The Effects of Different Plant Configuration Modes on Soil Organic Carbon Fractions in the Lakeshore of Hongze Lake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Plot Setting and Sample Collection
2.3. Indicator Measurement
2.4. Data Analysis
3. Results
3.1. Soil Organic Carbon and Its Fraction Content in Different Plant Configuration Modes
3.2. Characteristics of Soil Organic Carbon Fraction Ratios and Carbon Pool Activity for Different Plant Configuration Modes
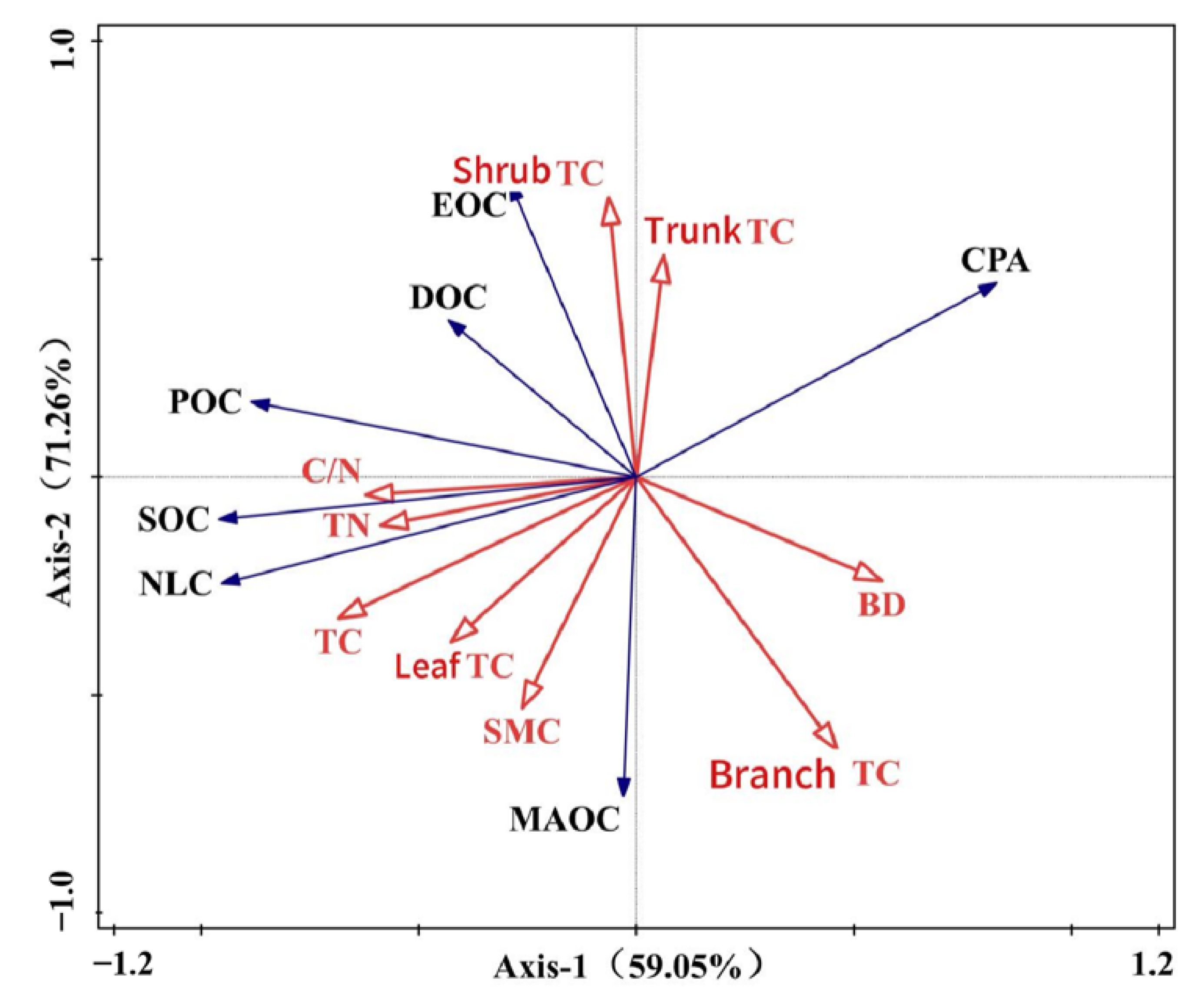
3.3. Relationships Between Soil Organic Carbon Fractions, Carbon Pool Activity, Soil Physical and Chemical Properties, and Vegetation Carbon Content
4. Discussions
4.1. Soil Total Organic Carbon Distribution in Response to Plant Configuration Modes
4.2. Variability and Sensitivity Analysis of Soil Organic Carbon Fraction in Different Plant Configuration Modes
4.3. Characteristics of Soil Organic Carbon Partitioning Ratio and Carbon Pool Activity in Different Plant Configuration Modes
4.4. Main Factors Influencing the Accumulation of Soil Organic Carbon Fractions and the Distribution of Their Components
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, K.; Zhang, Y.; Tang, S.; Liu, J. Spatial distribution of soil organic carbon in the Zoige alpine wetland, northeastern Qinghai–Tibet Plateau. CATENA 2016, 144, 102–108. [Google Scholar] [CrossRef]
- Wang, L.L.; Song, C.C.; Ge, R.J. Soil Organic Carbon Storage under Different Land-use Types in Sanjian Plain. China Environ. Sci. 2009, 29, 656–660. [Google Scholar]
- Liang, C.; Balser, T.C. Warming and nitrogen deposition lessen microbial residue contribution to soil carbon pool. Nat. Commun. 2012, 3, 1222. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Cao, Z.; Hu, Z. Characteristics and Ecological Effects of the Active Organic Carbon in Soil. Chin. J. Ecol. 1999, 3, 32–38. [Google Scholar]
- Blair, G.; Lefroy, R.D.B.; Lisle, L. Soil Carbon Fractions Based on their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Crop Pasture Sci. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Yu, J.; Fang, L.; Bian, Z.; Wang, Q.; Yu, Y. A review of the composition of soil carbon pool. Acta Ecol. Sin. 2014, 34, 4829–4838. [Google Scholar]
- Zhang, J.; Song, C.; Yang, W. Effects of land use patterns on soil water-dissolved organic carbon. China Environ. Sci. 2005, 343–347. [Google Scholar]
- Ji, H.; Han, J.; Li, P.; Zhu, Y.; Guo, Y.; Hao, D.; Cui, H. Effects of different vegetation types on soil organic carbon particle size distribution and microbial community structure in Hongze Lake Wetland. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 141–150. [Google Scholar]
- Xiao, Y.; Huang, Z.; Wu, H.; Lv, X. Compositions and contents of active organic carbon in different wetland soils in Sanjiang Plain, Northeast China. Acta Ecol. Sin. 2015, 35, 7625–7633. [Google Scholar]
- Chen, X.; Chen, Z.; Lei, J.; Wu, T.; Li, W. Contents of Organic Carbon and Its Active Components in Surface Soil of Different Community Areas of Mangrove Forest in Dongzhai Port. Wetl. Sci. 2022, 20, 499–506. [Google Scholar] [CrossRef]
- Rodríguez-Murillo, J.C.; Almendros, G.; Knicker, H. Wetland soil organic matter composition in a Mediterranean semiarid wetland (Las Tablas de Daimiel, Central Spain): Insight into different carbon sequestration pathways. Org. Geochem. 2011, 42, 762–773. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Dong, Y.; Chang, X.; Nie, X.; Liu, L.; Xiao, H.; Lu, Y.; Zeng, G. Response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly–gully region of China. Soil Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]
- Lai, L.; Zhao, J.; Dou, Y. Fungal necromass carbon contributes more to POC and MAOC under different forest types of Qinling Mountains. Plant Soil 2024. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.; Sun, W.; Zhu, J.; Liu, G.; Zhou, W.; Zhang, Q.; Shi, P.; Liu, X.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef] [PubMed]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [PubMed]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Tian, H.; Fu, B.; Yu, H.; Piao, S.; Chen, S.; Li, Y.; Li, X.; Wang, M.; Li, Z.; et al. Biophysical and economic constraints on China’s natural climate solutions. Nat. Clim. Change 2022, 12, 847–853. [Google Scholar] [CrossRef]
- Yu, Z.; Dong, Y.; Lu, C.; Agathokleous, E.; Zhang, L.; Liu, S.; Zhou, G. China’s forestation on marginal land was less efficient in carbon sequestration compared with non-marginal land. One Earth 2023, 6, 1692–1702. [Google Scholar] [CrossRef]
- Xu, H.; Yue, C.; Zhang, Y.; Liu, D.; Piao, S. Forestation at the right time with the right species can generate persistent carbon benefits in China. Proc. Natl. Acad. Sci. USA 2023, 120, e2304988120. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Chen, J.M.; Xu, M.; Lin, X.; Li, P.; Yu, G.; He, N.; Xu, L.; Gong, P.; Liu, L.; et al. China’s current forest age structure will lead to weakened carbon sinks in the near future. Innovation 2023, 4, 100515. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, P.; Huettmann, F.; Liu, S. Where should China practice forestry in a warming world? Glob. Change Biol. 2022, 28, 2461–2475. [Google Scholar] [CrossRef]
- Benz, J.P.; Chen, S.; Dang, S.; Dieter, M.; Labelle, E.R.; Liu, G.; Hou, L.; Mosandl, R.M.; Pretzsch, H.; Pukall, K.; et al. Multifunctionality of forests: A white paper on challenges and opportunities in China and Germany. Forests 2020, 11, 266. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.-h.; Wang, M.-l.; Zhao, L.; Zhao, Y.-c.; Zhang, Q.-p.; Zhang, C.-y. Decoupling analysis to assess the impact of land use patterns on carbon emissions: A case study in the Yellow River Delta efficient eco-economic zone, China. J. Clean. Prod. 2023, 412, 137415. [Google Scholar] [CrossRef]
- Wu, Y.; Dai, R.; Xu, Y.; Han, J.; Li, P. Spatial and temporal changes in wetland use in the meeting place of Hung-tse Lake and Huaihe River. J. Nanjing For. Univ. Nat. Sci. Ed. 2016, 40, 22–28. [Google Scholar]
- Ding, W.-X.; Cai, Z.-C. Methane Emission from Natural Wetlands in China: Summary of Years 1995–2004 Studies. Pedosphere 2007, 17, 475–486. [Google Scholar] [CrossRef]
- Song, C.; Xu, X.; Tian, H.; Wang, Y. Ecosystem–atmosphere exchange of CH4 and N2O and ecosystem respiration in wetlands in the Sanjiang Plain, Northeastern China. Glob. Change Biol. 2009, 15, 692–705. [Google Scholar] [CrossRef]
- Xie, Y.; Xie, Y.; Chen, X.; Li, F. Progress in research on the water-holding function of wetland soils. Wetl. Sci. 2012, 10, 109–115. [Google Scholar] [CrossRef]
- Hao, L.; Lv, X. Effect of different reclamation patterns on soil organic carbon distribution of aggregates in the topsoil of the Calamagrostis angustifolia wetland in Sanjiang Plain, China. China Environ. Sci. 2011, 31, 1711–1717. [Google Scholar]
- He, L.; Gao, B.; Xia, H.; Long, Y.; Wang, C.; He, Z. Water quantity dispatch of Jiangsu Province’s Water Diversion from the Yangtze River to the North. S. N. Water Transf. Water Sci. Technol. 2024, 22, 896–908. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Luo, Z.; Feng, W.; Luo, Y.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Change Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef] [PubMed]
- Nel, J.A.; Cramer, M.D. Soil microbial anaplerotic CO2 fixation in temperate soils. Geoderma 2019, 335, 170–178. [Google Scholar] [CrossRef]
- Huang, L.; Wang, F.; Zhou, L.; Huang, R.; Qi, D.; Wei, G. Root distribution in the different forest types and their relationship to soil properties. Acta Ecol. Sin. 2012, 32, 6110–6119. [Google Scholar] [CrossRef]
- Kätterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2020, 230, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Shangguan, Z. Specific root length characteristics of three plant species, Bothriochloa ischaemunm, Hippophae rhamnoidess and Quercus liaotungensis in the Loess Platean. Acta Ecol. Sin. 2006, 26, 4164–4170. [Google Scholar]
- Guo, Y. Effects of Land Use/Cover Types on Organic Carbon Stocks and Stability in Hongze Lake Wetlands. Ph.D. Thesis, Department of Ecology, Nanjing Forestry University, Nanjing, China, 2024. [Google Scholar]
- Zhang, S.; Xiong, D.; Xlao, L.; Yang, D.; Zhang, B.; Han, W. Influence of Dry-Wet Cycling on Soil Properties. Chin. J. Soil Sci. 2017, 48, 762–768. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, B.; Xu, Z.; Gong, L.; Yu, D.; Zhan, H.; Shen, R. Decomposition rate and temperature sensitivity of soil organic carbon in a typical shoal wetland of Lake Poyang. J. Lake Sci. 2024, 36, 1425–1435. [Google Scholar]
- Keuskamp, D. Limited effects of grazer exclusion on the epiphytes of Posidonia sinuosa in South Australia. Aquat. Bot. 2004, 78, 3–14. [Google Scholar] [CrossRef]
- Combs-Giroir, R.; Gschwend, A.R. Physical and molecular responses to flooding in Brassicaceae. Environ. Exp. Bot. 2024, 219, 105664. [Google Scholar] [CrossRef]
- He, Y.; Yu, Q.; Wang, G.; Hao, M.; Fan, S.; Hu, D.; Li, Z.; Gao, P. Tree Ring Width Responses of Pinus densiflora and Robinia pseudoacacia to Climate Variation in the Mount Tai Area of Northern China. Forests 2023, 14, 2087. [Google Scholar] [CrossRef]
- Kalks, F.; Liebmann, P.; Wordell-Dietrich, P.; Guggenberger, G.; Kalbitz, K.; Mikutta, R.; Helfrich, M.; Don, A. Fate and stability of dissolved organic carbon in topsoils and subsoils under beech forests. Biogeochemistry 2020, 148, 111–128. [Google Scholar] [CrossRef]
- da Silva, S.F.; Spaccini, R.; de Rezende, C.E.; Canellas, L.P. Influence of land use and different plant residues on isotopic carbon distribution of total and water extractable organic matter in an incubation experiment with weathered tropical soil. Land Degrad. Dev. 2023, 34, 1363–1374. [Google Scholar] [CrossRef]
- Mikutta, R.; Turner, S.; Schippers, A.; Gentsch, N.; Meyer-Stüve, S.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Eger, A.; Hempel, G. Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci. Rep. 2019, 9, 10294. [Google Scholar] [CrossRef]
- Hu, Y.; Ao, G.; Feng, J.; Chen, X.; Zhu, B. The patterns of forest soil particulate and mineral associated organic carbon characteristics with latitude and soil depth across eastern China. For. Ecosyst. 2025, 12, 100291. [Google Scholar] [CrossRef]
- Zhai, D.; Wang, Y.; Liao, C.; Men, X.; Wang, C.; Cheng, X. Soil Carbon Accumulation Under Afforestation Is Driven by Contrasting Responses of Particulate and Mineral-Associated Organic Carbon. Glob. Biogeochem. Cycles 2024, 38, e2024GB008116. [Google Scholar] [CrossRef]
- Xiao, R.; Ruan, H.; Liu, H.; Fang, Y.; Cha, Q.; Xie, Y.; Xu, Y.; Cao, G.; Shen, C. Effects of drought on soil particulate organic carbon and mineral-associated organic carbon in poplar plantations. J. Nanjing For. Univ. Nat. Sci. Ed. 2024, pp. 1–12. Available online: https://link.cnki.net/urlid/32.1161.s.20240408.1611.008 (accessed on 24 March 2025).
- Kaiser, M.; Kleber, M.; Berhe, A.A. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- De Neve, S.; Hofman, G. Quantifying soil water effects on nitrogen mineralization from soil organic matter and from fresh crop residues. Biol. Fertil. Soils 2002, 35, 379–386. [Google Scholar] [CrossRef]
- Anh, P.T.Q.; Gomi, T.; MacDonald, L.H.; Mizugaki, S.; Van Khoa, P.; Furuichi, T. Linkages among land use, macronutrient levels, and soil erosion in northern Vietnam: A plot-scale study. Geoderma 2014, 232, 352–362. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.-P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Niu, Z.; Li, Y.; Li, C.; Feng, W. Responses of soil organic and inorganic carbon vary at different soil depths after long-term agricultural cultivation in Northwest China. Land Degrad. Dev. 2019, 30, 1229–1242. [Google Scholar] [CrossRef]
- Guan, Y.; Bai, J.; Wang, J.; Wang, W.; Wang, X.; Zhang, L.; Li, X.; Liu, X. Effects of groundwater tables and salinity levels on soil organic carbon and total nitrogen accumulation in coastal wetlands with different plant cover types in a Chinese estuary. Ecol. Indic. 2021, 121, 106969. [Google Scholar] [CrossRef]
- Mendham, D.; Sankaran, K.; O’connell, A.; Grove, T. Eucalyptus globulus harvest residue management effects on soil carbon and microbial biomass at 1 and 5 years after plantation establishment. Soil Biol. Biochem. 2002, 34, 1903–1912. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Sun, X.; Cai, T. Responses of Soil Microbial Biomass and Enzyme Activities to Natural Restoration of Reclaimed Temperate Marshes After Abandonment. Front. Environ. Sci. 2021, 9, 701610. [Google Scholar] [CrossRef]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef]
- Chen, X.; Hu, H.; Wang, Q.; Wang, X.; Ma, B. Exploring the Factors Affecting Terrestrial Soil Respiration in Global Warming Manipulation Experiments Based on Meta-Analysis. Agriculture 2024, 14, 1581. [Google Scholar] [CrossRef]
- Dietterich, L.H.; Karpman, J.; Neupane, A.; Ciochina, M.; Cusack, D.F. Carbon content of soil fractions varies with season, rainfall, and soil fertility across a lowland tropical moist forest gradient. Biogeochemistry 2021, 155, 431–452. [Google Scholar] [CrossRef]


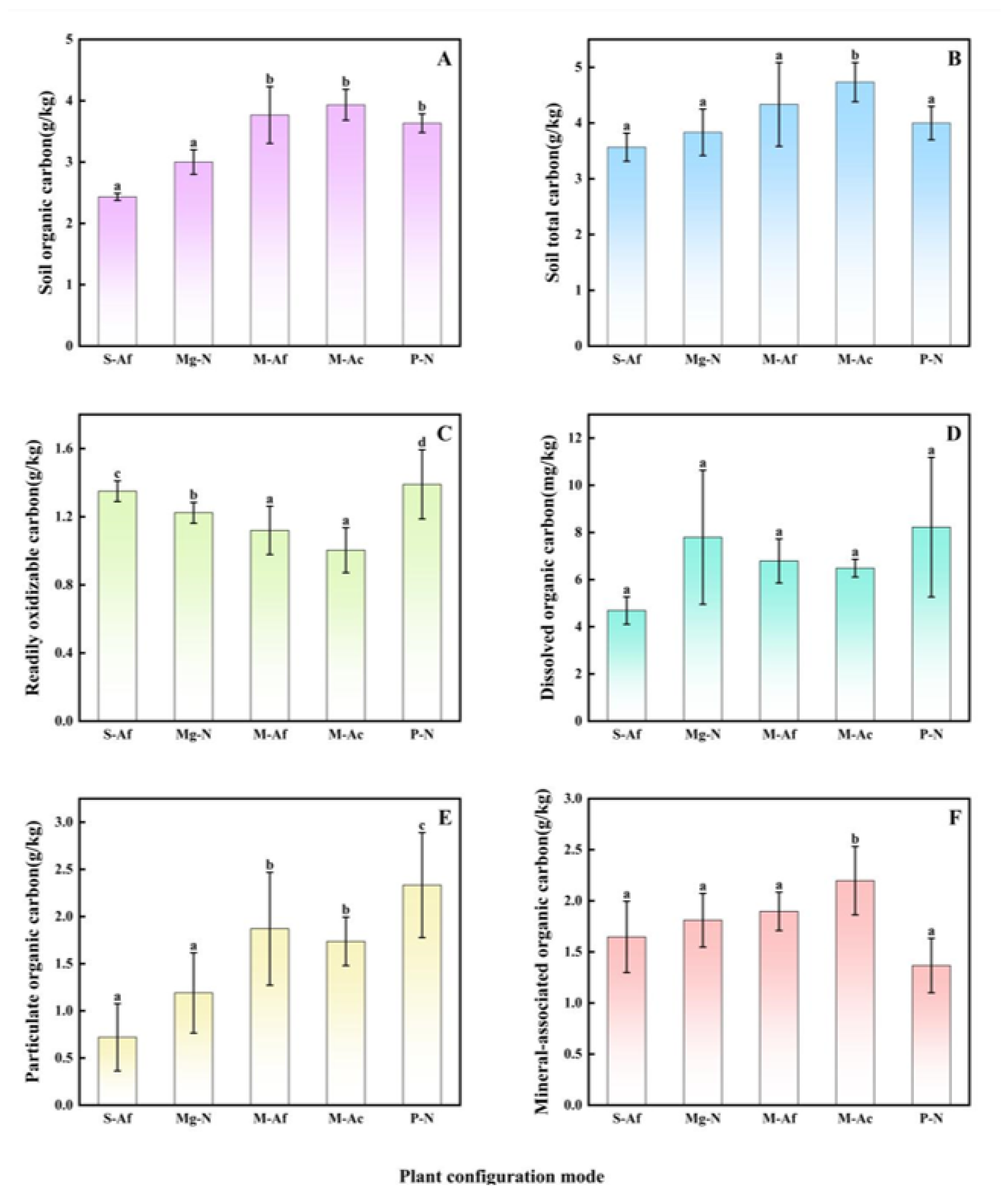
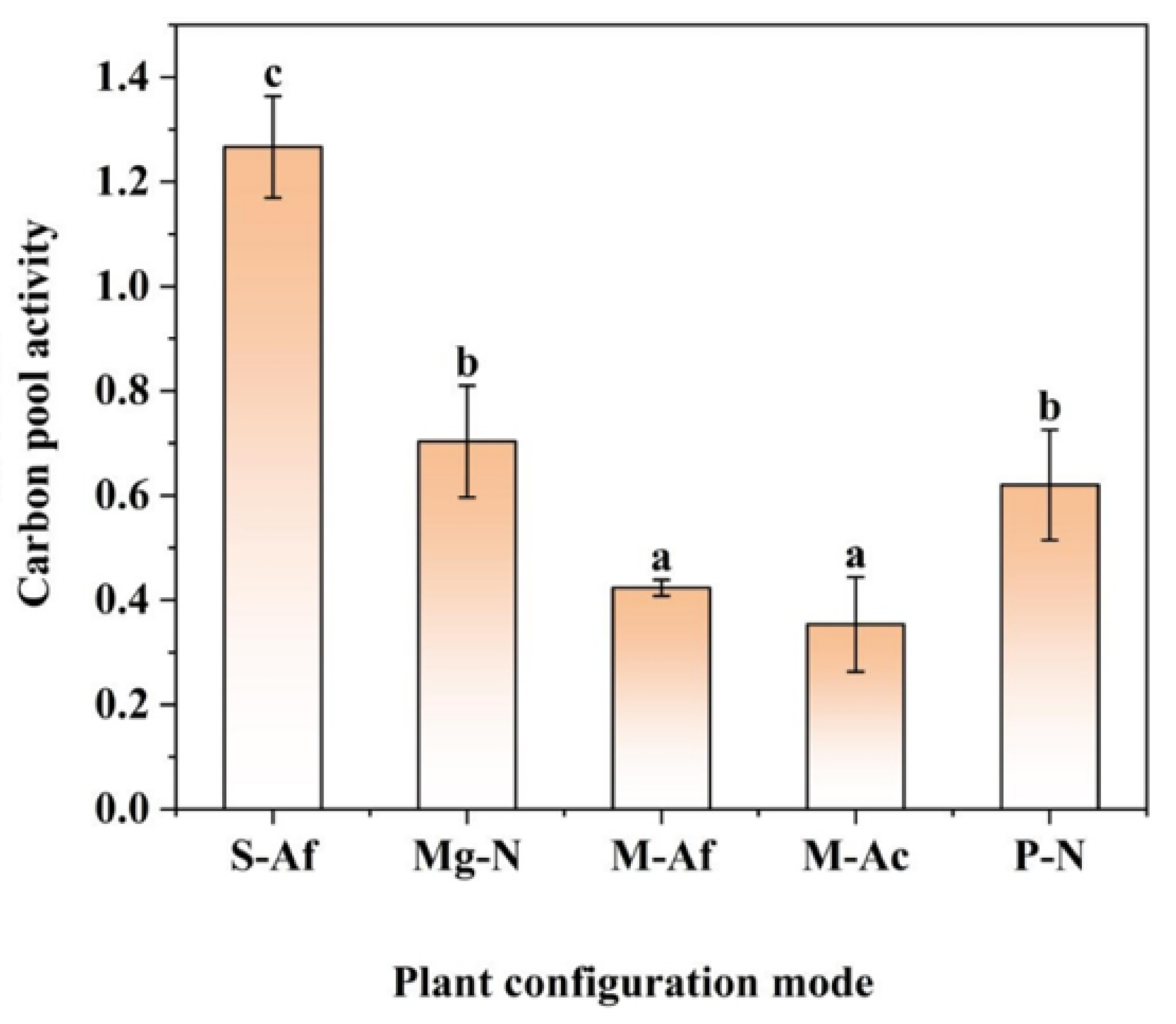
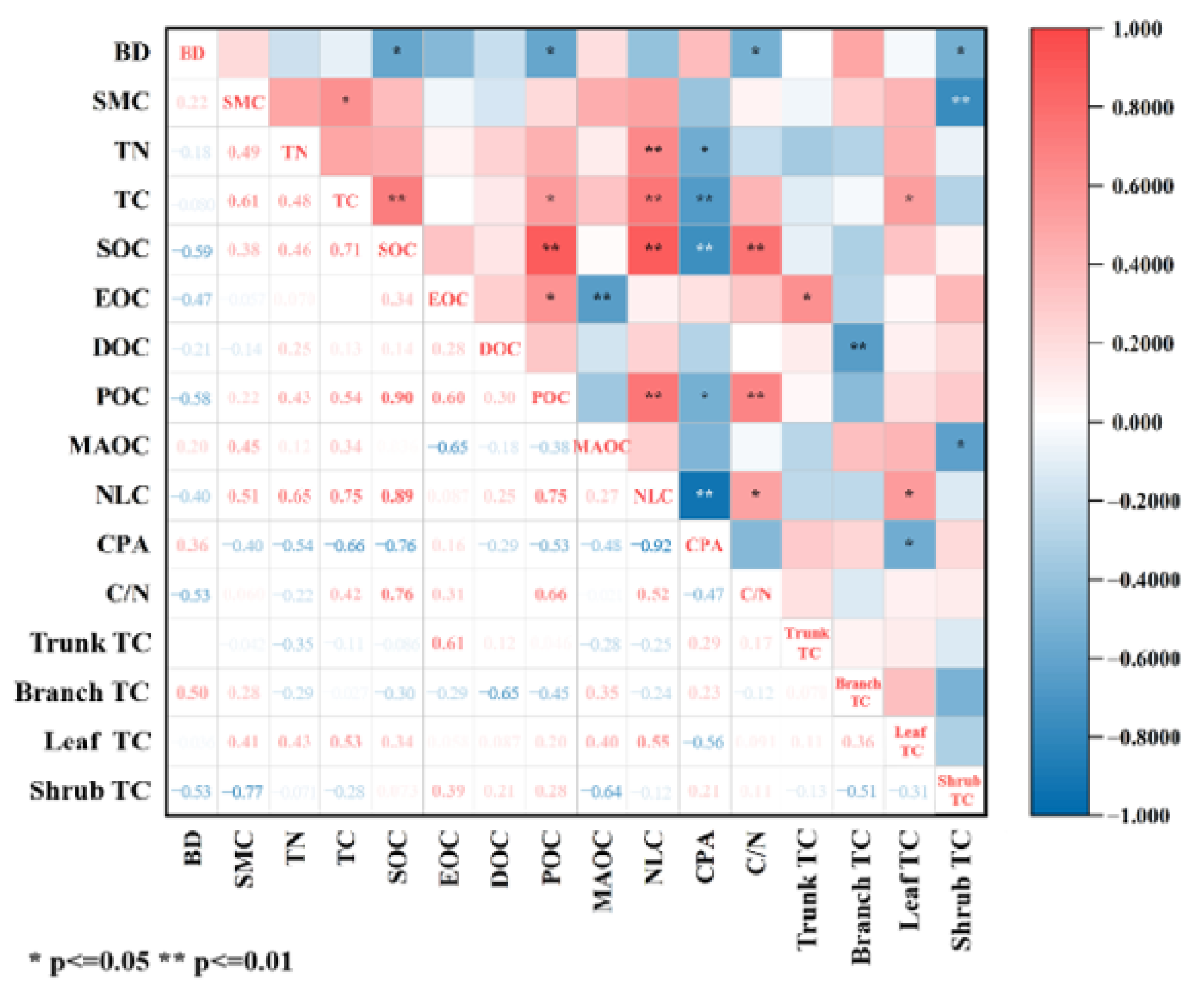
| SOC | ROC | DOC | POC | MAOC | NLC | |
|---|---|---|---|---|---|---|
| Sensitivity Index | 0.63 | 0.48 | 0.75 | 1.95 | 0.61 | 1.74 |
| Index (%) | S-Af | Mg-N | M-Af | M-Ac | P-N |
|---|---|---|---|---|---|
| ROC/SOC | 55.90 ± 6.08 c | 41.00 ± 3.87 b | 29.67 ± 4.54 a | 25.79 ± 4.93 a | 38.01 ± 4.15 b |
| DOC/SOC | 0.19 ± 0.03 a | 0.25 ± 0.09 a | 0.18 ± 0.04 a | 0.17 ± 0.02 a | 0.22 ± 0.10 a |
| POC/SOC | 32.57 ± 13.19 a | 39.22 ± 12.33 a | 48.94 ± 9.68 a | 44.24 ± 6.98 a | 58.53 ± 11.69 a |
| MAOC/SOC | 67.53 ± 13.19 b | 60.78 ± 12.33 b | 51.06 ± 9.68 a | 55.73 ± 6.98 a | 35.71 ± 11.69 a |
| NLC/SOC | 44.10 ± 2.13 a | 58.99 ± 3.99 b | 70.33 ± 8.76 c | 74.21 ± 8.14 c | 62.00 ± 6.30 b |
| Items | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|
| Eigenvalues | 0.5905 | 0.1221 | 0.1142 | 0.0112 |
| Explained variation (cumulative) (%) | 59.05 | 71.26 | 82.68 | 83.80 |
| Pseudo-canonical correlation | 0.9726 | 0.8215 | 0.8244 | 0.8211 |
| Explained fitted variation (cumulative) (%) | 70.02 | 84.50 | 98.05 | 99.38 |
| Canonical eigenvalues | 0.843259 | |||
| The sum of all eigenvalues | 1.000000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Li, X.; He, Y.; Jiang, J. The Effects of Different Plant Configuration Modes on Soil Organic Carbon Fractions in the Lakeshore of Hongze Lake. Forests 2025, 16, 611. https://doi.org/10.3390/f16040611
Guo T, Li X, He Y, Jiang J. The Effects of Different Plant Configuration Modes on Soil Organic Carbon Fractions in the Lakeshore of Hongze Lake. Forests. 2025; 16(4):611. https://doi.org/10.3390/f16040611
Chicago/Turabian StyleGuo, Tianyi, Xinrui Li, Yuan He, and Jiang Jiang. 2025. "The Effects of Different Plant Configuration Modes on Soil Organic Carbon Fractions in the Lakeshore of Hongze Lake" Forests 16, no. 4: 611. https://doi.org/10.3390/f16040611
APA StyleGuo, T., Li, X., He, Y., & Jiang, J. (2025). The Effects of Different Plant Configuration Modes on Soil Organic Carbon Fractions in the Lakeshore of Hongze Lake. Forests, 16(4), 611. https://doi.org/10.3390/f16040611





