Comparison of Growth Strategies and Biomass Allocation in Chinese Fir Provenances from the Subtropical Region of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
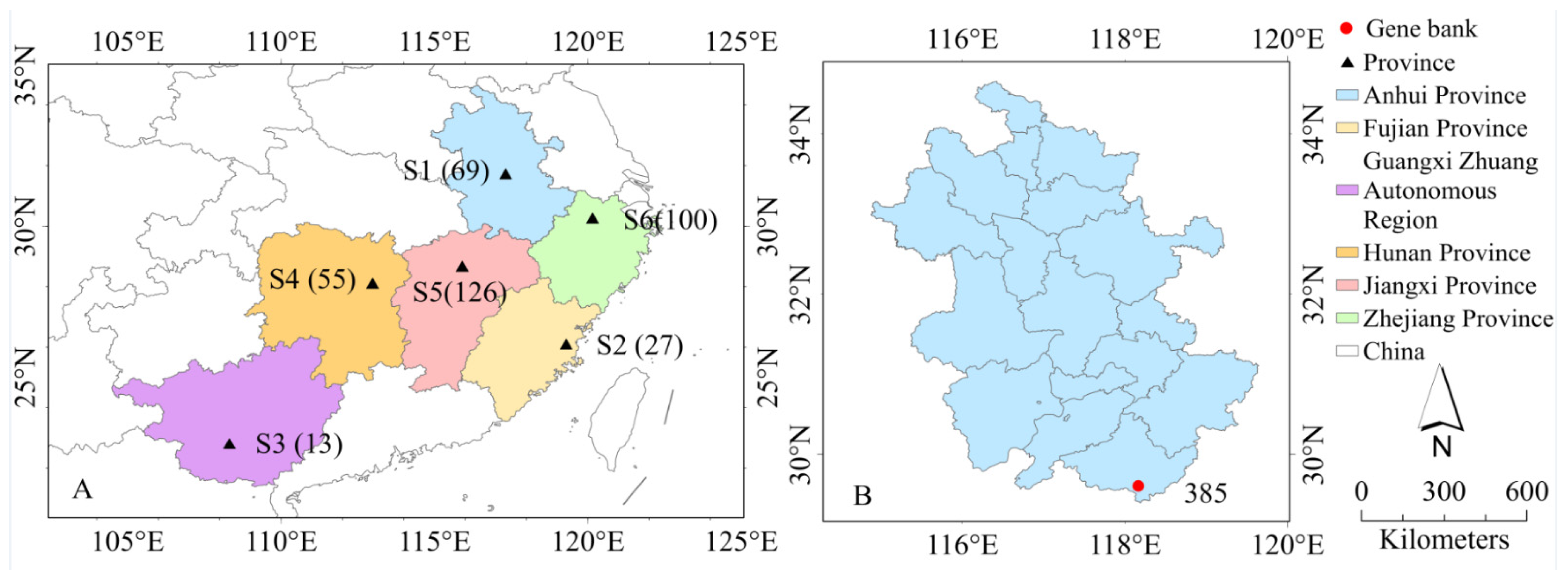
| Site | Characteristics of Stand | Characteristics of Climate | Soil Environment (0–40 cm) | |||
|---|---|---|---|---|---|---|
| Xitian Forest Farm, Xiuning County, Anhui Province, China | FC | Artificial pure forest | TC | North subtropical humid monsoon climate | TS | Red-yellow soil |
| MMP | 1613 mm | pH | 6.2 | |||
| AT | Since 1965 | AAT | 16.3 °C | SC | 12% | |
| FP | 230 d | TN | 5.29 mg·g−1 | |||
| SD | 4 m × 4 m | LD | 1937.42 h | TP | 0.83 mg·g−1 | |
| RAH | 72%–81% | OM | 36.38 mg·g−1 | |||
| Main Vegetation under the Forest | Loropetalum chinense, Rhododendron simsii, Eurya japonica, Actinodaphne nantoensis, Lespedeza bicolor; Rhus chinensis, Buxus sinica, Cyathea spinulosa, Zoysia japonica, Miscanthus floridulus, Setaria viridis, and Houttuynia cordata et al. | |||||
| Provenances | Climate Type | Elevation | Temp. | Precip. | Frost-Free Period | Sunshine Duration | Soil Type |
|---|---|---|---|---|---|---|---|
| S1 | Transitional areas between warm temperate and subtropical | 120 m | 16.3 °C | 1784.2 mm | 235 d | 1900 h | Yellow-brown soil, paddy soil, and red soil |
| S2 | Subtropical marine monsoon climate | 500 m | 19.0 °C | 1833.7 mm | 295 d | 1850 h | Red soil and latosolic red soil |
| S3 | Subtropical monsoon climate | 225 m | 18.8 °C | 1540.2 mm | 300 d | 1600 h | Red soil, yellow soil, and latosol |
| S4 | Continental subtropical monsoon humid climate | 543 m | 15.7 °C | 1463.3 mm | 290 d | 1550 h | Red soil, paddy soil, yellow soil, and fluvo-aquic soil |
| S5 | Warm and humid monsoon climate in central subtropical | 355 m | 19.8 °C | 1674.1 mm | 275 d | 1800 h | Red soil, yellow soil, and paddy soil |
| S6 | Subtropical monsoon humid climate | 410 m | 16.6 °C | 1478.2 mm | 255 d | 1950 h | Red soil, yellow soil, and paddy soil |
2.2. Data Collection
2.3. Growth Equations and the Fast-Growth Periods
2.4. BAI and Latewood Percentage
2.5. Biomass and Root-Shoot Ratio
3. Results
3.1. Growth Equations of DBH Across Different Provenances
3.2. Comparison of Fast-Growth Period
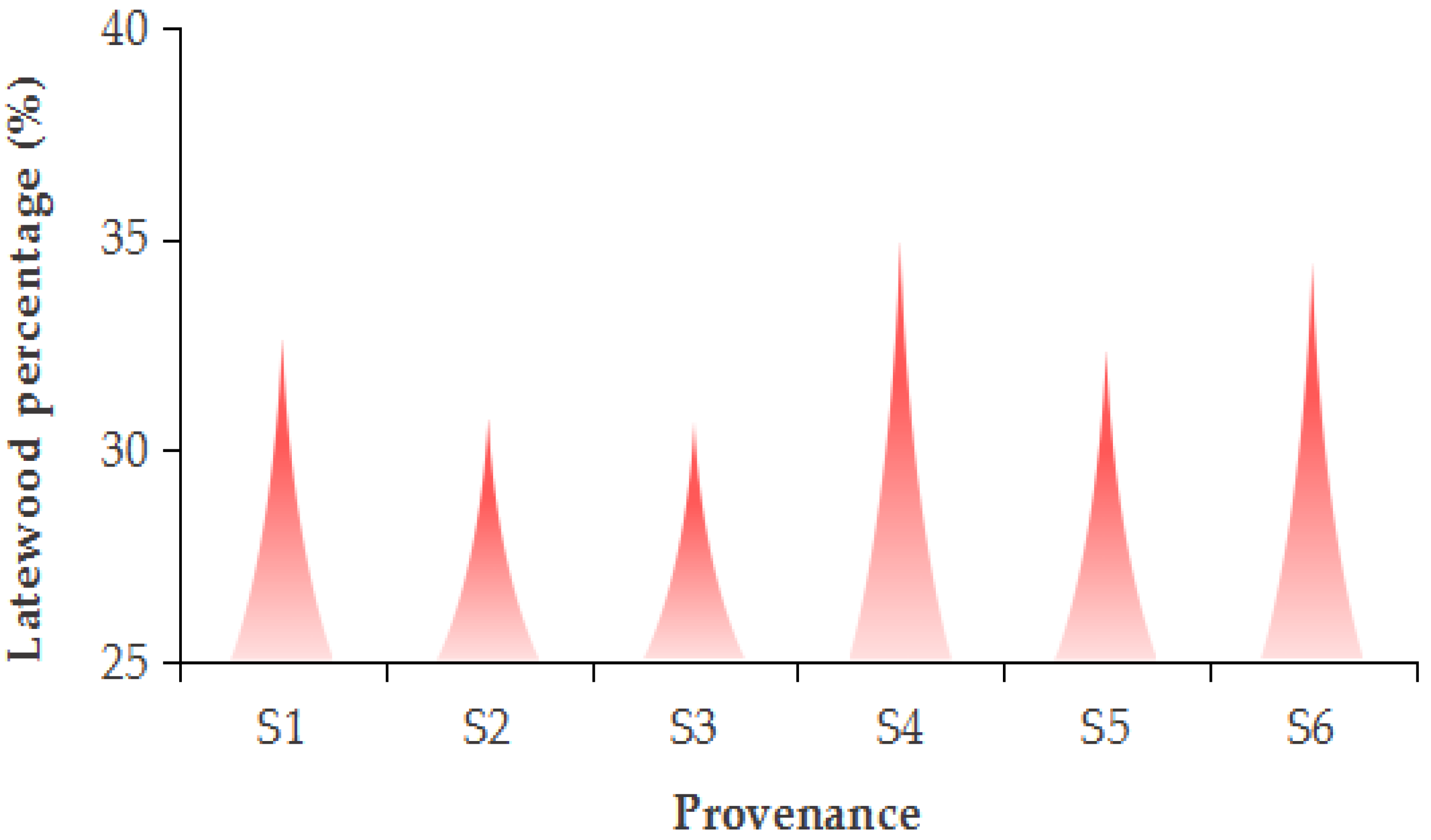
3.3. The BAI and Latewood Percentage of Different Provenances
3.4. Biomass of Different Provenances
3.5. The Root/Shoot Ratio of Different Provenances Changes with Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, S.; Li, Y.; Chang, S.X.; Li, Y.; Yang, W.; Fu, W.; Liu, J.; Jiang, P.; Lin, Z. Soil autotrophic and heterotrophic respiration respond differently to land-use change and variations in environmental factors. Agric. For. Meteorol. 2018, 250, 290–298. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; Roberge, G.; Feng, L.; Pan, C.; McNie, P.; Yu, Y. The negative effect of Chinese fir (Cunninghamia lanceolata) monoculture plantations on soil physicochemical properties, microbial biomass, fungal communities, and enzymatic activities. For. Ecol. Manag. 2022, 519, 120297. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, H.; Tao, S.; Su, Y.; Guan, H.; Ren, Y.; Hu, T.; Li, W.; Xu, G.; Chen, M.; et al. Carbon storage through China’s planted forest expansion. Nat. Commun. 2024, 15, 4106. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shrestha, A.; Wang, G.; Wang, T. Site-based climate-smart tree species selection for forestation under climate change. Clim. Smart Agric. 2024, 1, 100019. [Google Scholar] [CrossRef]
- Van Dijk, A.I.J.M.; Keenan, R.J. Planted forests and water in perspective. For. Ecol. Manag. 2007, 251, 1–9. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.U.; Tigabu, M.; Gilani, M.M.; Zou, X.H.; Wu, P.F. Chinese fir (Cunninghamia lanceolata) a green gold of china with continues decline in its productivity over the successive rotations: A review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- Forest, F.; Moat, J.; Baloch, E.; Brummitt, N.A.; Bachman, S.P.; Ickert-Bond, S.; Ickert-Bond, P.M.; Liston, A.; Little, D.P.; Mathews, S.; et al. Gymnosperms on the EDGE. Sci. Rep. 2018, 8, 6053. [Google Scholar] [CrossRef]
- Xiang, W.; Li Xu, L.; Lei, P.; Ouyang, S.; Deng, X.; Chen, L.; Zeng, Y.; Hu, Y.; Zhao, Z.; Wu, H.; et al. Rotation age extension synergistically increases ecosystem carbon storage and timber production of Chinese fir plantations in southern China. J. Environ. Manag. 2022, 317, 115426. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Aguila, L.C.R.; Wu, D.; Lie, Z.; Xu, W.; Tang, X.; Liu, J. Carbon sequestration and storage capacity of Chinese fir at different stand ages. Sci. Total Environ. 2023, 904, 166962. [Google Scholar] [CrossRef]
- Ma, X.Q.; Liu, C.J.; Ilvesniemi, H.; Carl, J.W.; Liu, A.Q. Biomass, litterfall and the nutrient fluxes in Chinese fir stands of different age in subtropical China. J. For. Res. 2002, 13, 165–170. [Google Scholar]
- Wang, Z.; Zhang, X.; Chhin, S.; Zhang, J.; Duan, A. Disentangling the effects of stand and climatic variables on forest productivity of Chinese fir plantations in subtropical China using a random forest algorithm. Agric. For. Meteorol. 2021, 304–350, 108412. [Google Scholar] [CrossRef]
- Wei, X.H.; Blanco, J.A.; Jiang, H.; Kimmins, J.P.H. Effects of nitrogen deposition on carbon sequestration in Chinese fir forest ecosystems. Sci. Total Environ. 2012, 416, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, Q.V.; Duan, A.; Zhang, J. Self-thinning trajectories of Chinese fir plantations in southern China. For. Sci. 2012, 62, 594–599. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; Shao, Q.; Xu, X. Carbon sequestration by forestation across China: Past, present, and future. Renew. Sustain. Energy Rev. 2012, 16, 1291–1299. [Google Scholar] [CrossRef]
- Chen, X.; Ju, Q.; Lin, K. Development status, issues and countermeasures of China’s plantation. World For. Res. 2014, 27, 54–59. [Google Scholar]
- Libby, W.J.; Rauter, R.M. Advantages of clonal forestry. For. Chron. 1984, 60, 145–149. [Google Scholar] [CrossRef]
- Kang, X. Cognition and suggestions on some issues related to clonal forestry: Taking poplar as an example. J. Beijing For. Univ. 2017, 9, 1–7. (In Chinese) [Google Scholar]
- Zhang, J.; Li, M.; Li, Y.; Liu, L.; Fei, Y.; Cao, G. Effects of mechanical damage treatment on the tillering ability and endogenous hormone content of Chinese fir clones. J. Nanjing For. Univ. 2021, 2, 153–158. (In Chinese) [Google Scholar]
- Jing, Y.; Bian, L.; Zhang, X.; Zhao, B.; Zheng, R.; Su, S.; Ye, D.; Zheng, X.; El-Kassaby, Y.A.; Shi, J. Genetic diversity and structure of the 4th cycle breeding population of Chinese fir (Cunninghamia lanceolata (lamb.) hook). Front. Plant Sci. 2023, 14, 1106615. [Google Scholar] [CrossRef]
- Qi, M. Achievements, problems and countermeasures of clonal selection and breeding of Chinese Fir in China. World For. Res. 2007, 20, 50–55. (In Chinese) [Google Scholar]
- Chen, X.; Li, B. Testing the allometric scaling relationships with seedlings of two tree species. Acta Oecol. 2003, 24, 125–129. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Magni, C.R.; Santelices, R.E.; Ivković, M.; Cabrera, A.M. Changes in drought tolerance of Pinus radiata in Chile associated with provenance and breeding generation. Ann. For. Sci. 2016, 73, 267–275. [Google Scholar] [CrossRef]
- Kaya, Z.; Lindgren, D. The genetic variation of inter-provenance hybrids of Picea abies and possible breeding consequences. Scand. J. For. Res. 1992, 7, 15–26. [Google Scholar] [CrossRef]
- Gao, C.; Liu, C.; Chen, C.; Liu, N.; Liu, F.; Su, X.; Huang, Q. Genetic evaluation of water use efficiency and nutrient use efficiency in Populus deltoides Bartr. ex Marsh. Seedlings in China. Plants 2024, 13, 2228. [Google Scholar] [CrossRef]
- Buras, A.; Sass-Klaassen, U.; Verbeek, I.; Copini, P. Provenance selection and site conditions determine growth performance of pedunculate oak. Dendrochronologia 2020, 61, 125705. [Google Scholar] [CrossRef]
- Leštianska, A.; Fleischer, P., Jr.; Merganičová, K.; Fleischer, S.P.; Nalevanková, P.; Střelcová, K. Effect of provenance and environmental factors on tree growth and tree water status of Norway spruce. Forests 2023, 14, 156. [Google Scholar] [CrossRef]
- Park, A.; Rodgers, J.L. Provenance trials in the service of forestry assisted migration: A review of North American field trials and experiments. For. Ecol. Manag. 2023, 537, 120854. [Google Scholar] [CrossRef]
- Hao, Z.; Hao, F.; Singh, V.P.; Zhang, X. Changes in the severity of compound drought and hot extremes over global land areas. Environ. Res. Lett. 2018, 13, 124022. [Google Scholar] [CrossRef]
- Xu, R.; Liu, J.; Wang, L.; Yan, Y.; Ma, X.; Li, M. Analysis of root and leaf functional traits and C, N, P stoichiometry of Cunninghamia lanceolate from different provenances. Acta Ecol. Sin. 2022, 42, 6298–6310. [Google Scholar]
- O’brien, E.K.; Mazanec, R.A.; Krauss, S.L. Provenance variation of ecologically important traits of forest trees: Implications for restoration. J. Appl. Ecol. 2007, 44, 583–593. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, X.; Wang, B. Study on Leaf morphological and stoichiometric traits of Cunninghamia lanceolata based on different provenances. Sustainability 2024, 16, 4236. [Google Scholar] [CrossRef]
- Luo, H.; Luo, J.; Feng, H.; Xiao, M.; Yang, S.; Li, Y.; Liu, J.; Li, X. Restoration dynamics of medicinal plant species diversity in Cunninghamia. Agric. Technol. 2024, 30, 35–45. [Google Scholar]
- Qu, Y.; Wang, H.; Dean, T.J.; Zhang, J.; Zhang, X. Growth dominance and growth efficiency in response to thinning treatments in Chinese fir plantations with long-term spacing trials. For. Ecol. Manag. 2022, 521, 120438. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; McNie, P.; Liu, Q.; Xu, X.; Pan, C.; Yan, K.; Feng, L.; Goitom, E.A.; Yu, Y. Species mixing improves soil properties and enzymatic activities in Chinese fir plantations: A meta-analysis. Catena 2023, 220, 106723. [Google Scholar] [CrossRef]
- Yan, Y.; Li, B.; He, Z.; Liu, Y.; Li, M.; Yu, X.; Ma, X. Comparative sStudy on wood characteristics of Cunninghamia lanceolata from different provenances. For. Res. 2021, 34, 49–57. [Google Scholar]
- Li, B.; Yan, Y.; Zhang, H.; He, Z.; Liu, Y.; Ma, X. Comparison of fine root morphology and carbon and nitrogen content in Chinese fir from different provenances. J. For. Environ. 2019, 39, 561–567. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in treering dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Dale, V.H.; Doyle, T.W.; Shugart, H.H. A comparison of tree growth models. Ecol. Model. 1985, 29, 145–169. [Google Scholar] [CrossRef]
- Shoda, T.; Imanishi, J.; Shibata, S. Growth characteristics and growth equations of the diameter at breast height using tree ring measurements of street trees in Kyoto City, Japan. Urban For. Urban Gree. 2020, 49, 126627. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Z.; Chen, P.; Chen, X. Development of a tree growth difference equation and its application in forecasting the biomass carbon stocks of Chinese forests in 2050. Forests 2019, 10, 582. [Google Scholar] [CrossRef]
- Gregorczyk, A. The logistic function-its application to the description and prognosis of plant growth. Acta Soc. Bot. Pol. 1991, 60, 67–76. [Google Scholar] [CrossRef]
- Sun, F.; Kuang, Y.; Wen, D.; Xu, Z.; Li, J.; Zuo, W.; Hou, E. Long-term tree growth rate, water use efficiency, and tree ring nitrogen isotope composition of Pinus massoniana L. in response to global climate change and local nitrogen deposition in Southern China. J. Soils. Sediment. 2010, 10, 1453–1465. [Google Scholar] [CrossRef]
- Francisco-Fernández, M.; Tarrío-Saavedra, J.; Naya, S.; López-Beceiro, J.; Artiaga, R. Statistical classification of early and late wood through the growth rings using thermogravimetric analysis. J. Therm. Anal. Calorim. 2017, 127, 499–506. [Google Scholar] [CrossRef]
- Ågren, G.I.; Ingestad, T. Root: Shoot ratio as a balance between nitrogen productivity and photosynthesis. Plant Cell Environ. 1987, 10, 579–586. [Google Scholar] [CrossRef]
- Lü, Z.; Duan, A. Biomass and carbon sturage modcl of Cunninghamia lancculata in diffcrent production areas. Sci. Silv. Sin. 2024, 60, 1–11. (In Chinese) [Google Scholar]
- Anderegg, L.D.L.; HilleRisLambers, J. Drought stress limits the geographic ranges of two tree species via different physiological mechanisms. Glob. Change Biol. 2016, 22, 1029–1045. [Google Scholar] [CrossRef]
- Hodge, G.R.; Dvorak, W.S. Provenance variation and within-provenance genetic parameters in Eucalyptus urophylla across 125 test sites in Brazil, Colombia, Mexico, South Africa and Venezuela. Tree Genet. Genomes 2015, 11, 1–18. [Google Scholar] [CrossRef]
- Matyas, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Yu, Z.; Ning, Z.; Yang, H. Carbon sequestration benefit and optimal rotation period determination of Cunninghamia lanceolata plantation under stochastic price. J. Nat. Resour. 2022, 37, 753–768. (In Chinese) [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, T.; Zhao, M.; Deng, Y.; Yang, G.; Ban, Y.; Lei, T.; Yu, X.; Huang, Y. Influence of soil properties and near-surface roots on soil infiltration process in short-rotation eucalyptus plantations in southern subtropical China. Catena 2024, 234, 107606. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, P.; Wang, D.; Xu, Z. Density-and age-dependent influences of droughts and intrinsic water use efficiency on growth in temperate plantations. Agric. For. Meteorol. 2022, 325, 109134. [Google Scholar] [CrossRef]
- Liang, R.; Sun, Y.; Qiu, S.; Wang, B.; Xie, Y. Relative effects of climate, stand environment and tree characteristics onannual tree growth in subtropical Cunninghamia lanceolata forests. Agric. For. Meteorol. 2023, 342, 109711. [Google Scholar] [CrossRef]
- Mensah, A.A.; Holmström, E.; Petersson, H.; Nyström, K.; Mason, E.G.; Nilsson, U. The millennium shift: Investigating the relationship between environment and growth trends of Norway spruce and Scots pine in northern Europe. For. Ecol. Manag. 2021, 481, 118727. [Google Scholar] [CrossRef]
- Muigg, B.; Skiadaresis, G.; Tegel, W.; Herzig, F.; Krusic, P.J.; Schmidt, U.E.; Büntgen, U. Tree rings reveal signs of Europe’s sustainable forest management long before the first historical evidence. Sci. Rep. 2020, 10, 21832. [Google Scholar] [CrossRef]
- Smith, J.H.G.; Heger, L.; Hejjas, J. Patterns in growth of earlywood, latewood, and percentage latewood determined by complete analysis of 18 Douglas-fir trees. Can. J. Bot. 1966, 44, 453–466. [Google Scholar] [CrossRef]
- Bertaud, F.; Holmbom, B. Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci. Technol. 2004, 38, 245–256. [Google Scholar] [CrossRef]
- Sun, F.; Li, R.; Zhu, J.; Peng, H.; Li, Z.; Jiang, J.; Zhan, T.; Cai, L.; Lyu, J. Comparison analysis of moisture-dependent orthotropic elasticity between earlywood and latewood in Chinese fir using digital image correlation. Ind. Crop. Prod. 2024, 220, 119185. [Google Scholar] [CrossRef]
- Lasserre, J.P.; Mason, E.G.; Watt, M.S.; Moore, J.R. Influence of initial planting spacing and genotype on microfibril angle, wood density, fibre properties and modulus of elasticity in Pinus radiata D. Don corewood. For. Ecol. Manag. 2009, 258, 1924–1931. [Google Scholar] [CrossRef]
- Jiang, F.; Kutia, M.; Ma, K.; Chen, S.; Long, J.; Sun, H. Estimating the aboveground biomass of coniferous forest in Northeast China using spectral variables, land surface temperature and soil moisture. Sci. Total Environ. 2021, 785, 147335. [Google Scholar] [CrossRef] [PubMed]
- LaRue, E.A.; Knott, J.A.; Domke, G.M.; Chen, H.Y.H.; Guo, Q.; Hisano, M.; Oswalt, C.; Oswalt, S.; Kong, N.; Potter, K.M.; et al. Structural diversity as a reliable and novel predictor for ecosystem productivity. Front. Ecol. Environ. 2023, 21, 33–39. [Google Scholar] [CrossRef]
- Verónica, G.; Luis, P.P.; Gerardo, R. Allometric relations for biomass partitioning of Nothofagus antarctica trees of different crown classes over a site quality gradient. For. Ecol. Manag. 2010, 259, 1118–1126. [Google Scholar]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Ruger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef]
- Anderson, K.J.; Allen, A.P.; Gillooly, J.F.; Brown, J.H. Temperature-dependence of biomass accumulation rates during secondary succession. Ecol. Lett. 2006, 9, 673–682. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, D.W. Morphological canalization, integration, and plasticity in response to population density in Abutilon theophrasti: Influences of soil conditions and growth stages. Ecol. Evol. 2021, 11, 11945–11959. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Morgan, J.; Cannell, M.G.R. Shape of tree stems—A re-examination of the uniform stress hypothesis. Tree Physiol. 1994, 14, 49–62. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turne, N.C.; Ong, C.K. The redistribution of soil water by tree root systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef]
- He, D.; Deane, D.C. The relationship between trunk-and twigwood density shifts with tree size and species stature. For. Ecol. Manag. 2016, 372, 137–142. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, Z.; Li, H.; Wang, W.; Xu, F.; Sun, K.; Chu, J.; Qian, Y.; Loake, G.J.; Chu, C.; et al. An activated form of NB-ARC protein RLS1 functions with cysteine-rich receptor-like protein RMC to trigger cell death in rice. Plant Commun. 2022, 4, 100459. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lei, X.; Xu, Q.; Lan, J.; Wu, B.; Guo, H.; Gao, W.; Liu, D. Combining multiple feature selection methods and structural equation modelling for exploring factors affecting stand biomass of natural coniferous-broad leaved mixed forests. Sci. Total Environ. 2024, 954, 176669. [Google Scholar] [CrossRef] [PubMed]
- Teklehaimanot, Z.; Lanek, J.; Tomlinson, H.F. Provenance variation in morphology and leaflet anatomy of Parkia biglobosa and its relation to drought tolerance. Trees 1998, 13, 96–102. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Liu, W.; Feng, B.; Lv, W.; Zhang, Z.; Yang, X.; Dong, Q. Plant biomass partitioning in alpine meadows under different herbivores as influenced by soil bulk density and available nutrients. Catena 2024, 240, 108017. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, R.; Lin, L.; Russo, S.E. Functional traits and ecological niches as correlates of the interspecific growth-mortality trade-off among seedlings of 14 tropical tree species. Funct. Ecol. 2024, 38, 1888–1901. [Google Scholar] [CrossRef]
- Cong, Y.; Saurer, M.; Bai, E.; Siegwolf, R.; Gessler, A.; Liu, K.; Han, H.; Dang, Y.; Xu, W.; He, H.S.; et al. In situ 13CO2 labeling reveals that alpine treeline trees allocate less photoassimilates to roots compared with low-elevation trees. Tree Physiol. 2022, 42, 1943–1956. [Google Scholar] [CrossRef]
- Geng, Q.; Ma, X.; Liao, J.; Wu, W.; Niu, S.; Luo, Y.; Xu, X. Contrasting nutrient-mediated responses between surface and deep fineroot biomass to N addition in poplar plantations on the east coast of China. For. Ecol. Manag. 2021, 490, 119152. [Google Scholar] [CrossRef]
- Fang, C.; Verbrigghe, N.; Sigurdsson, B.D.; Ostonen, I.; Leblans, N.I.W.; Mara~non-Jimenez, S.; Fuchslueger, L.; Sigurðsson, P.; Meeran, K.; Portillo-Estrada, M.; et al. Decadal soil warming decreased vascular plant above andbelowground production in a subarctic grassland by inducingnitrogen limitation. New Phytol. 2023, 240, 565–576. [Google Scholar] [CrossRef]
- Bacher, H.; Sharaby, Y.; Walia, H.; Peleg, Z. Modifying root-to-shoot ratio improves root water influxes in wheat under drought stress. J. Exp. Bot. 2022, 73, 1643–1654. [Google Scholar] [CrossRef]
- Kelly, J.W.G.; Landhäusser, S.M.; Chow, P.S. The impact of light quality and quantity on root-to-shoot ratio and root carbon reserves in aspen seedling stock. New Forest. 2015, 46, 527–545. [Google Scholar] [CrossRef]
- Zadworny, M.; Mucha, J.; Bagniewska-Zadworn, A.; Żytkowiak, R.; Mąderek, E.; Danusevičius, D.; Oleksyn, J.; Wyka, T.P.; McCormack, M.L. Higher biomass partitioning to absorptive roots improves needle nutrition but does not alleviate stomatal limitation of northern Scots pine. Global Change Biol. 2021, 27, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Z.; Su, Z.; Du, G.; Bai, W.; Wang, Q.; Wang, R.; Nie, J.; Sun, T.; Feng, Z.; et al. Root plasticity and interspecific complementarity improve yields and water use efficiency of maize/soybean intercropping in a water-limited condition. Field Crop. Res. 2022, 282, 108523. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B. Multi-element composition of wheat grain and provenance soil and their potentialities as fingerprints of geographical origin. J. Cereal Sci. 2013, 57, 391–397. [Google Scholar] [CrossRef]
- Persson, B.; Beuker, E. Distinguishing between the effects of changes in temperature and light climate using provenance trials with Pinus sylvestris in Sweden. Can. J. For. Res. 1997, 27, 572–579. [Google Scholar] [CrossRef]
- Pandey, H.N.; Dubey, S.K. Achene germination of Parthenium hysterophorus L.: Effects of light, temperature, provenance and achene size. Weed Res. 1988, 28, 185–190. [Google Scholar] [CrossRef]
- Sun, S.; Xiang, W.; Zhao, Z.; Zhao, Z.; Deng, X.; Ouyang, S.; Chen, L.; Hu, Y.; Zeng, Y.; Peng, C. Long-term field observations of the impacts of drought and stand development on runoff in a forested watershed. Agric. For. Meteorol. 2025, 367, 110519. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, Q.; Sun, J.; Robinson, D.; Yang, Z.; Yao, X.; Wang, X.; Dai, X.; Chen, T.; Wu, D.; et al. Contrasting depth-related fine root plastic responses to soil warming in a subtropical Chinese fir plantation. J. Ecol. 2024, 112, 1058–1073. [Google Scholar] [CrossRef]
- Qu, Z.; Lin, C.; Zhao, H.; Chen, T.; Yao, X.; Wang, X.; Yang, Y.; Chen, G. Above-and belowground phenology responses of subtropical Chinese fir (Cunninghamia lanceolata) to soil warming, precipitation exclusion and their interaction. Sci. Total Environ. 2024, 933, 173147. [Google Scholar] [CrossRef]
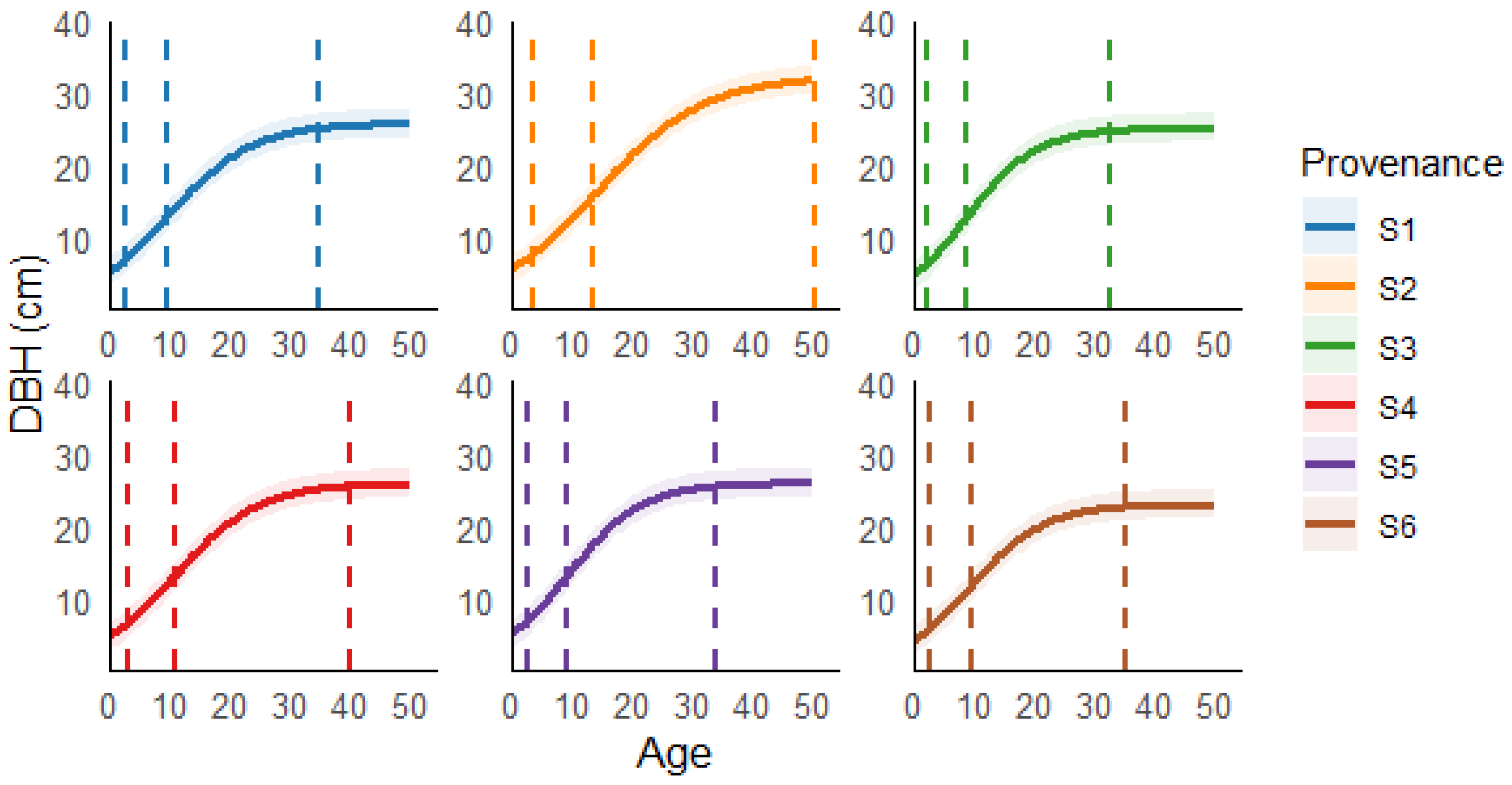



| Provenance | a | b | c | R2 | RMSE | AIC | TRE | MAE |
|---|---|---|---|---|---|---|---|---|
| S1 | 26.00 | 3.83 | 0.14 | 0.67 | 4.68 | 12,450.86 | 21.61 | 3.62 |
| S2 | 32.55 | 4.72 | 0.11 | 0.85 | 3.32 | 4273.59 | 15.52 | 2.74 |
| S3 | 25.30 | 4.37 | 0.17 | 0.85 | 2.83 | 1787.44 | 12.99 | 2.18 |
| S4 | 26.07 | 4.51 | 0.14 | 0.77 | 3.77 | 9606.61 | 18.41 | 3.01 |
| S5 | 26.08 | 4.33 | 0.16 | 0.74 | 4.16 | 22,795.11 | 19.11 | 3.33 |
| S6 | 23.12 | 4.55 | 0.16 | 0.72 | 3.87 | 16,701.82 | 19.86 | 2.98 |
| Provenance | (ta, ga) | (ti, gi) | (td, gd) | ΔT |
|---|---|---|---|---|
| S1 | (3, 5) | (9, 13) | (35, 21) | 33 |
| S2 | (4, 7) | (14, 16) | (51, 26) | 47 |
| S3 | (2, 5) | (9, 13) | (33, 20) | 30 |
| S4 | (3, 6) | (11, 13) | (40, 21) | 37 |
| S5 | (2, 6) | (9, 13) | (34, 21) | 32 |
| S6 | (3, 5) | (9, 12) | (35, 18) | 33 |
| Age Group | Provenance | Biomass Levels of the Different Organs (kg) | |||
|---|---|---|---|---|---|
| Root | Branch | Leaf | Trunk | ||
| 0–10 | S1 | 6.03 ± 5.93 bc | 1.49 ± 1.95 bc | 1.35 ± 1.53 bc | 17.05 ± 17.96 c |
| S2 | 5.43 ± 4.85 a | 1.27 ± 1.47 a | 1.19 ± 1.20 a | 15.27 ± 15.03 a | |
| S3 | 5.76 ± 4.95 bc | 1.36 ± 1.53 bc | 1.27 ± 1.24 bc | 16.30 ± 15.26 bc | |
| S4 | 4.58 ± 4.49 c | 1.04 ± 1.34 c | 0.99 ± 1.11 c | 12.66 ± 13.84 bc | |
| S5 | 5.94 ± 5.61 b | 1.46 ± 1.77 b | 1.33 ± 1.42 b | 16.85 ± 17.24 b | |
| S6 | 4.35 ± 4.54 d | 0.98 ± 1.40 d | 0.94 ± 1.13 d | 11.96 ± 13.85 d | |
| 11–20 | S1 | 20.24 ± 12.44 bc | 6.76 ± 5.53 bc | 5.22 ± 3.69 bc | 57.89 ± 32.05 c |
| S2 | 19.84 ± 9.18 a | 6.39 ± 3.83 a | 5.04 ± 2.65 a | 57.80 ± 25.02 a | |
| S3 | 19.70 ± 7.55 bc | 6.25 ± 3.13 bc | 4.97 ± 2.17 bc | 57.91 ± 20.72 bc | |
| S4 | 17.87 ± 9.26 c | 5.63 ± 3.81 c | 4.49 ± 2.65 c | 52.19 ± 25.57 bc | |
| S5 | 21.10 ± 10.75 b | 7.00 ± 4.67 b | 5.43 ± 3.16 b | 60.83 ± 28.42 b | |
| S6 | 15.79 ± 9.54 d | 4.85 ± 3.96 d | 3.92 ± 2.74 d | 46.09 ± 26.16 d | |
| 21–30 | S1 | 31.46 ± 17.13 bc | 11.92 ± 8.68 bc | 8.60 ± 5.40 bc | 85.64 ± 38.45 c |
| S2 | 36.26 ± 12.58 a | 13.92 ± 6.34 a | 9.99 ± 3.96 a | 98.55 ± 28.19 a | |
| S3 | 32.32 ± 9.26 bc | 11.87 ± 4.36 bc | 8.73 ± 2.83 bc | 90.13 ± 22.42 bc | |
| S4 | 28.93 ± 12.14 c | 10.45 ± 5.77 c | 7.75 ± 3.72 c | 81.05 ± 29.20 bc | |
| S5 | 33.66 ± 14.62 b | 12.80 ± 7.20 b | 9.23 ± 4.55 b | 91.73 ± 33.82 b | |
| S6 | 26.79 ± 12.94 d | 9.53 ± 6.13 d | 7.12 ± 3.96 d | 75.32 ± 31.35 d | |
| 31–40 | S1 | 41.84 ± 19.06 bc | 17.10 ± 10.25 bc | 11.86 ± 6.18 bc | 109.12 ± 39.58 c |
| S2 | 59.48 ± 14.09 a | 26.42 ± 8.14 a | 17.53 ± 4.73 a | 146.13 ± 26.31 a | |
| S3 | 42.60 ± 10.40 bc | 17.00 ± 5.58 bc | 11.96 ± 3.37 bc | 113.29 ± 21.33 bc | |
| S4 | 41.77 ± 15.66 c | 16.85 ± 8.28 c | 11.77 ± 5.04 c | 110.06 ± 32.98 bc | |
| S5 | 44.86 ± 18.18 b | 18.61 ± 9.75 b | 12.80 ± 5.89 b | 115.85 ± 37.75 b | |
| S6 | 33.49 ± 13.12 d | 12.62 ± 6.47 d | 9.15 ± 4.08 d | 91.86 ± 30.45 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Z.; Liu, N.; Liu, C.; Zhang, M.; Gao, C.; Yang, L.; Yao, L.; Zhang, X. Comparison of Growth Strategies and Biomass Allocation in Chinese Fir Provenances from the Subtropical Region of China. Forests 2025, 16, 687. https://doi.org/10.3390/f16040687
Wan Z, Liu N, Liu C, Zhang M, Gao C, Yang L, Yao L, Zhang X. Comparison of Growth Strategies and Biomass Allocation in Chinese Fir Provenances from the Subtropical Region of China. Forests. 2025; 16(4):687. https://doi.org/10.3390/f16040687
Chicago/Turabian StyleWan, Zhibing, Ning Liu, Chenggong Liu, Meiman Zhang, Chengcheng Gao, Lingyu Yang, Liangjin Yao, and Xueli Zhang. 2025. "Comparison of Growth Strategies and Biomass Allocation in Chinese Fir Provenances from the Subtropical Region of China" Forests 16, no. 4: 687. https://doi.org/10.3390/f16040687
APA StyleWan, Z., Liu, N., Liu, C., Zhang, M., Gao, C., Yang, L., Yao, L., & Zhang, X. (2025). Comparison of Growth Strategies and Biomass Allocation in Chinese Fir Provenances from the Subtropical Region of China. Forests, 16(4), 687. https://doi.org/10.3390/f16040687






