Clonal Variation in Growth, Physiology and Ultrastructure of Populus alba L. Seedlings Under NaCl Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Growth Conditions
2.2. Determination of Relative Growth Rate, Gas Exchange and Chlorophyll Concentrations
2.3. Determination of Water Relations and Osmotic Adjustment
2.4. Determination of Soluble Sugars, Starch and Proline
2.5. Determination of Na+, K+ and Cl− Concentrations
2.6. Ultrastructure of Chloroplasts
2.7. Statistical Analyses
3. Results
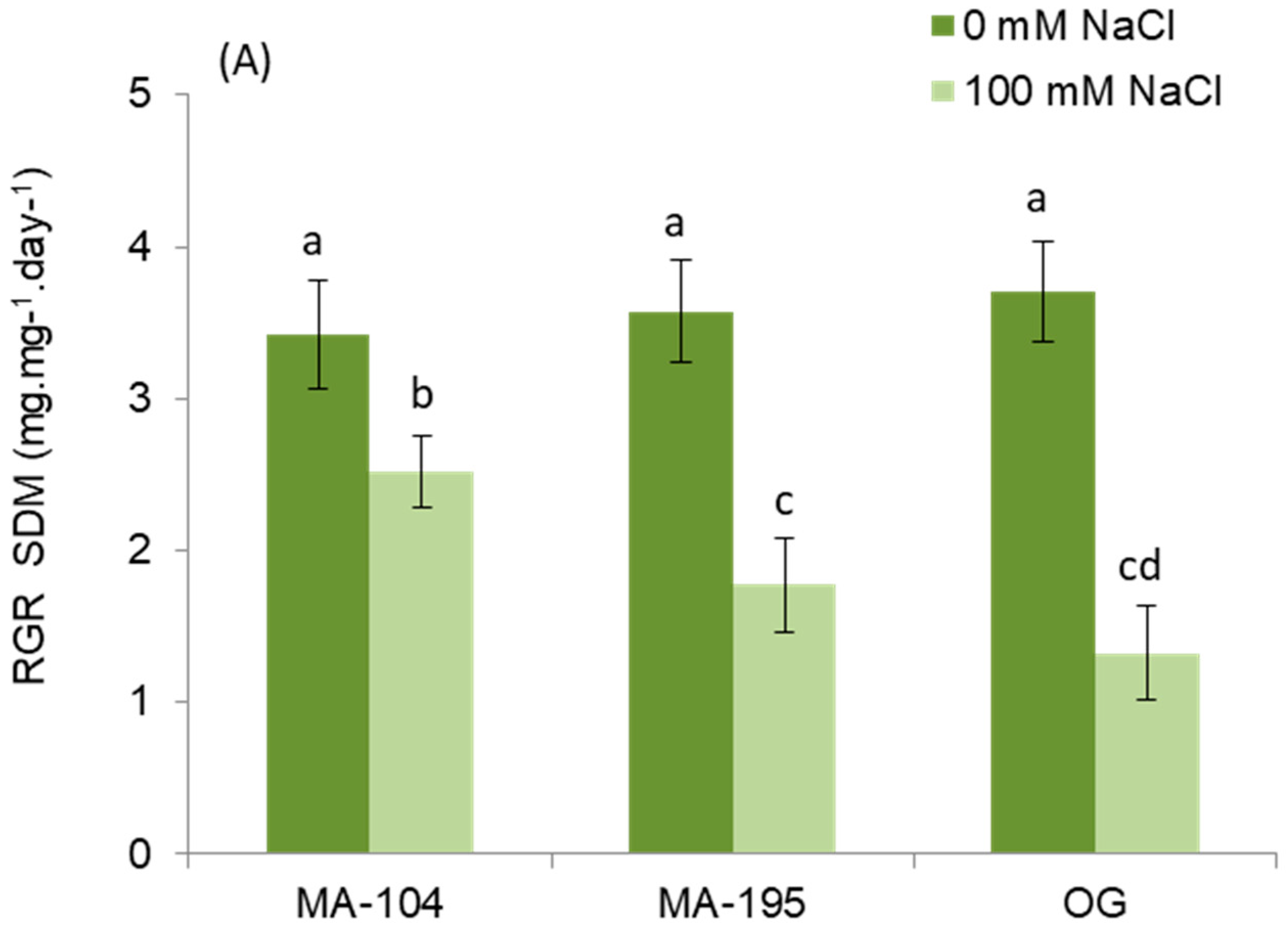
3.1. Effect of NaCl Salinity on Dry Mass Production
3.2. Effect of NaCl Salinity on Gas Exchanges and Chlorophyll Concentration
3.3. Effect of NaCl Salinity on Water Relations
3.4. Effects of NaCl Salinity on Soluble Sugars, Starch and Proline Accumulation
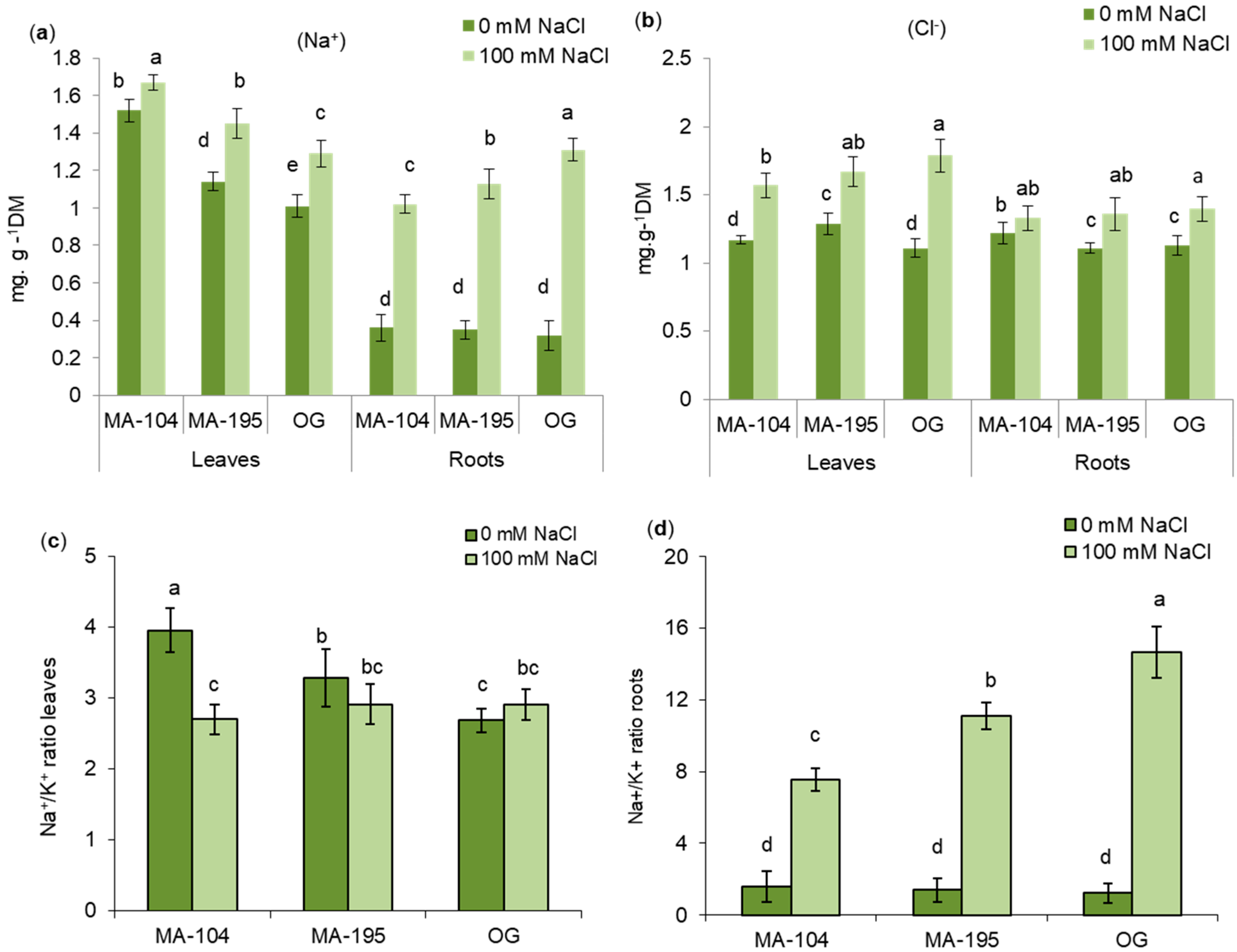
3.5. Effects of NaCl Salinity on Na+, K+ and Cl− Distribution
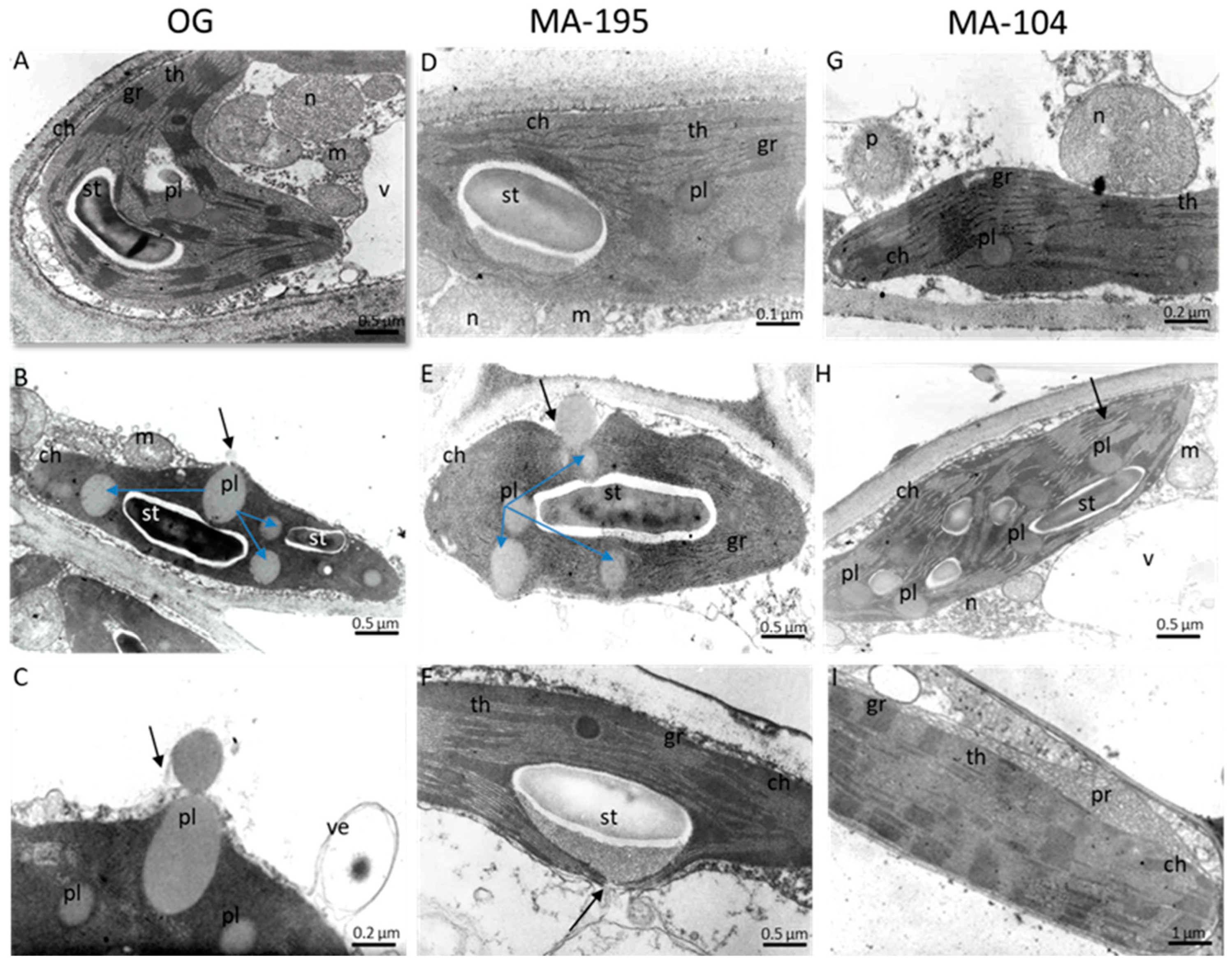
3.6. Effects of NaCl Salinity on Chloroplast Ultrastructure
4. Discussion
4.1. Clonal Variation in Dry Mass Production and Na+, K+ and Cl− Distribution
4.2. Osmotic Adjustment and Water Relations
4.3. Gas Exchange, Chlorophyll Concentration and Chloroplast Ultrastructure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PGIP. Projet de Gestion Intégrée des Paysages dans les Régions les moins Développées en Tunisie, 2017—Banque Mondiale, Environnement & Ressources Naturelles-Moyen Orient et Afrique du Nord. Available online: https://documents.banquemondiale.org/fr/publication/documents-reports/documentdetail/311681484719969266/cadre-de-gestion-environnementale-et-sociale (accessed on 4 March 2025).
- IPCC. In-Depth Q&A: The IPCC’s Special Report on Climate Change and Land; IPCC: Paris, France, 2019. [Google Scholar]
- FAO. Global Forest Resources Assessment. 2020, Main Report. Rome, Food and Agriculture Organization of the UnitedNations: 184. Available online: https://openknowledge.fao.org/items/d6f0df61-cb5d-4030-8814-0e466176d9a1 (accessed on 18 November 2020).
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- FAO; ICBA. Thematic 1: Farmers’ Guidelines on Soil and Water Management in Salt-Affected Areas; FAO: Rome, Italy, 2023. [Google Scholar]
- Hachicha, M. Les sols salés et leur mise en valeur en Tunisie. Sécheresse 2007, 18, 45–50. [Google Scholar]
- PIF. Programme D’investissement du PIF de la Tunisie, Version du 30 Septembre 2016. Available online: https://www.cif.org/sites/default/files/meeting-documents/fip_tunisia_joint_mission_aide_memoire_june_20-24_2016french.pdf (accessed on 4 March 2025).
- Minhas, P.; Yadav, R.; Bali, A. Perspectives on reviving waterlogged and saline soils through plantation forestry. Agric. Water Manag. 2020, 232, 106063. [Google Scholar] [CrossRef]
- Imada, S.; Yamanaka, N.; Tamai, S. Effects of salinity on the growth, Na partitioning, and Na dynamics of a salt-tolerant tree, Populus alba L. J. Arid Environ. 2009, 73, 245–251. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Response of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xia, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in Understanding the Physiological and Molecular Responses of Populus to Salt Stress. Int. J. Mol. Sci. 2019, 20, 1312. [Google Scholar] [CrossRef]
- Rood, S.B.; Braatne, J.H.; Hughes, F.M.R. Ecophysiology of riparian cottonwoods: Stream flow dependency, water relations and restoration. Tree Physiol. 2003, 23, 1113–1124. [Google Scholar] [CrossRef]
- Sixto, H.; González-González, B.D.; Molina-Rueda, J.J.; Garrido-Aranda, A.; Sanchez, M.M.; López, G.; Gallardo, F.; Cañellas, I.; Mounet, F.; Grima-Pettenati, J.; et al. Eucalyptus spp. and Populus spp. coping with salinity stress: An approach on growth, physiological and molecular features in the context of short rotation coppice (SRC). Trees 2016, 30, 1873–1891. [Google Scholar] [CrossRef]
- Abassi, M.; Mguis, K.; Béjaoui, Z.; Albouchi, A. Morphogenetic responses of Populus alba L. under salt stress. J. For. Res. 2014, 25, 155–161. [Google Scholar] [CrossRef]
- Sixto, H.; Grau, J.M.; Alba, N.; Alia, R. Response to sodium chloride in different species and clones of genus Populus L. Forestry 2005, 78, 93–104. [Google Scholar] [CrossRef]
- Beritognolo, I.; Piazzai, M.; Bencci, S.; Kuzminsky, E.; Sabatti, M.; Scarascia, G.; Muleo, R. Functional characterization of three Italian Populus alba L. genotypes under salinity stress. Trees 2007, 21, 465–477. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effect on plants: A review. Exotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429–430. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, S.; Li, Y. Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. J. Exp. Bot. 2019, 70, 5259–5269. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Min, Y.; Kunetsov, V.V.; Alakhverdiev, S.I.; Shabala, S.S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2020, 51, 791–825. [Google Scholar] [CrossRef]
- Dourado, P.R.M.; de Souza, E.R.; Santos, M.A.d.; Lins, C.M.T.; Monteiro, D.R.; Paulino, M.K.S.S.; Schaffer, B. Stomatal Regulation and Osmotic Adjustment in Sorghum in Response to Salinity. Agriculture 2022, 12, 658. [Google Scholar] [CrossRef]
- Navarro, A.; Bañón, E.; Olmos, E.; Sánchez-Blanco, M.J. Effects of sodium chloride on water potential components, hydraulic conductivity, gas exchange and leaf ultrastructure of Arbutus unedo plants. Plant Sci. 2007, 172, 473–480. [Google Scholar] [CrossRef]
- Al Yazi, H.; Attia, H.; Alamer, K.; Hassan, F.K.; Siddique, H.M.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Massange-Sanchez, J.A.; Sanchez-Hernandez, C.V.; Hernandez-Herrera, M.H.; Palmeros-Suarez, P.A. Chapter: The Biochemical Mechanisms of Salt Tolerance in Plants. In Plant Stress Physiology-Perspectives in Agriculture; Intech Open: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Marshner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: San Diego, CA, USA, 1995; 423p. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Abassi, M.; Albouchi, A.; Ben Mansoura, A.; Béjaoui, Z.; Rejeb, M.N.; Mougou, A. Tolérance de divers clones de Peuplier à la salinité. Annales l’INRGREF 2004, 6, 17–34. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Hunt, R. Plant Growth Curves: A Functional Approach to Plant Growth Analysis; Edward Arnold: London, UK, 1982. [Google Scholar]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Berry, J.A.; Downton, W.J.S. Environmental regulation of photosynthesis. In Photosynthesis: Development, Carbon Metabolism, and Plant Productivity; Govindjee, Ed.; Academic Press: New York, NY, USA, 1982; pp. 263–343. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Ritchie, G.A.; Hinckley, T.M. The pressure chamber as an instrument for ecological research. Adv. Ecol. Res. 1975, 9, 165–254. [Google Scholar]
- Hachani, C.; Lamhamedi, M.S.; Zine El Abidine, A.; Abassi, M.; Khasa, D.P.; Béjaoui, Z. Water Relations, Gas Exchange, Chlorophyll Fluorescence and Electrolyte Leakage of Ectomycorrhizal Pinus halepensis Seedlings in Response to Multi-Heavy Metal Stresses (Pb, Zn, Cd). Microorganisms 2022, 10, 57. [Google Scholar] [CrossRef]
- Nabil, M.; Coudret, A. Effects of sodium chloride on growth, tissue elasticity and solute adjustment in two Acacia subspecies. Physiol. Plant. 1995, 93, 217–224. [Google Scholar] [CrossRef]
- Albouchi, A.; Ghrir, R.; El Aouni, M.H. Endurcissement à la sécheresse et accumulation de glucides solubles et d’acides aminés libres dans les phyllodes d’Acacia cyanophylla Lindl. Ann. Sci. For. 1977, 54, 155–168. [Google Scholar] [CrossRef]
- Troll, W.; Lindsey, J. A photometric method for the determination of proline. J.Biol. Chem. 1955, 215, 655–660. [Google Scholar] [CrossRef]
- Dreir, W.; Goring, M. Der Einfluß hoher Salzkonzentration auf verschiedene physiologische Parameter von Maiswurzeln. Wiss. Z. Humboldt-Univ. Berlin, Reine/Math. Naturwiss 1974, 23, 641–644. [Google Scholar]
- Sabatini, D.D.; Bensch, K.; Barnett, R.J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. Cell Biol. 1963, 17, 19–58. [Google Scholar] [CrossRef] [PubMed]
- Spurr, A.R. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultras. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Levigneron, A.; Lopez, F.; Vansuyt, G.; Berthomieu, P.; Fourcroy, P.; Casse-Delbart, F. Les plantes face au stress salin. Cah. Agric. 1995, 4, 263–273. [Google Scholar]
- Abrar, M.M.; Saquib, M.; Abbas, G.; Atiqur Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mhmood, K.; Maitlo, A.A.; Hassan, M.; Sun, N. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in Jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Sun, P.; Zhang, J.; Xia, Y.; Hu, J.; Wang, L.; Lu, M. The WUSCHELa (PtoWUSa) is involved in developmental plasticity of adventitious root in poplar. Genes 2020, 11, 176. [Google Scholar] [CrossRef]
- Breckle, S.W. The significance of salinity. In Desertification and Development: Dryland Ecology in Social Perspective, 1st ed.; Spooner, B., Mann, H.S., Eds.; Academic Press: New York, NY, USA, 1982; pp. 277–292. [Google Scholar]
- Wu, H.; Li, Z. The importance of Cl− exclusion and vacuolar Cl− sequestration: Revisiting the role of Cl− transport in plant salt tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Beritognolo, I.; Muleo, R.; Piazzai, M.; Sabatti, M.; Mugnozza, G.S.; Kuzminsky, E. Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environ. Exp. Bot. 2009, 66, 381–388. [Google Scholar] [CrossRef]
- Martinez, V.; Läuchli, A. Effects of Ca2+ on the salt-stress response of barley roots as observed by in vivo 31P-nuclear magnetic resonance and in vitro-analysis. Planta 1993, 190, 519–524. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Abbas, G.; Muhammad, S.; Javaid, A.; Ghulam, M. Physiological and biochemical characterization of Acacia stenophylla and Acacia albida exposed to salinity under hydroponic conditions. Can. J. For. Res. 2017, 47, 1293–1301. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Balti, H.; Abassi, M.; Dietz, K.J.; Kumar, V. Differences in Ionic, Enzymatic and photosynthetic Features Characterize distinct Salt tolerance in Eucalyptus Species. Plants 2021, 10, 1401. [Google Scholar] [CrossRef]
- Epron, D.; Toussaint, M.; Badot, P.M. Effects of sodium chloride salinity on root growth and respiration in oak seedlings. Ann. For. Sci. 1999, 56, 41–47. [Google Scholar] [CrossRef]
- Kumari, S.; Chhillar, H.; Chopra, P.; Khanna, R.R.; Khan, M.I.R. Potassium: A Trak to develop salinity tolerant plants. Plant Physiol. Biochem. 2021, 167, 1011–1023. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Siaut, M.; Cuine, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylides, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Bolaños, J.A.; Longstreth, D.J. Salinity effect on water potential components and water bulk elastic modulus of Alternantherata philoxeroides (Mart.) Griseb. Plant Physiol. 1984, 75, 281–284. [Google Scholar] [CrossRef]
- Clifford, S.C.; Arndt, S.K.; Corlett, J.E.; Joshi, S.; Sankhla, N.; Popp, M.; Jones, H.G. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.). J. Exp. Bot. 1998, 49, 967–977. [Google Scholar] [CrossRef]
- Saltpeter, K.E.; Milleemann, D.R.; Capito, M.F.; White, B.L.; Touchette, B.W. Delayed modifications in plant–water relations in the coastal marsh halophyte Spartina patens following sudden increases in soil salinity. Bot. Mar. 2012, 55, 307–310. [Google Scholar] [CrossRef]
- Laamari, I.; Marques, I.; Ribeiro-Barros, A.I.; Abassi, M. Can saline preconditioning enhance plant survival in degraded soils? Physiological, biochemical, and molecular responses in Casuarina glauca saplings. Plant Ecol. 2023, 224, 905–919. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions 2.0. Int. J. Mol. Sci. 2023, 24, 15740. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, W.; Ma, C.; Liu, X. Physiological responses of different willow’s clones to NaCl stress. Acta Ecol. Sin. 2010, 30, 895–904. [Google Scholar]
- Chen, S.; Polle, A. Salinity tolerance of Populus. Plant Biol. 2010, 12, 317–333. [Google Scholar] [CrossRef]
- Ma, H.C.; Fung, L.; Wang, S.S.; Altman, A.; Huttermann, A. Photosynthetic response of Populus euphratica to salt stress. For. Ecol. Manag. 1997, 93, 55–61. [Google Scholar] [CrossRef]
- Papp, J.C.; Ball, M.C.; Terry, N. A comparative study of the effects of NaCl salinity on respiration, photosynthesis, and leaf extension growth in Beta vulgaris L. (sugar beet). Plant Cell Environ. 1983, 6, 675–677. [Google Scholar] [CrossRef]
- Abassi, M.; Albouchi, A.; Béjaoui, Z.; Rejeb, M.N.; Mougou, A. Adaptations anatomiques foliaires développées par le peuplier blanc (Populus alba L.) face au stress salin. Annales l’INRGREF 2009, 13, 153–163. [Google Scholar]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and responses of chloroplasts to salt stress. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.J.; Yang, H.Y.; Bai, J.P.; Liang, X.Y.; Lou, Y.; Zhang, J.L.; Di Wang, D.; Zhang, J.L.; Niu, S.Q.; Chen, Y.L. Ultrastructural and physiological responses of potato (Solanum tuberosum L.) plantlets to gradient saline stress. Front. Plant Sci. Sec. Plant Physiol. 2015, 5, 787. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity Response in Chloroplasts: Insights from Gene Characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef]
- Kutík, J.; Holá, D.; Kočová, M.; Rothová, O.; Haisel, D.; Wilhelmová, N.; Tichá, I. Ultrastructure and dimensions of chloroplasts in leaves of three maize (Zea mays L.) inbred lines and their F-1 hybrids grown under moderate chilling stress. Photosynthetica 2004, 42, 447–455. [Google Scholar] [CrossRef]
- Wise, R.R. The diversity of plastid form and function. In The Structure and Function of Plastids; Wise, R.R., Hoober, J.K., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 3–26. [Google Scholar]
- Szczepanik, J.; Sowinski, P. The occurrence of chloroplast peripheral reticulum in grasses: A matter of phylogeny or a matter of function? Acta Physiol. Plant. 2014, 36, 1133–1142. [Google Scholar] [CrossRef][Green Version]

| p-Values | |||
|---|---|---|---|
| Variables | Clone (C) | Treatment (T) | (C) × (T) |
| Shoot dry mass (SDM) | <0.001 | <0.001 | <0.001 |
| Root dry mass (RDM) | <0.001 | <0.001 | <0.001 |
| Net photosynthesis (A) | <0.001 | <0.001 | <0.001 |
| Stomatal conductance (Gs) | <0.001 | <0.001 | 0.0149 |
| Transpiration (E) | <0.001 | <0.001 | 0.0064 |
| Intercellular CO2 concentration (Ci) | <0.001 | 0.0304 | 0.0089 |
| Chlorophyll concentration (Chl) | 0.0279 | 0.0272 | 0.0174 |
| Osmotic potential at loss of turgor (Ψπ0) | 0.006 | 0.0321 | 0.0051 |
| Osmotic potential at saturation (Ψπ100) | <0.001 | 0.001 | 0.0065 |
| Relative water content at loss of turgor (RWC0) | 0.0061 | 0.0321 | 0.0051 |
| Apoplastic water content (AWC) | 0.001 | 0.001 | 0.005 |
| Modulus of elasticity (Ɛmax) | 0.001 | 0.001 | 0.002 |
| Variables | Treatments NaCl (mM) | Clone | ||
|---|---|---|---|---|
| MA-104 | MA-195 | OG | ||
| E (µmol H2O) | 0 | 4.32 ± 0.63 a | 5.17 ± 0.79 a | 4.95 ± 0.77 a |
| 100 | 2.81 ± 0.74 b | 3.03 ± 0.88 b | 2.76 ± 0.72 b | |
| Gs (mol.m−2.s−1) | 0 | 0.31 ± 0.06 a | 0.37 ± 0.09 a | 0.29 ± 0.07 a |
| 100 | 0.17 ± 0.04 b | 0.15 ± 0.03 b | 0.11 ± 0.03 b | |
| A (µmol.m−2.s−1) | 0 | 11.37 ± 1.54 a | 10.94 ± 1.27 a | 11.68 ± 1.47 a |
| 100 | 8.96 ± 1.07 b | 5.72 ± 1.85 c | 5.55 ± 0.92 c | |
| iWUE (µmol.mol−1) | 0 | 36.7 ± 1.5 d | 29.6 ± 1.3 e | 40.3 ± 2.5 c |
| 100 | 46.8 ± 2.1 b | 38.1 ± 2.4 cd | 50.6 ± 1.3 a | |
| Ci.µmol | 0 | 94 ± 1.55 d | 140 ± 1.25 b | 144 ± 1.98 b |
| CO2.mol−1) | 100 | 119 ± 2.67 c | 165 ± 2.35 a | 169 ± 2.88 a |
| Ls | 0 | 0.738 ± 0.018 a | 0.610 ± 0.015 a | 0.599 ± 0.012 a |
| 100 | 0.668 ± 0.011 b | 0.540 ± 0.013 b | 0.531 ± 0.014 b | |
| Chlorophyll (mg.g−1 FM) | 0 | 3.656 ± 0.318 d | 4.061 ± 0.323 cd | 4.488 ± 0.377 c |
| 100 | 4.358 ± 0.212 c | 5.225 ± 0.355 b | 6.473 ± 0.387 a | |
| Variables | Treatments NaCl (mM) | Clone | ||
|---|---|---|---|---|
| MA-104 | MA-195 | OG | ||
| Ψπ0 (MPa) | 0 | −2.37 ± 0.02 b | −2.34 ± 0.01 b | −2.23 ± 0.02 a |
| 100 | −2.61 ± 0.02 e | −2.56 ± 0.01 d | −2.41 ± 0.01 c | |
| Ψπ100 (MPa) | 0 | −1.67 ± 0.03 b | −1.61 ± 0.03 b | −1.52 ± 0.03 a |
| 100 | −1.93 ± 0.02 d | −1.75 ± 0.02 c | −1.64 ± 0.04 b | |
| RWC0 (%) | 0 | 74.16 ± 1.32 c | 74.52 ± 1.28 c | 76.31 ± 1.46 b |
| 100 | 81.28 ± 1.29 a | 80.42 ± 0.92 a | 80.75 ± 2.16 a | |
| AWC0 (%) | 0 | 24.75 ± 1.5 b | 17.18 ± 1.24 d | 18.32 ± 0.82 d |
| 100 | 34.26 ± 1.06 a | 24.23 ± 1.81 b | 21.53 ± 1.29 c | |
| Ɛmax (MPa) | 0 | 4.33 ± 0.14 c | 5.28 ± 0.15 b | 5.19 ± 0.62 b |
| 100 | 6.7 ± 0.21 a | 6.65 ± 0.12 a | 6.73 ± 0.88 a | |
| OA (MPa) | 100 | 0.26 ± 0.03 a | 0.14 ± 0.03 b | 0.12 ± 0.02 b |
| Variables | Treatments NaCl (mM) | Clone | ||
|---|---|---|---|---|
| MA-104 | MA-195 | OG | ||
| Starch (mg.g−1 DM) | 0 | 116.89 ± 3.29 a | 117.53 ± 4.44 a | 123.59 ± 6.35 a |
| 100 | 88.86 ± 3.22 b | 78.67 ± 2.35 c | 60.19 ± 2.35 d | |
| Soluble sugars (mg.g−1 DM) | 0 | 43.21 ± 2.09 b | 42.26 ± 3.54 b | 39.63 ± 2.52 b |
| 100 | 71.06 ± 4.31 a | 70.69 ± 3.45 a | 66.39 ± 3.84 a | |
| Proline (µmol.g−1 DM) | 0 | 7.70 ± 0.64 b | 4.27 ± 0.62 c | 3.95 ± 0.56 c |
| 100 | 10.78 ± 0.91 a | 9.66 ± 0.84 a | 4.45 ± 0.47 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abassi, M.; Lamhamedi, M.S.; Albouchi, A.; Khasa, D.; Bejaoui, Z. Clonal Variation in Growth, Physiology and Ultrastructure of Populus alba L. Seedlings Under NaCl Stress. Forests 2025, 16, 721. https://doi.org/10.3390/f16050721
Abassi M, Lamhamedi MS, Albouchi A, Khasa D, Bejaoui Z. Clonal Variation in Growth, Physiology and Ultrastructure of Populus alba L. Seedlings Under NaCl Stress. Forests. 2025; 16(5):721. https://doi.org/10.3390/f16050721
Chicago/Turabian StyleAbassi, Mejda, Mohammed S. Lamhamedi, Ali Albouchi, Damase Khasa, and Zoubeir Bejaoui. 2025. "Clonal Variation in Growth, Physiology and Ultrastructure of Populus alba L. Seedlings Under NaCl Stress" Forests 16, no. 5: 721. https://doi.org/10.3390/f16050721
APA StyleAbassi, M., Lamhamedi, M. S., Albouchi, A., Khasa, D., & Bejaoui, Z. (2025). Clonal Variation in Growth, Physiology and Ultrastructure of Populus alba L. Seedlings Under NaCl Stress. Forests, 16(5), 721. https://doi.org/10.3390/f16050721







