Solid Microbial Fertilizers Prepared with Different Carriers Have the Potential to Enhance Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carriers and Strains
2.2. Laboratory Preparation of Immobilized Microbial Inoculant and Assessment of Shelf Life
2.3. Design of Experiments and Sample Collection
2.4. Assessment of Soil Nutrients and Enzymatic Activities
2.5. The Multifunctionality of Soil and the Roles of Carbon, Nitrogen, Phosphorus, and Sulfur Cycles
2.6. Statistical Analysis
3. Results
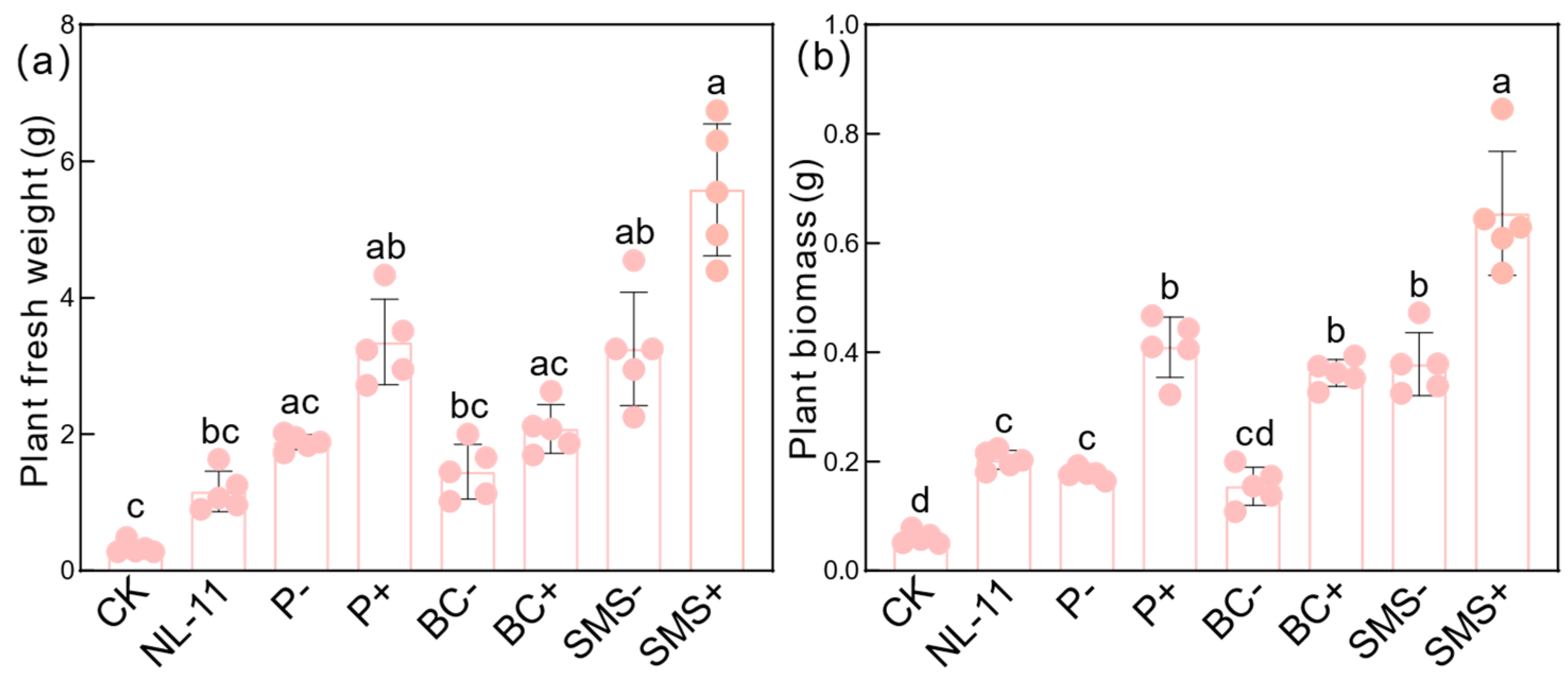
3.1. Responses of Alfalfa Biomass to Different Carriers Mediated by Microbial Inoculants
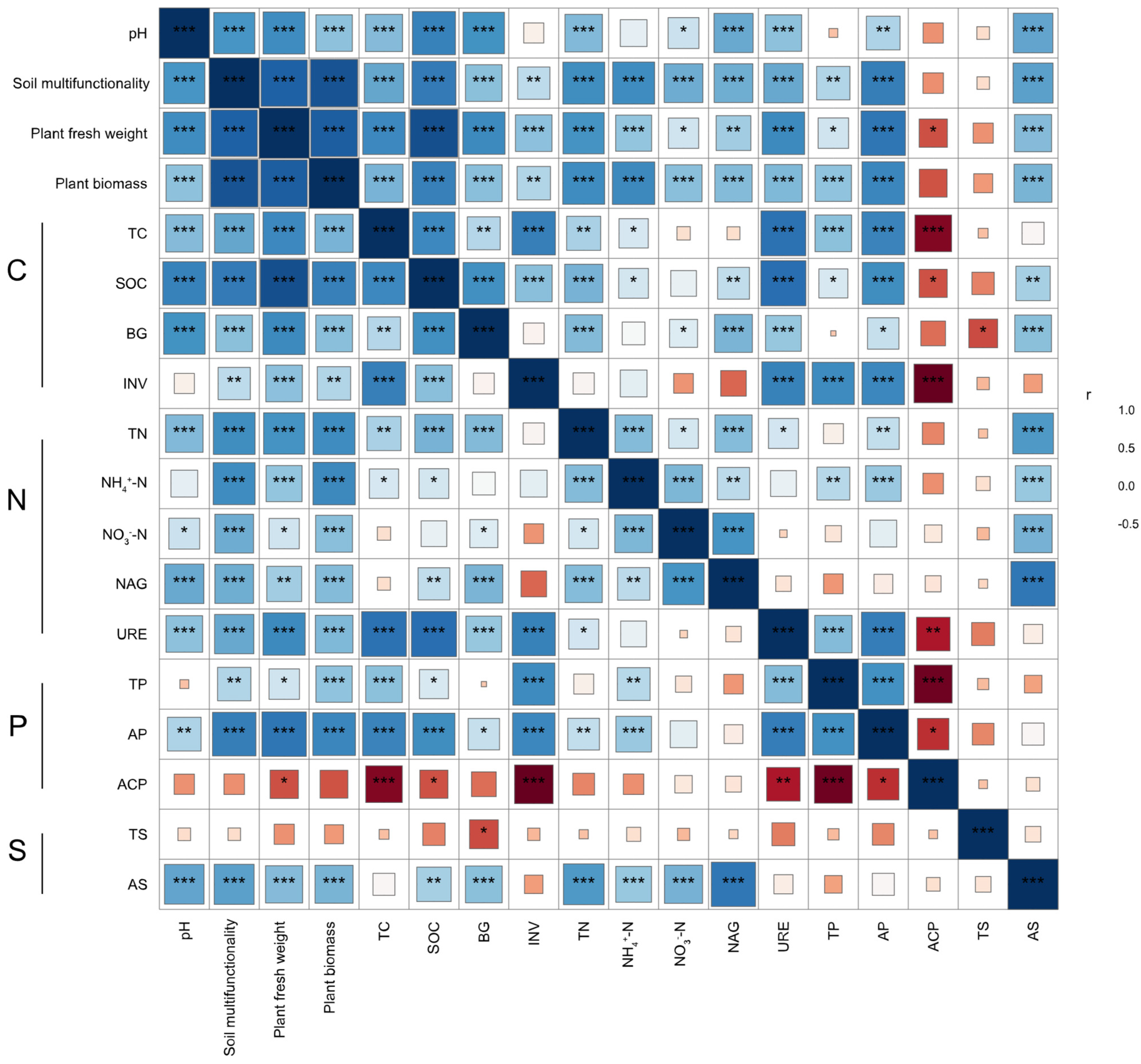
3.2. Responses of Rhizospheric Physicochemical Properties of Soil and Enzyme Activities Associated with Alfalfa to Different Carriers Mediated by Microbial Inoculants
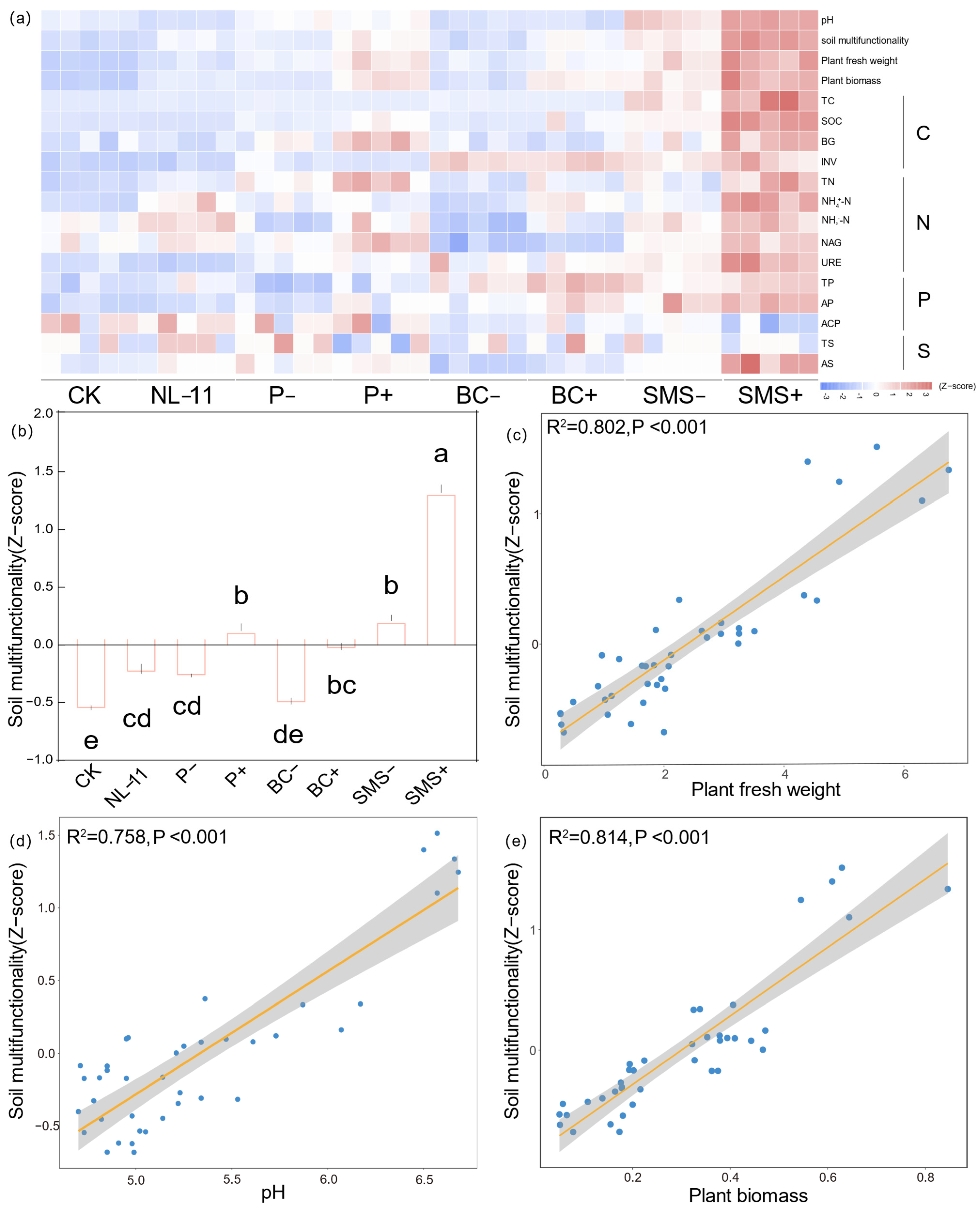
3.3. Responses of Multifunctionality in Rhizospheric Soil of Alfalfa to Different Carriers Mediated by Microbial Inoculants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peco, J.D.; Higueras, P.; Campos, J.A.; Esbrí, J.M.; Moreno, M.M.; Battaglia-Brunet, F.; Sandalio, L.M. Abandoned Mine Lands Reclamation by Plant Remediation Technologies. Sustainability 2021, 13, 27. [Google Scholar] [CrossRef]
- Fanin, N.; Gundale, M.J.; Farrell, M.; Ciobanu, M.; Baldock, J.A.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Consistent effects of biodiversity loss on multifunctionality across contrasting ecosystems. Nat. Ecol. Evol. 2018, 2, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.M.; Trexler, R.V.; Malik, R.J.; Hockett, K.L.; Bell, T.H. The Inherent Conflicts in Developing Soil Microbial Inoculants. Trends Biotechnol. 2019, 37, 140–151. [Google Scholar] [CrossRef]
- Li, C.; Sun, L.H.; Jia, Z.H.; Tang, Y.Z.; Liu, X.; Zhang, J.C.; Müller, C. Microbial Inoculants Drive Changes in Soil and Plant Microbiomes and Improve Plant Functions in Abandoned Mine Restoration. Plant Cell Environ. 2025, 48, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Liu, D.H.; Li, F.Q.; Dong, Y.H.; Jin, Z.L.; Liao, Y.W.K.; Li, X.H.; Peng, S.G.; Delgado-Baquerizo, M.; Li, X.G. Superiority of native soil core microbiomes in supporting plant growth. Nat. Commun. 2024, 15, 13. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.H.; Tang, Y.Z.; Zhang, S.F.; Li, T.; Ma, S.L.; Nie, H.; Zhai, L.; Zhang, B.; Liu, X.; et al. Mineral-solubilizing microbial inoculants facilitate the rejuvenation of soil multifunctionality and plant growth at abandoned mine sites. Land Degrad. Dev. 2024, 35, 442–454. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zhang, J.C.; Guo, X.P. An indigenous soil bacterium facilitates the mitigation of rocky desertification in carbonate mining areas. Land Degrad. Dev. 2017, 28, 2222–2233. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.C.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 14. [Google Scholar] [CrossRef]
- Gong, Y.Z.; Niu, Q.Y.; Liu, Y.G.; Dong, J.; Xia, M.M. Development of multifarious carrier materials and impact conditions of immobilised microbial technology for environmental remediation: A review. Environ. Pollut. 2022, 314, 19. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef]
- Wang, J.J.; Shi, G.J.; Zhu, H.B. Synergistic effect of ATMP, EDTMPS and PESA on the scale inhibition in the reinjected sewage. Desalin. Water Treat. 2020, 179, 38–44. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 7. [Google Scholar] [CrossRef]
- Subhashini, R. Suitability of amended vermiculite as a carrier for bacterial inoculants. Res. Crop. 2008, 9, 707–723. [Google Scholar]
- Phan, C.W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 17. [Google Scholar] [CrossRef]
- Yang, G.T.; Ma, Y.; Ma, X.C.; Wang, X.Q.; Lu, C.; Xu, W.Y.; Luo, J.; Guo, D.J. Changes in soil organic carbon components and microbial community following spent mushroom substrate application. Front. Microbiol. 2024, 15, 13. [Google Scholar] [CrossRef]
- Pan, X.; Deng, T.F.; Zhang, L.; Ge, L.J.; Li, L.Q.; Yang, L.S.; Gao, M.; Cao, J.F.; Wei, F.X.; Liu, X.L.; et al. Epimedium Herbal Residue as a Bulking Agent for Lignite and Spent Mushroom Substrate Co-composting. Waste Biomass Valorization 2023, 14, 2547–2555. [Google Scholar] [CrossRef]
- Seekram, P.; Thammasittirong, A.; Thammasittirong, S.N.R. Evaluation of spent mushroom substrate after cultivation of Pleurotus ostreatus as a new raw material for xylooligosaccharides production using crude xylanases from Aspergillus flavus KUB2. 3 Biotech 2021, 11, 9. [Google Scholar] [CrossRef]
- Han, S.; Delgado-Baquerizo, M.; Luo, X.S.; Liu, Y.R.; Van Nostrand, J.D.; Chen, W.L.; Zhou, J.Z.; Huang, Q.Y. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality. Soil Biol. Biochem. 2021, 154, 8. [Google Scholar] [CrossRef]
- Creamer, R.E.; Barel, J.M.; Bongiorno, G.; Zwetsloot, M.J. The life of soils: Integrating the who and how of multifunctionality. Soil Biol. Biochem. 2022, 166, 15. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 8. [Google Scholar] [CrossRef]
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated use of bio-organic fertilizers for enhancing soil fertility-plant nutrition, germination status and initial growth of corn (Zea mays L.). Environ. Technol. Innov. 2021, 21, 13. [Google Scholar] [CrossRef]
- Xue, S.; Yang, X.M.; Liu, G.B.; Gai, L.T.; Zhang, C.S.; Ritsema, C.J.; Geissen, V. Effects of elevated CO2 and drought on the microbial biomass and enzymatic activities in the rhizospheres of two grass species in Chinese loess soil. Geoderma 2017, 286, 25–34. [Google Scholar] [CrossRef]
- LY/T 1230-1999; Determination of Available Phosphorus in Forest Soil—Ammonium Molybdate Colorimetry. Standards Press: Beijing, China, 1999.
- LY/T 1231-1999; Determination of Ammonium Nitrogen in Forest Soil. Standards Press: Beijing, China, 1999.
- LY/T 1236-1999; Determination of Available Potassium in Forest Soil. Standards Press: Beijing, China, 1999.
- LY/T 1234-1999; Determination of Total Potassium in Forest Soil. Standards Press: Beijing, China, 1999.
- Lessard, I.; Renella, G.; Sauvé, S.; Deschênes, L. Metal toxicity assessment in soils using enzymatic activity: Can water be used as a surrogate buffer? Soil Biol. Biochem. 2013, 57, 256–263. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Luo, X.S.; Fu, X.Q.; Yang, Y.; Cai, P.; Peng, S.B.; Chen, W.L.; Huang, Q.Y. Microbial communities play important roles in modulating paddy soil fertility. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, W.; Delgado-Baquerizo, M.; Song, W.Z.; Ma, J.Y.; Wang, K.Y.; Ling, X.L. Phosphorus addition regulates the responses of soil multifunctionality to nitrogen over-fertilization in a temperate grassland. Plant Soil 2022, 473, 73–87. [Google Scholar] [CrossRef]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The Plant Growth-Promoting Rhizobacterium Bacillus cereus AR156 Induces Systemic Resistance in Arabidopsis thaliana by Simultaneously Activating Salicylate- and Jasmonate/Ethylene-Dependent Signaling Pathways. Mol. Plant-Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef]
- Pindi, P.K.; Sultana, T.; Vootla, P.K. Plant growth regulation of Bt-cotton through Bacillus species. 3 Biotech 2014, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Naskar, S.K.; Ray, R.C. Indole-3-acetic acid production and effect on sprouting of yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol. J. Microbiol. 2007, 56, 103–110. [Google Scholar] [PubMed]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef]
- Korenblum, E.; Aharoni, A. Phytobiome metabolism: Beneficial soil microbes steer crop plants’ secondary metabolism. Pest Manag. Sci. 2019, 75, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.R.; Ge, Y.H.; Lei, Y.H.; Wei, X.C.; Xu, Y.; Zheng, X.Q. Partial substitution of nitrogen fertilizers by organic products of rural waste co-composting impacts on farmland soil quality. Environ. Technol. Innov. 2024, 33, 14. [Google Scholar] [CrossRef]
- Li, H.Y.; Qiu, Y.Z.; Yao, T.; Ma, Y.C.; Zhang, H.R.; Yang, X.L. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 10. [Google Scholar] [CrossRef]
- Gu, Y.B.; Meng, D.L.; Yang, S.; Xiao, N.W.; Li, Z.Y.; Liu, Z.H.; Li, L.Z.; Zeng, X.X.; Zeng, S.R.; Yin, H.Q. Invader-resident community similarity contribute to the invasion process and regulate biofertilizer effectiveness. J. Clean. Prod. 2019, 241, 11. [Google Scholar] [CrossRef]
- Mallon, C.A.; Poly, F.; Le Roux, X.; Marring, I.; van Elsas, J.D.; Salles, J.F. Resource pulses can alleviate the biodiversity-invasion relationship in soil microbial communities. Ecology 2015, 96, 915–926. [Google Scholar] [CrossRef]
- Martin, C.; Zervakis, G.I.; Xiong, S.J.; Koutrotsios, G.; Straetkvern, K.O. Spent substrate from mushroom cultivation: Exploitation potential toward various applications and value-added products. Bioengineered 2023, 14, 40. [Google Scholar] [CrossRef]
- Wang, Z.C.; Liu, Y.; Li, J.H.; Meng, G.Q.; Zhu, D.Y.; Cui, J.D.; Jia, S.R. Efficient Immobilization of Enzymes on Amino Functionalized MIL-125-NH2 Metal Organic Framework. Biotechnol. Bioprocess Eng. 2022, 27, 135–144. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 13. [Google Scholar] [CrossRef]
- Gao, X.Y.; Rodrigues, S.M.; Spielman-Sun, E.; Lopes, S.; Rodrigues, S.; Zhang, Y.L.; Avellan, A.; Duarte, R.; Duarte, A.; Casman, E.A.; et al. Effect of Soil Organic Matter, Soil pH, and Moisture Content on Solubility and Dissolution Rate of CuO NPs in Soil. Environ. Sci. Technol. 2019, 53, 4959–4967. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.M.; Lu, L.X.; Ye, J.R.; Shi, L.N. Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana. Int. J. Mol. Sci. 2022, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Jing, G.H.; Zhao, N.; Lu, Q.Q.; Zhang, Z.; Chen, Z.K.; Huang, B.; Ding, X.Z. Soil nutrients and the responses of microbial community structure to pine bark and vinegar residues in blueberry cultivation. Appl. Soil Ecol. 2023, 189, 9. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.Y.; Ren, G.X.; Khan, A.; Feng, Y.Z.; Yang, G.H. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Dumigan, C.R.; Deyholos, M.K. Soil and seed both influence bacterial diversity in the microbiome of the Cannabis sativa seedling endosphere. Front. Plant Sci. 2024, 15, 13. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.W.; Arafat, Y.; Lin, W.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Characterizing rhizosphere microbial communities in long-term monoculture tea orchards by fatty acid profiles and substrate utilization. Eur. J. Soil Biol. 2017, 81, 48–54. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.H.; Zhang, S.F.; Li, T.; Ma, S.L.; Cheng, X.F.; Chen, M.L.; Nie, H.; Zhai, L.; Zhang, B.; et al. The positive effects of mineral-solubilizing microbial inoculants on asymbiotic nitrogen fixation of abandoned mine soils are driven by keystone phylotype. Sci. Total Environ. 2023, 882, 13. [Google Scholar] [CrossRef]
- Li, C.; Nie, H.; Zhang, S.F.; Jia, Z.H.; Ma, S.L.; Li, T.; Zhai, L.; Zhang, B.; Liu, X.; Zhang, J.C.; et al. Mineral-solubilizing microbial inoculant positively affects the multifunctionality of anthropogenic soils in abandoned mining areas. J. Environ. Manag. 2023, 344, 11. [Google Scholar] [CrossRef]
- Bai, B.X.; Yang, X.; Zhao, Q.S.; Liu, R.X.; Ren, J.H. Inoculations with Pseudomonas fluorescens and Bacillus cereus affect the soil enzyme activity, growth and rhizosphere microbial diversity of Taxus chinensis var. mairei. Plant Soil 2020, 455, 41–52. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Rathore, A.P.; Sharma, S. Effect of halotolerant plant growth promoting rhizobacteria inoculation on soil microbial community structure and nutrients. Appl. Soil Ecol. 2020, 150, 6. [Google Scholar] [CrossRef]
- Hidri, R.; Metoui-Ben Mahmoud, O.; Debez, A.; Abdelly, C.; Barea, J.M.; Azcon, R. Modulation of C:N:P stoichiometry is involved in the effectiveness of a PGPR and AM fungus in increasing salt stress tolerance of Sulla carnosa Tunisian provenances. Appl. Soil Ecol. 2019, 143, 161–172. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.L.; Wu, X.B.; Cui, R.Z.; Li, G.S.; Zheng, F.L.; Tan, D.S. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Ul Hassan, T.; Tahir, M.I.; Ali, A.; Jeyasundar, P.; Hussain, Q.; Bashir, S.; Mehmood, S.; Zhang, Z.Q. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 2021, 157, 13. [Google Scholar] [CrossRef]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Zhang, Y.Y.; Li, Z.J.; Shi, J.W.; Zhang, G.X.; Zhang, H.; Yang, L.J. Vermicompost improves microbial functions of soil with continuous tomato cropping in a greenhouse. J. Soils Sediments 2020, 20, 380–391. [Google Scholar] [CrossRef]
- Ren, H.; Lv, C.Q.; Fernández-García, V.; Huang, B.L.; Yao, J.M.; Ding, W. Biochar and PGPR amendments influence soil enzyme activities and nutrient concentrations in a eucalyptus seedling plantation. Biomass Convers. Biorefinery 2021, 11, 1865–1874. [Google Scholar] [CrossRef]
- Garland, G.; Banerjee, S.; Edlinger, A.; Oliveira, E.M.; Herzog, C.; Wittwer, R.; Philippot, L.; Maestre, F.T.; van der Heijden, M.G.A. A closer look at the functions behind ecosystem multifunctionality: A review. J. Ecol. 2021, 109, 600–613. [Google Scholar] [CrossRef]
- Manning, P.; van der Plas, F.; Soliveres, S.; Allan, E.; Maestre, F.T.; Mace, G.; Whittingham, M.J.; Fischer, M. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Jia, Z.H.; Meng, M.J.; Li, C.; Zhang, B.; Zhai, L.; Liu, X.; Ma, S.L.; Cheng, X.F.; Zhang, J.C. Rock-Solubilizing Microbial Inoculums Have Enormous Potential as Ecological Remediation Agents to Promote Plant Growth. Forests 2021, 12, 13. [Google Scholar] [CrossRef]
- Shu, X.Y.; He, J.; Zhou, Z.H.; Xia, L.L.; Hu, Y.F.; Zhang, Y.L.; Zhang, Y.Y.; Luo, Y.Q.; Chu, H.Y.; Liu, W.J.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Chen, P.; Wang, F.H.; Han, W.X.; Qiao, M.; Dong, W.X.; Hu, C.S.; Zhu, D.; Chu, H.Y.; Zhu, Y.G. The ecological clusters of soil organisms drive the ecosystem multifunctionality under long-term fertilization. Environ. Int. 2022, 161, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Wang, T.T.; Sun, C.W.; Liu, P.; Chen, J.; Hou, X.; Yu, T.; Gao, Y.; Liu, Z.G.; Yang, L.; et al. Eugenol improves salt tolerance via enhancing antioxidant capacity and regulating ionic balance in tobacco seedlings. Front. Plant Sci. 2024, 14, 13. [Google Scholar] [CrossRef]
- Gou, J.Y.; Suo, S.Z.; Shao, K.Z.; Zhao, Q.; Yao, D.; Li, H.P.; Zhang, J.L.; Rensing, C. Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World J. Microbiol. Biotechnol. 2020, 36, 12. [Google Scholar] [CrossRef] [PubMed]
- Su, H.F.; Lin, J.F.; Chen, H.; Wang, Q.Y. Production of a novel slow-release coal fly ash microbial fertilizer for restoration of mine vegetation. Waste Manag. 2021, 124, 185–194. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Lv, X.; Song, J.H.; Li, W.D.; Wang, H.J. Application of cotton straw biochar and compound Bacillus biofertilizer decrease the bioavailability of soil cd through impacting soil bacteria. BMC Microbiol. 2022, 22, 13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Zhou, Y.; Nie, H.; Li, C.; Liu, X.; Lin, J.; Zhang, X.; Zhang, J. Solid Microbial Fertilizers Prepared with Different Carriers Have the Potential to Enhance Plant Growth. Forests 2025, 16, 539. https://doi.org/10.3390/f16030539
Sun L, Zhou Y, Nie H, Li C, Liu X, Lin J, Zhang X, Zhang J. Solid Microbial Fertilizers Prepared with Different Carriers Have the Potential to Enhance Plant Growth. Forests. 2025; 16(3):539. https://doi.org/10.3390/f16030539
Chicago/Turabian StyleSun, Lianhao, Yuexiang Zhou, Hui Nie, Chong Li, Xin Liu, Jie Lin, Xiongfei Zhang, and Jinchi Zhang. 2025. "Solid Microbial Fertilizers Prepared with Different Carriers Have the Potential to Enhance Plant Growth" Forests 16, no. 3: 539. https://doi.org/10.3390/f16030539
APA StyleSun, L., Zhou, Y., Nie, H., Li, C., Liu, X., Lin, J., Zhang, X., & Zhang, J. (2025). Solid Microbial Fertilizers Prepared with Different Carriers Have the Potential to Enhance Plant Growth. Forests, 16(3), 539. https://doi.org/10.3390/f16030539





