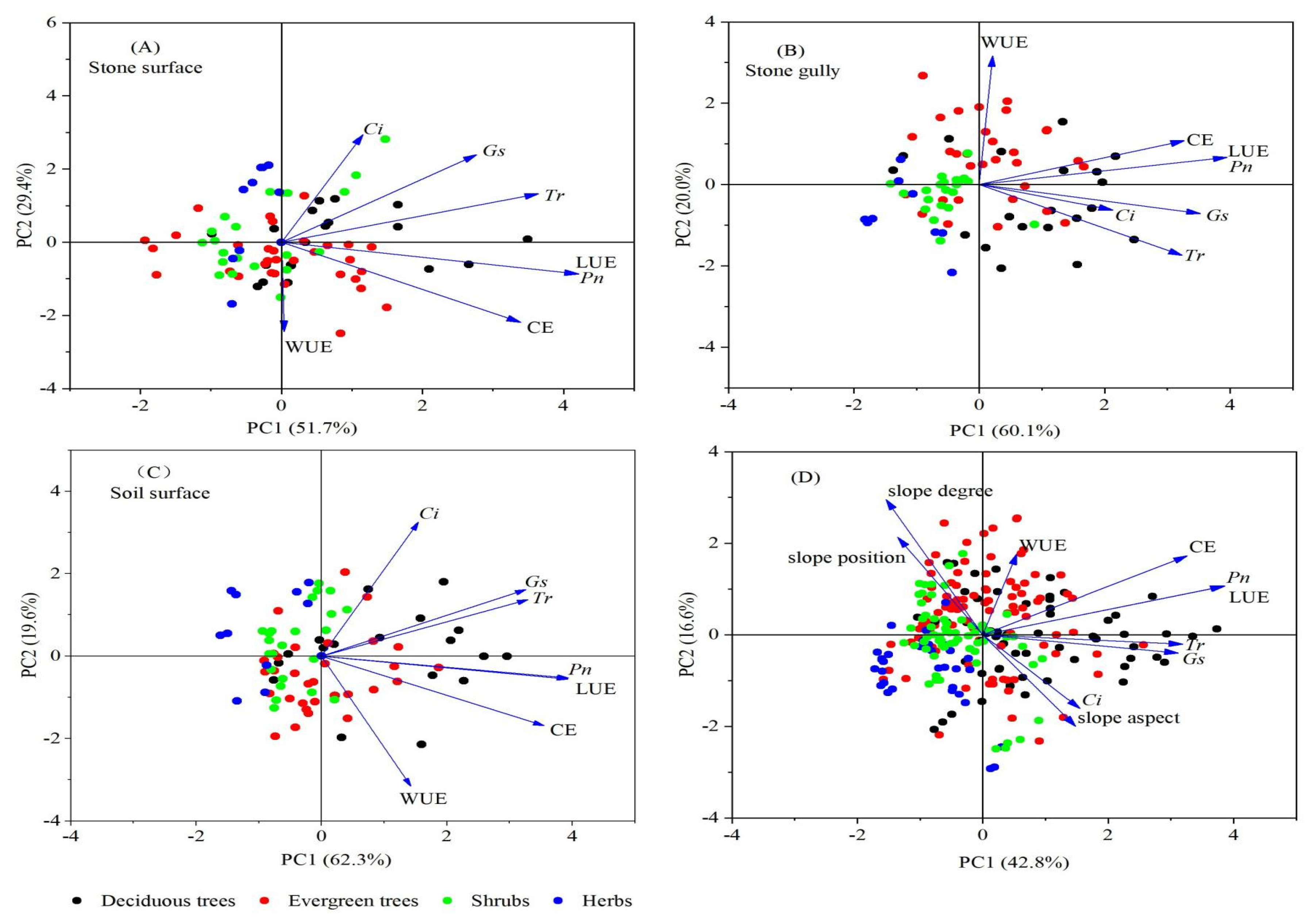
3.5. PCA of Plant Photosynthetic Characteristics
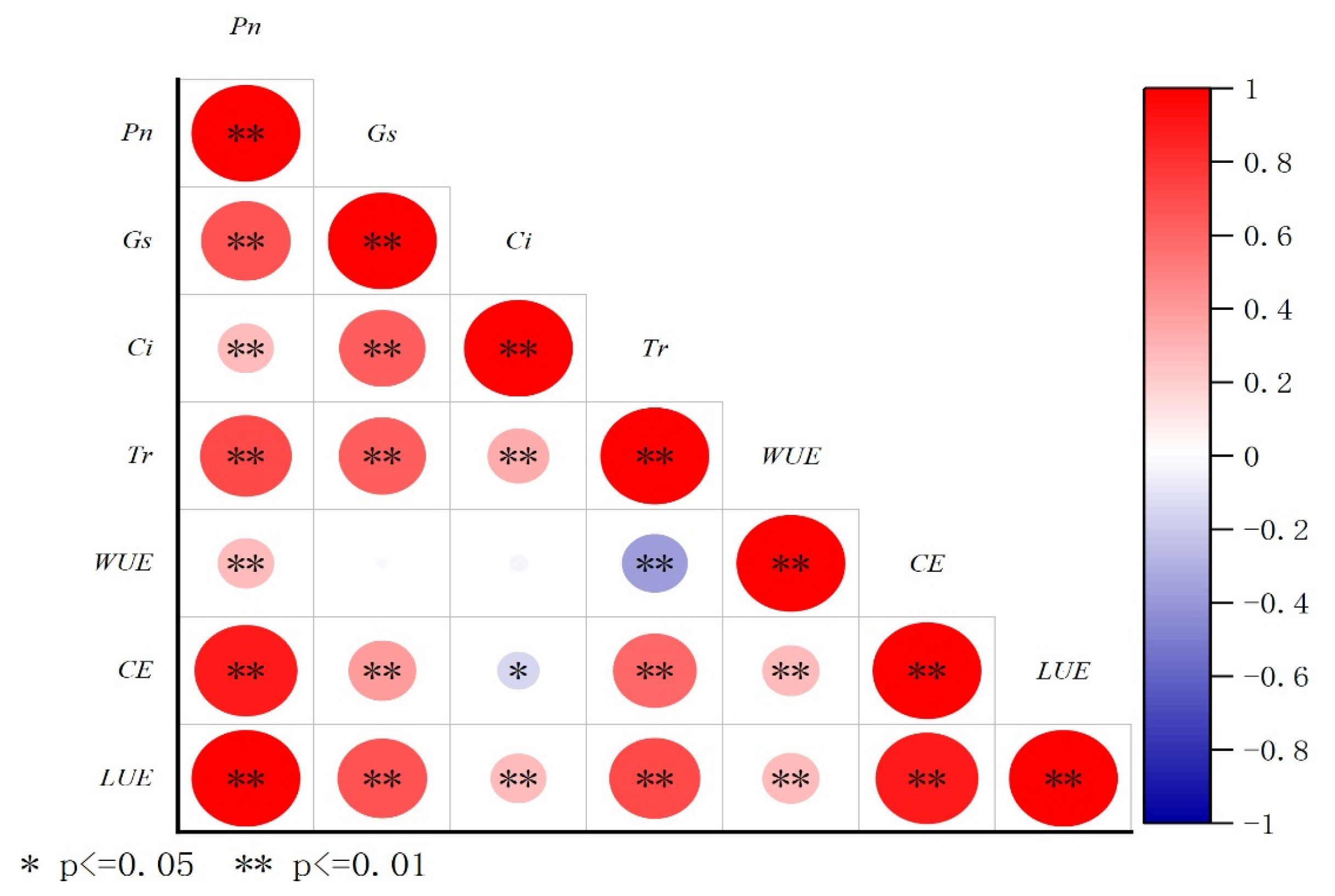
3.6. The Correlation of Plant Photosynthetic Characteristics
4. Discussion
4.1. Response of Plant Photosynthetic Characteristics to Microhabitat
4.2. Response of Plant Physiological Characteristics to Microtopography
4.3. Strategies of Plants for Adapting to Microhabitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Wang, M.; Kuang, S.; Wang, W. Study on Geoscience Model of Karst Rocky Desertification Control; Geological Publishing House: Beijing, China, 2010. [Google Scholar]
- Long, J.; Wu, Q.; Li, J.; Liao, H.; Liu, L.; Huang, B.; Zhang, J. Effects of different microhabitat types on rock dissolution in Maolan karst forest, Southwest China. Acta Pedol. Sin. 2021, 58, 151–161. [Google Scholar]
- Chen, F.; Liu, F.; Bai, X.; Wu, L.; Chen, Z.; Wang, J. Spatial heterogeneity and ecological stoichiometry characteristics of soil carbon, nitrogen and phosphorus under different micro-geomorphology in karst mountains. Acta Ecol. Sin. 2022, 42, 10201–10213. [Google Scholar]
- An, M.T. Studies on Maintenance Mechanism of Plant Species Diversity and Soil Moisture and Nutrient Pattern in Karst Forest. Ph.D. Thesis, Guizhou University, Guiyang, China, 2019. [Google Scholar]
- Wu, P.; Zhou, H.; Cui, Y.; Zhao, W.; Hou, Y.; Tan, C.; Yang, G.; Ding, F. Stoichiometric characteristics of leaf, litter and soil during vegetation succession in Maolan national nature reserve, Guizhou, China. Sustainability 2022, 14, 16517. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. Cell Mol. Biol. 2020, 101, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wu, J.; Chen, J.; Shi, Z. Research progress on limiting factors of plant photosynthesis and vegetation productivity. Acta Ecol. Sin. 2023, 43, 5186–5199. [Google Scholar]
- Yu, G.; Wang, S.; Rong, L. Microclimate characteristics of different microhabitats in successional stages of Maolan karst forest. Earth Environ. 2011, 39, 469–477. [Google Scholar]
- Jin, T.; Fu, B.; Liu, G.; Hu, C.; Su, C.; Liu, Y. Diurnal changes of photosynthetic characteristics of Hippophae rhamnoides and the relevant environment factors at different slope locations. Acta Ecol. Sin. 2011, 31, 1783–1793. [Google Scholar]
- Pu, Y.; Liu, J.; Yang, Y. Study on the effect of different site conditions on the growth of Taxus chinensis seedlings. Hortic. Seed 2024, 44, 4–6. [Google Scholar]
- Zhu, S.; Xu, W.; Liu, G.; Zhao, J.; Chen, Y. Effect of slope gradient on physiological characteristic of Medicago sativa and Cynodon dactylon. Res. Soil Water Conserv. 2012, 19, 218–221. [Google Scholar]
- Zhou, T.; Cui, Y.; Ye, Y.; Zhao, W.; Hou, Y.; Wu, P.; Ding, F. Leaf functional traits of typical karst forest plants under different niches. J. Cent. South Univ. For. Technol. 2022, 42, 129–140. [Google Scholar]
- Zhu, S.Q. Study on Karst Forest Ecosystem III; Guizhou Science and Technology Press: Guiyang, China, 2014; p. 72. [Google Scholar]
- Liao, W.; Wei, Y.; Huang, S.; Wei, Z.; Hu, X. Association analysis of the spatial and temporal changes in vegetation photosynthesis levels with land cover changes in China based on solar-induced fluorescence. Sustainability 2024, 16, 5107. [Google Scholar] [CrossRef]
- Coşkun, Ö.F.; Toprak, S.; Mavi, K. Some seed properties and molecular analysis with inter-primary binding site (iPBS) etrotranposons markers of edible-seeded watermelon genotypes. Genet. Resour. Crop Ev. 2024, 71, 3151–3162. [Google Scholar] [CrossRef]
- Coskun, O.F.; Gulsen, O. Determination of markers associated with important agronomic traits of Watermelon (Citrullus lanatus L.). J. Agric. Sci. Tech. 2024, 26, 1359–1371. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Li, S.; Yang, Q.; Cheng, X. Growth and photosynthetic characteristics of Bombax ceiba seedlings in different habitats. J. Northeast. For. Univ. 2023, 51, 6–10. [Google Scholar]
- Jiang, X.; Su, C.; Wu, S.; Zhang, X. Photosynthetic and physiological characteristics of Cladrastis platycarpa (Maxim.) Makino in spring in dolomite rocky hills of karst region. Guangdong Agric. Sci. 2017, 44, 41–47. [Google Scholar]
- Liu, H.; Yu, Y.; Xiong, K.; Zhang, S.; Yang, S. Response characteristics of photosynthesis to light intensity of three non-wood forests tree species in karst habitat. J. South. Agric. 2021, 52, 2507–2515. [Google Scholar]
- Xing, D.; Wu, Y.; Fu, W.; Hang, H.; Zhao, Y.; Wu, Y. Photosynthetic physiological response of three plant species to different slope environments in karst forest of Guizhou, China. Guihaia 2016, 36, 1147–1155. [Google Scholar]
- Zhang, Z.; Hu, B.; Hu, G. Spatial heterogeneity of soil chemical properties in a subtropical karst forest, Southwest China. Sci. World J. 2014, 2014, 473651. [Google Scholar] [CrossRef]
- Wen, P.; Jin, G. Effects of topography on species diversity in a typical mixed broadleaved-Korean pine forest. Acta Ecol. Sin. 2019, 39, 945–956. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C. A feasible method for measuring photosynthesis in vitro for major tree species in northeastern China. Chin. J. Plant Ecol. 2011, 35, 452–462. [Google Scholar] [CrossRef]
- Huang, B.; Zhu, J.; He, C.; Li, W.; Chen, Z.; Fan, Y.; Hong, T. Effects of tides on leaf phenotypic modeling and leaf economics spectrum of Kandelia obovata. Sci. Silvae Sin. 2021, 57, 63–72. [Google Scholar]
- Liu, W.; Li, D.; Ji, Q.; Chen, H.; Lai, S.; Geng, S.; Yun, X.; Chen, Q. Leaf nitrogen allocation of evergreen and deciduous broad-leaved tree species and their relationships with photosynthetic capacity in the two babitats. Ecol. Sci. 2015, 34, 1–8. [Google Scholar]
- Soudani, K.; Hmimina, G.; Dufrêne, E.; Berveiller, D.; Delpierre, N.; Ourcival, J.M.; Rambal, S.; Joffre, R. Relationships between photochemical reflectance index and light-use efficiency in deciduous and evergreen broadleaf forests. Remote Sens. Environ. 2014, 144, 73–84. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, G.; Yang, P.; Zhao, J. Photosynthetic characteristics and ecological adaptability of different landscape tree species in Karst mountainous area. Guizhou Agric. Sci. 2013, 41, 65–68. [Google Scholar]
- Ma, F.; Li, M.; Rong, Y.; Jiang, J.; Dong, S.; Jun, T. A Study of effect of water deficit of three period during cotton anthesis on canopy apparent photosynthesis and WUE. Sci. Agric. Sin. 2002, 35, 1467–1472. [Google Scholar]
- Wang, Y.; Wu, S. Effects of different cultivation methods on the photosynthetic characteristics and water-utilizing efficiency of Populus euphratica. J. Gansu Agric. Univ. 2023, 58, 156–164. [Google Scholar]
- Zhang, S.; Xiong, K.; Zhang, Y.; Ji, C.; Ma, X.; Lai, J.; Min, X. Leaf functional traits characteristics of dominant species and influencing factors in succession of the typical rocky desertification ecosystems. Ecol. Environ. Sci. 2019, 28, 2165–2175. [Google Scholar]
- Yuan, C.; Wang, H.; Dai, X.; Chen, M.; Luo, J.; Yang, R.; Ding, F. Effect of karst microhabitats on the structure and function of the rhizosphere soil microbial community of Rhododendron pudingense. Sustainability 2023, 15, 7104. [Google Scholar] [CrossRef]
- Yan, L.B. Response Mechanism of Soil Microbial Community and Plant Functional Traits to Ecological Factors of Water and Heat in Karst Micro-Habitats. Ph.D. Thesis, Guizhou University, Guiyang, China, 2020. [Google Scholar]
- Qiao, S.; Ma, C.; Li, H.; Zhang, Y.; Zhang, M.; Zhao, W.; Liu, B. Responses of growth and photosynthesis to alkaline stress in three willow species. Sci. Rep. 2024, 14, 14672. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Xu, D. Thinking about the relationship between net photosynthetic rate and intercellular CO2 concentration. Plant Physiol. Commun. 2010, 46, 64–66. [Google Scholar]
- Falcioni, R.; Moriwaki, T.; Rodrigues, M.; de Oliveira, K.M.; Furlanetto, R.H.; Dos Reis, A.S.; Dos Santos, G.L.A.A.; Mendonça, W.A.; Crusiol, L.G.T.; Gonçalves, J.V.F.; et al. Nutrient deficiency lowers photochemical and carboxylation efficiency in tobacco. Theor. Exp. Plant Physiol. 2023, 35, 81–97. [Google Scholar] [CrossRef]
- Zhao, S.; Han, L.; Zhang, Y.; Zhang, C.; Xiao, K. Study on photosynthetic characteristics and its influencing factors of Lespedeza bicolor Turcz. under different slope situation. Acta Bot. Boreal. Occident. Sin. 2008, 28, 342–347. [Google Scholar]
- Zhang, Z.; Cai, Y.; Zhao, L.; Zu, Y.; Lin, S.; He, K. Effects of stand structure and environmental factors on understory herbaceous plant diversity in eastern Oilian Mountains. Acta Ecol. Sin. 2024, 44, 2089–2099. [Google Scholar]
- Choudhury, B.U.; Fiyaz, A.R.; Mohapatra, K.P.; Ngachan, S. Impact of land uses, agrophysical variables and altitudinal gradient on soil organic carbon concentration of North-eastern Himalayan region of India. Land Degrad. Dev. 2016, 27, 1163–1174. [Google Scholar] [CrossRef]
- Carletti, P.; Vendramin, E.; Pizzeghello, D.; Concheri, G.; Zanella, A.; Nardi, S.; Squartini, A. Soil humic compounds and microbial communities in six spruce forests as function of parent material, slope aspect and stand age. Plant Soil 2009, 315, 47–65. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Ma, J.; Mo, Y. The leaf photosynthetic characteristics of 50 common plants on karst rocky mountain in Guilin. Acta Ecol. Sin. 2020, 40, 8649–8659. [Google Scholar]
- Pang, S.; Ou, Z.; Hui, S.; He, F.; Lu, G. Leaf function trait variation and adaptive strategies of dominant woody economic plants in karst area of Southwest Guangxi. Guihaia 2021, 41, 707–714. [Google Scholar]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]
- Bai, K.; Jiang, D.; Wan, X. Photosynthesis-nitrogen relationship in evergreen and deciduous tree species at different altitudes on Mao’er Mountain, Guangxi. Acta Ecol. Sin. 2013, 16, 4930–4938. [Google Scholar]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]


| Microtopography | Division Basis |
|---|---|
| Slope positions | upslope, midslope, downslope, and depression |
| Slope degrees | slope ≤ 5°, slope 5°~15°, slope 15°~25°, slope 25°~35°, slope ≥ 35° |
| Slope aspects | shady slope (337.5°~22.5°, 22.5°~67.5°) |
| semi-shady slope (67.5°~112.5°, 292.5°~337.5°) | |
| flat land (slope degrees ≤ 5°) | |
| semi-sunny slope (112.5°~157.5°, 247.5°~292.5°) | |
| sunny slope (157.5°~247.5°) |
| Types | Pn (μmol·m−2·s−1) | WUE (μmol·mmol−1) | Ci (μmol·mol−1) | Tr (mmol·m−2·s−1) | Gs (mol·m−2·s−1) | CE (μmol·m−2·s−1) | LUE (%) |
|---|---|---|---|---|---|---|---|
| Stone gully | 6.11 ± 0.31 a | 7.66 ± 0.35 a | 294.07 ± 6.36 a | 0.89 ± 0.05 a | 0.136 ± 0.014 a | 0.021 ± 0.001 a | 0.51 ± 0.03 a |
| Stone surface | 5.73 ± 0.30 a | 6.14 ± 0.35 b | 277.60 ± 7.69 a | 1.02 ± 0.06 a | 0.106 ± 0.009 a | 0.022 ± 0.001 a | 0.48 ± 0.02 a |
| Soil surface | 6.36 ± 0.43 a | 6.62 ± 0.30 b | 279.95 ± 6.34 a | 0.99 ± 0.06 a | 0.127 ± 0.015 a | 0.023 ± 0.001 a | 0.54 ± 0.04 a |
| F | 0.821 | 6.628 | 1.674 | 1.511 | 1.402 | 0.732 | 1.158 |
| p | 0.441 | 0.002 | 0.190 | 0.223 | 0.248 | 0.482 | 0.316 |
| Component | Eigenvector | Eigenvalue | Cumulative/ % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x1 (Pn) | x2 (Gs) | x3 (Ci) | x4 (Tr) | x5 (WUE) | x6 (CE) | x7 (LUE) | |||
| y1 | 0.49 | 0.39 | 0.19 | 0.40 | 0.07 | 0.41 | 0.49 | 4.03 | 57.59% |
| y2 | 0.15 | −0.33 | −0.54 | −0.28 | 0.57 | 0.38 | 0.15 | 1.54 | 22.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zhou, H.; Zhao, W.; Cui, Y.; Hou, Y.; Zhou, T.; Hu, F.; Wu, P. The Impact of Microhabitat and Microtopography on the Photosynthetic Characteristics of Typical Karst Forest Plants in Guizhou, China. Forests 2025, 16, 532. https://doi.org/10.3390/f16030532
Jiang X, Zhou H, Zhao W, Cui Y, Hou Y, Zhou T, Hu F, Wu P. The Impact of Microhabitat and Microtopography on the Photosynthetic Characteristics of Typical Karst Forest Plants in Guizhou, China. Forests. 2025; 16(3):532. https://doi.org/10.3390/f16030532
Chicago/Turabian StyleJiang, Xia, Hua Zhou, Wenjun Zhao, Yingchun Cui, Yiju Hou, Ting Zhou, Fangcai Hu, and Peng Wu. 2025. "The Impact of Microhabitat and Microtopography on the Photosynthetic Characteristics of Typical Karst Forest Plants in Guizhou, China" Forests 16, no. 3: 532. https://doi.org/10.3390/f16030532
APA StyleJiang, X., Zhou, H., Zhao, W., Cui, Y., Hou, Y., Zhou, T., Hu, F., & Wu, P. (2025). The Impact of Microhabitat and Microtopography on the Photosynthetic Characteristics of Typical Karst Forest Plants in Guizhou, China. Forests, 16(3), 532. https://doi.org/10.3390/f16030532





