Cold-Season Precipitation and Latitudinal Differences Are Key Drivers of Salix alba Genetic Diversity in Arid Zones
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design of Experiments
2.2.1. Sample Collection
2.2.2. DNA Extraction and SSR Primer Selection
2.2.3. Characterization of Genetic Diversity and Analysis of Population Genetic Structure
2.2.4. Research on Drivers of Genetic Diversity
3. Results and Analyses
3.1. Analysis of Genetic Diversity and Population Genetic Structure
3.1.1. Analysis of Genetic Diversity Indicators
3.1.2. Genetic Distance and Similarity
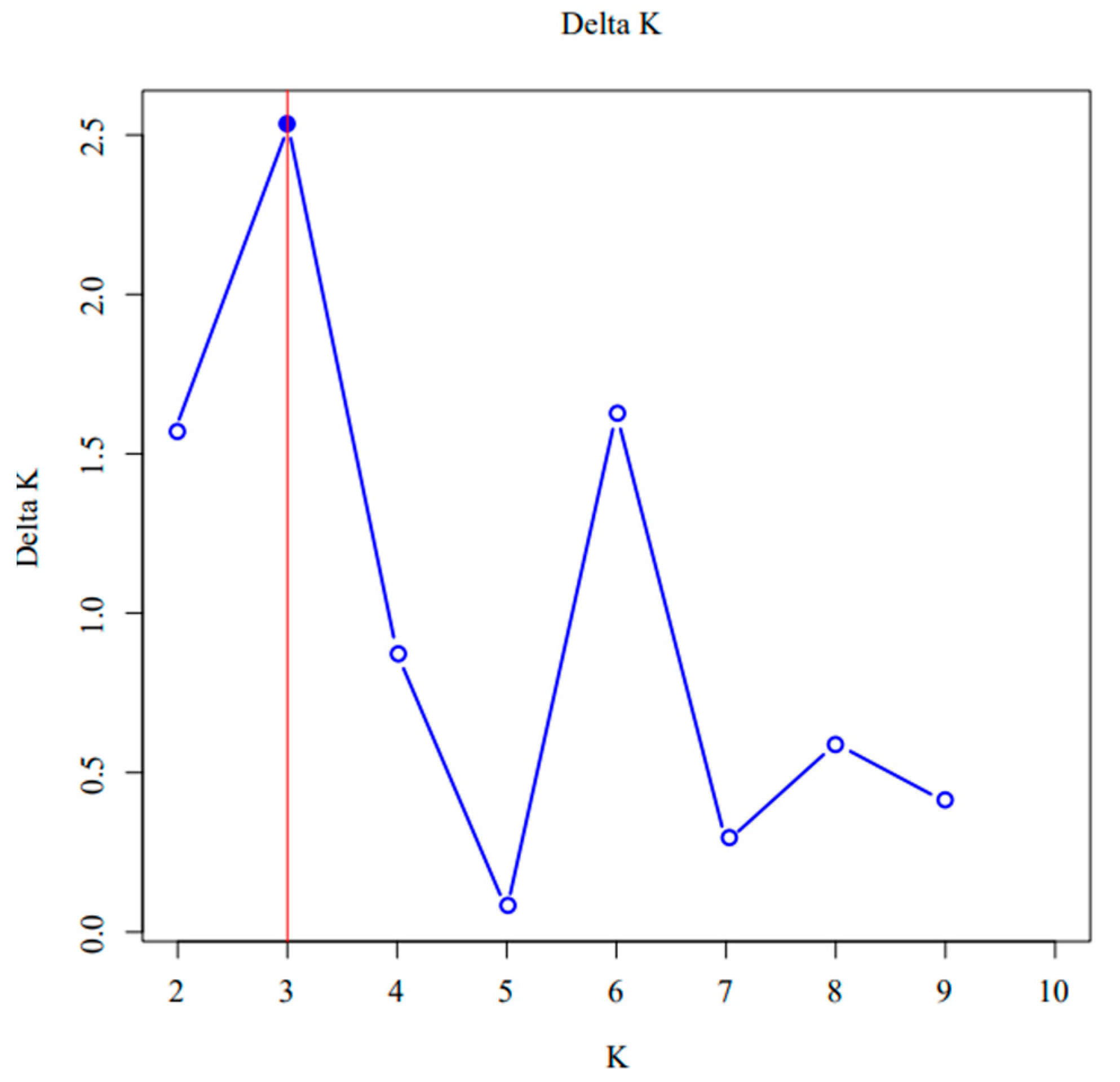
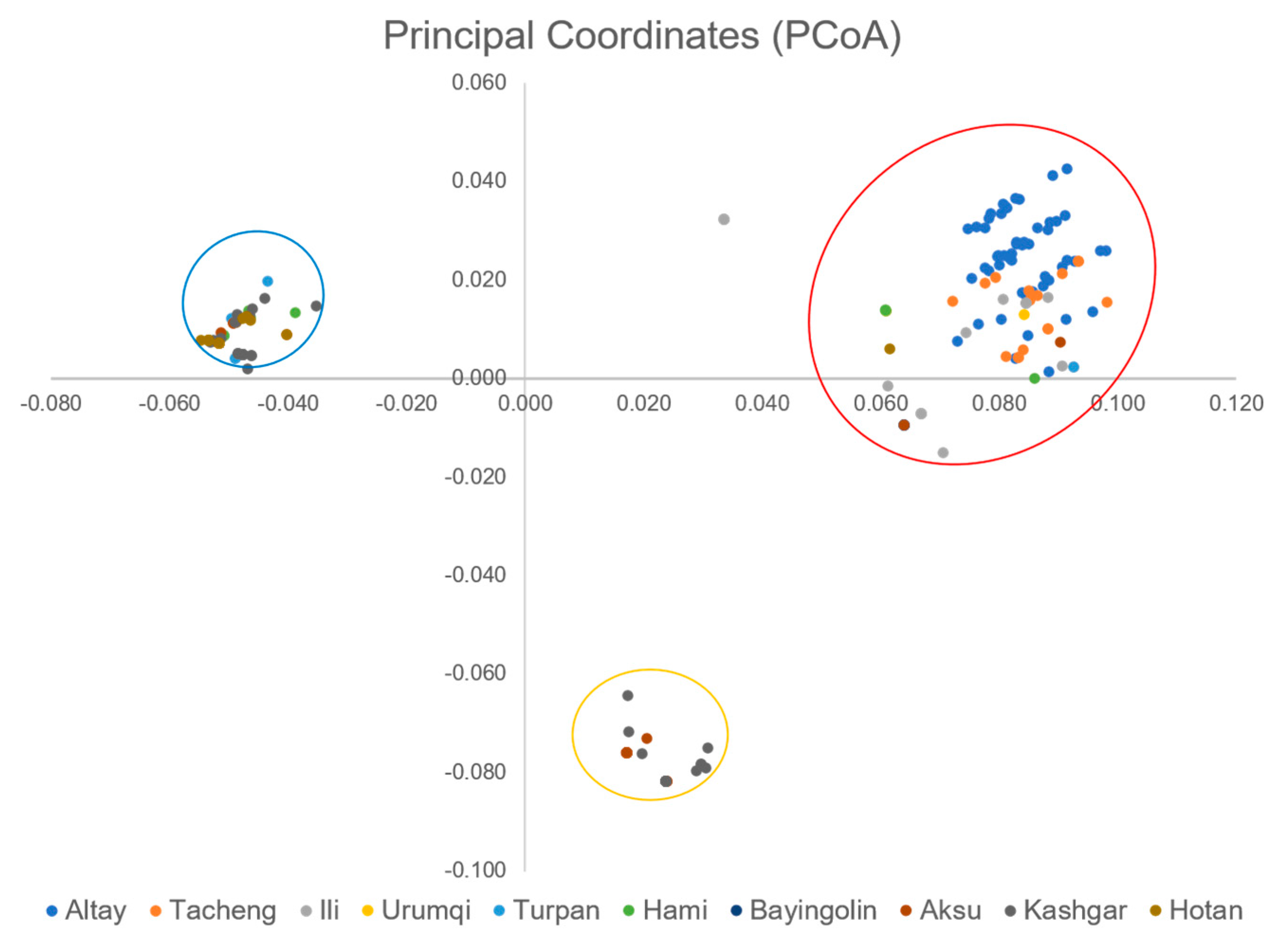
3.1.3. Genetic Clustering Analysis
3.2. Results of Genetic Diversity Driver Analysis
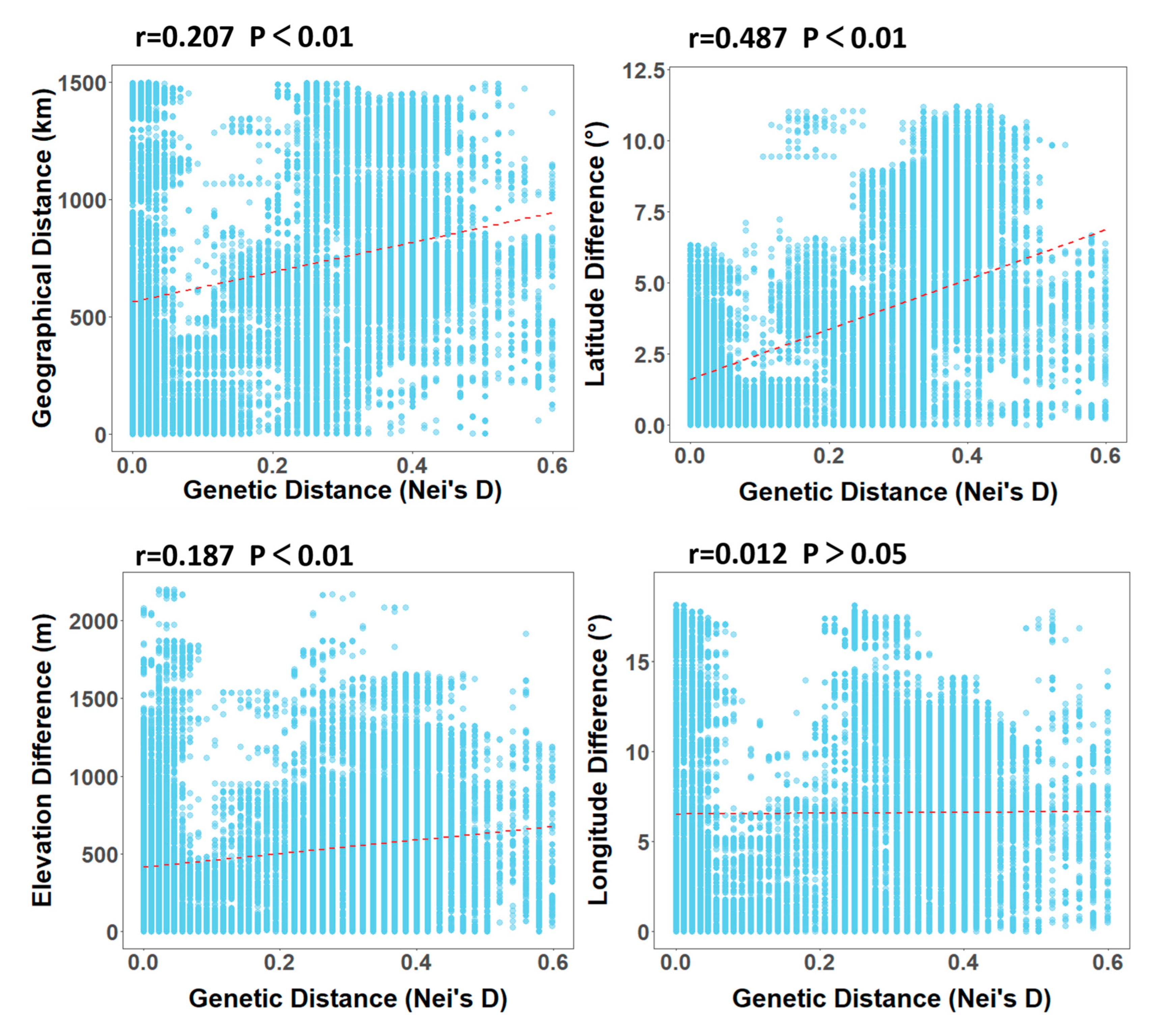
3.2.1. Analysis of Geographical Factors
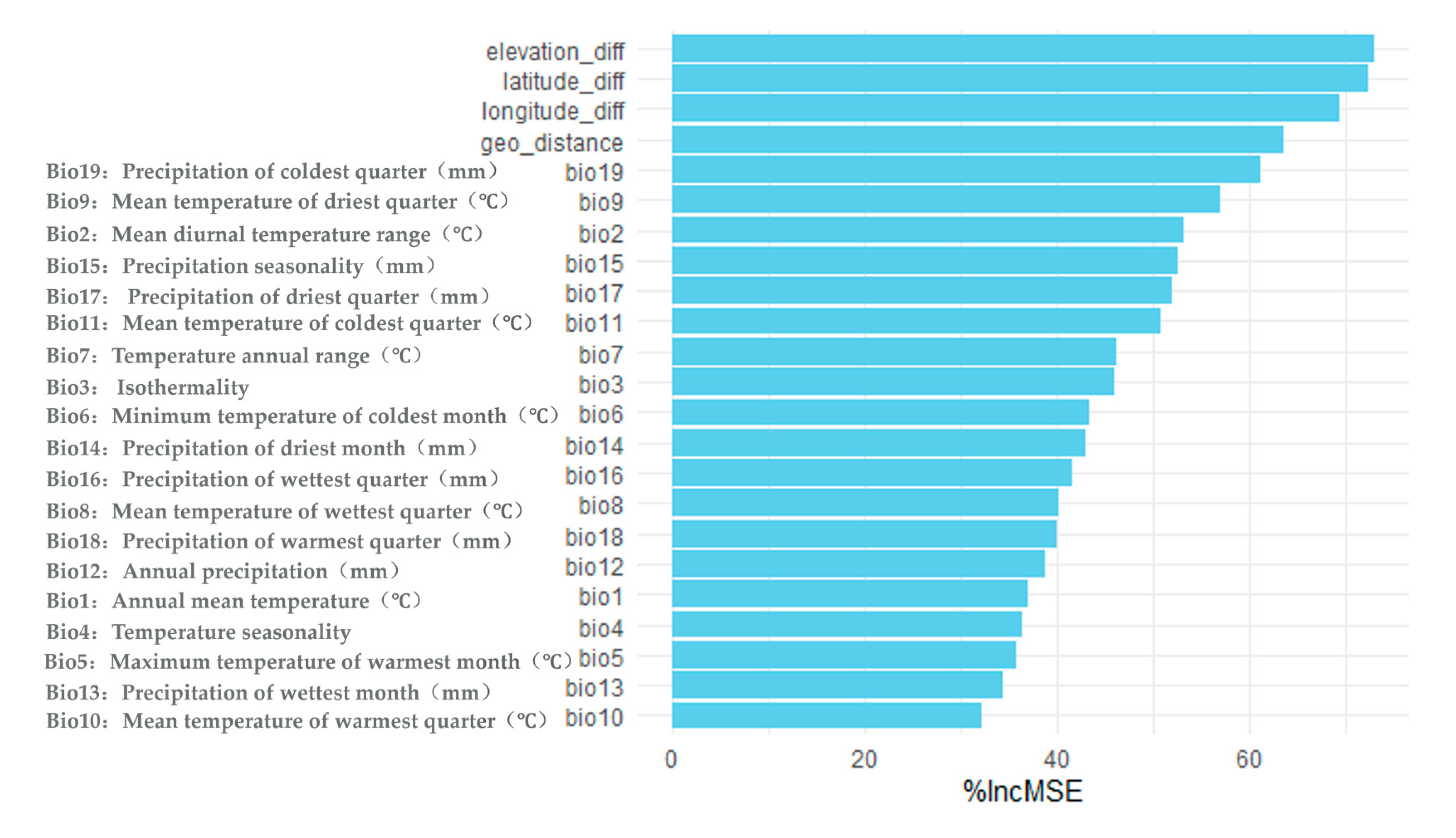
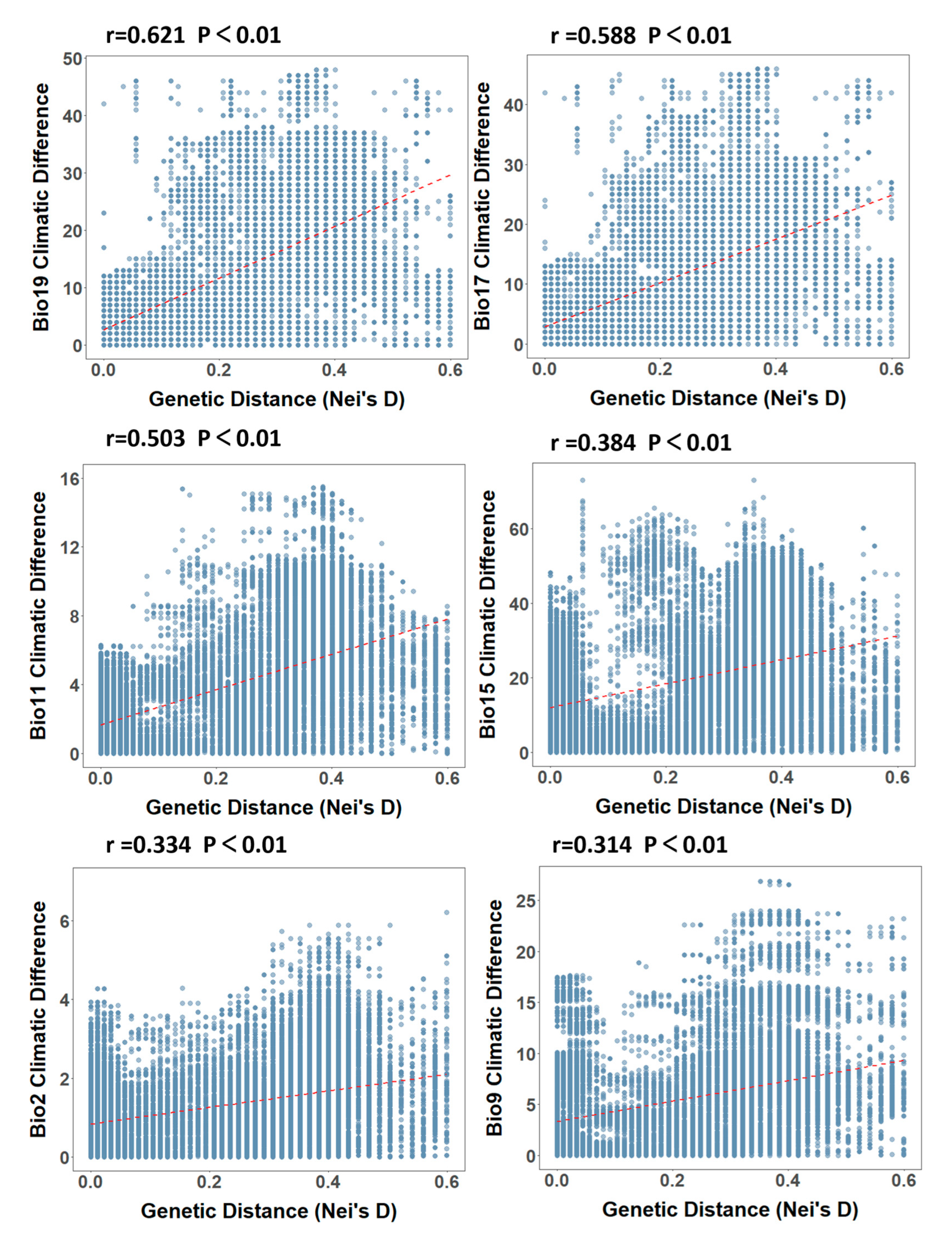
3.2.2. Climate Factor Analyses
4. Discussion
4.1. Characteristics of Salix alba Genetic Diversity in the Arid Zone
4.2. Study on the Driving Factors Affecting Genetic Diversity
4.3. Recommendations for the Conservation of Salix alba in Arid Zones
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golubkina, N.; Plotnikova, U.; Amagova, Z.; Sheshnitsan, S. Peculiarities of Willow (Salix alba) Bark Mineral Composition. Trace Elem. Med. 2023, 24, 47–52. [Google Scholar] [CrossRef]
- Markus-Michalczyk, H.; Smith, Z.; Bouma, T.J. Floodplain Forest Foundation Species Salix alba L. Is Resilient to Seawater Pulses during Winter. Limnol. Rev. 2024, 24, 250–265. [Google Scholar] [CrossRef]
- Axmanová, I.; Chytrý, K.; Boublík, K.; Chytrý, M.; Dřevojan, P.; Ekrtová, E.; Fajmon, K.; Hájková, P.; Härtel, H.; Hejda, M.; et al. Catalogue of Expansive Plants of the Czech Republic. Preslia 2024, 96, 299–327. [Google Scholar] [CrossRef]
- Karrenberg, S.; Edwards, P.J.; Kollmann, J. The Life History of Salicaceae Living in the Active Zone of Floodplains. Freshw. Biol. 2002, 47, 733–748. [Google Scholar] [CrossRef]
- Meneghetti, S.; Barcaccia, G.; Paiero, P.; Lucchin, M. Genetic Characterization of Salix alba L. and Salix fragilis L. by Means of Different PCR-Derived Marker Systems. Plant Biosyst. 2007, 141, 283–291. [Google Scholar] [CrossRef]
- Stalažs, A. To Be or Not to Be Native: From the Discourse on the Official Status of Salix alba to the Recommendation to Strengthen the Protection of Marginal Populations in Latvia. J. Nat. Conserv. 2022, 67, 126158. [Google Scholar] [CrossRef]
- Du, W. An Excellent Shelterbelt Species: White Willow (Salix alba). Agric. For. Sci. Exp. 1978, 4, 19–20. [Google Scholar]
- Song, J.; Xue, Z.; Yang, B.; Liu, T.; Yuan, Y.; Xu, L.; Zhang, Z. Spatial Distribution Patterns and Influencing Factors of Dominant Species in Plain Valley Forests of the Irtysh River Basin. Forests 2024, 15, 1237. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, Z.D.; Xue, Z.F.; Zeng, S.J.; Liu, T. Characteristics of Plant Diversity in the Haba River Valley Forest, a Tributary of the Irtysh River. J. Plant Resour. Environ. 2024, 33, 89–96. [Google Scholar]
- Sitzia, T.; Barcaccia, G.; Lucchin, M. Genetic Diversity and Stand Structure of Neighboring White Willow (Salix alba L.) Populations along Fragmented Riparian Corridors: A Case Study. Silvae Genet. 2018, 67, 79–88. [Google Scholar] [CrossRef]
- Tokdemir, Y.; Değirmenci, F.Ö.; Uluğ, A.; Acar, P.; Kaya, Z. Genetic Diversity of Salix caprea L. Populations in Fragmented Habitats of Northeastern Türkiye. Biologia 2024, 79, 2013–2023. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological Consequences of Genetic Diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Ørsted, M.; Hoffmann, A.A.; Sverrisdóttir, E.; Nielsen, K.L.; Kristensen, T.N. Genomic Variation Predicts Adaptive Evolutionary Responses Better than Population Bottleneck History. PLoS Genet. 2019, 15, e1008205. [Google Scholar] [CrossRef]
- Liu, H.; Zang, F.; Wu, Q.; Ma, Y.; Zheng, Y.; Zang, D. Genetic Diversity and Population Structure of the Endangered Plant Salix taishanensis Based on CDDP Markers. Glob. Ecol. Conserv. 2020, 24, e01242. [Google Scholar] [CrossRef]
- Gong, G.; Wang, Y.; Zhu, Z.; Wang, Y.; Hörandl, E.; Wang, X.; Xue, Z.; He, L. Evolutionary Population Dynamics and Conservation Strategies for Salix baileyi—A Species with Extremely Small Populations. Glob. Ecol. Conserv. 2025, 58, e03504. [Google Scholar] [CrossRef]
- Yuan, S.; Zeng, G.; Zhang, K.; Wu, M.; Zhang, D.; Harder, L.D.; Barrett, S.C.H. Diverse Mating Consequences of the Evolutionary Breakdown of the Sexual Polymorphism Heterostyly. Proc. Natl. Acad. Sci. USA 2023, 120, e2214492120. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Xie, H.; Zhao, D.; Huang, M. RAPD Analysis of Genetic Diversity in Tamarix Populations from the Yellow River Delta. J. Nanjing For. Univ. 2008, 05, 56–60. [Google Scholar]
- Gross, N.; Maestre, F.T.; Liancourt, P.; Berdugo, M.; Martin, R.; Gozalo, B.; Ochoa, V.; Delgado-Baquerizo, M.; Maire, V.; Saiz, H.; et al. Unforeseen Plant Phenotypic Diversity in a Dry and Grazed World. Nature 2024, 632, 808–814. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, W.; Wu, H.; Qu, Z. Genetic Diversity Analysis of Elymus sibiricus under Drought Stress. Grassl. Turf 2014, 34, 11–17+22. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, L.; Zhang, G.; Wang, Y.; Han, S.; Bai, Y. Genetic Diversity and Population Structure Analysis of Salix psammophila. Acta Bot. Boreali-Occident. Sin. 2017, 37, 1507–1516. [Google Scholar]
- Zhang, R.; Gong, Y.; Tan, M. Spatiotemporal Variation and Regional Comparison of NDVI in Arid Central Asia. Res. Environ. Sci. 2025, 38, 90–100. [Google Scholar] [CrossRef]
- Zeng, J.; Zou, Y.; Bai, J.; Zheng, H. Preparation of Total DNA from “Recalcitrant Plant Taxa”. Acta Bot. Sin. 2002, 44, 694–697. [Google Scholar]
- Zhang, X. Genetic Diversity Analysis and Cutting Propagation of Wild Willows in Chifeng Region. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2015. [Google Scholar]
- Raja, R.; Singh, N.; Bhat, S. Assessment of Genetic Diversity among High Yielding Selected Salix Clones Using RAPD and SSR Markers. Genetika 2018, 50, 983–994. [Google Scholar] [CrossRef]
- King, R.A.; Harris, S.L.; Karp, A.; Barker, J.H.A. Characterisation and Inheritance of Nuclear Microsatellite Loci for Use in Population Studies of the Allotetraploid Salix alba–Salix fragilis Complex. Tree Genet. Genomes 2010, 6, 247–258. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Malekmohammadi, L.; Sheidai, M.; Ghahremaninejad, F.; Danehkar, A.; Koohdar, F. Studies on Genetic Diversity, Gene Flow and Landscape Genetics in Avicennia marina: Spatial PCA, Random Forest, and Phylogeography Approaches. BMC Plant Biol. 2023, 23, 459. [Google Scholar] [CrossRef]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient Forests: Calculating Importance Gradients on Physical Predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Li, L.; Li, J.; Yin, H.; Zhao, Y.; Chen, X. Evaluation of Environmental Factors Affecting the Genetic Diversity, Genetic Structure, and the Potential Distribution of Rhododendron aureum Georgi under Changing Climate. Ecol. Evol. 2021, 11, 12294–12306. [Google Scholar] [CrossRef]
- Amiteye, S. Basic Concepts and Methodologies of DNA Marker Systems in Plant Molecular Breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef]
- Lander, T.A.; Klein, E.K.; Roig, A.; Oddou-Muratorio, S. Weak Founder Effects but Significant Spatial Genetic Imprint of Recent Contraction and Expansion of European Beech Populations. Heredity 2021, 126, 491–504. [Google Scholar] [CrossRef]
- Zwaenepoel, A.; Sachdeva, H.; Fraïsse, C. The Genetic Architecture of Polygenic Local Adaptation and Its Role in Shaping Barriers to Gene Flow. Genetics 2024, 228, iyae140. [Google Scholar] [CrossRef]
- Adams, N.E.; Bandivadekar, R.R.; Battey, C.J.; Clark, M.W.; Epperly, K.; Ruegg, K.; Tell, L.A.; Bay, R.A. Widespread Gene Flow Following Range Expansion in Anna’s Hummingbird. Mol. Ecol. 2023, 32, 3089–3101. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, P.; Gazol, A.; Valbuena-Carabaña, M.; Sangüesa-Barreda, G.; Moreno-Urbano, A.; Zavala, M.A.; Camarero, J.J. Remaking a Stand: Links between Genetic Diversity and Tree Growth in Expanding Mountain Pine Populations. For. Ecol. Manag. 2020, 472, 118244. [Google Scholar] [CrossRef]
- Adams, R.V.; Burg, T.M. Influence of Ecological and Geological Features on Rangewide Patterns of Genetic Structure in a Widespread Passerine. Heredity 2015, 114, 143–154. [Google Scholar] [CrossRef]
- Dias, E.F.; Moura, M.; Schaefer, H.; Silva, L. Geographical Distance and Barriers Explain Population Genetic Patterns in an Endangered Island Perennial. AoB Plants 2016, 8, plw072. [Google Scholar] [CrossRef]
- Pfeifer, M.; Jetschke, G. Influence of Geographical Isolation on Genetic Diversity of Himantoglossum hircinum (Orchidaceae). Folia Geobot. 2006, 41, 3–20. [Google Scholar] [CrossRef]
- Hindley, J.A.; Graham, B.A.; Pulgarin-R., P.C.; Burg, T.M. The Influence of Latitude, Geographic Distance, and Habitat Discontinuities on Genetic Variation in a High Latitude Montane Species. Sci. Rep. 2018, 8, 11846. [Google Scholar] [CrossRef]
- Hirao, A.S.; Watanabe, M.; Tsuyuzaki, S.; Shimono, A.; Li, X.; Masuzawa, T.; Wada, N. Genetic Diversity within Populations of an Arctic–Alpine Species Declines with Decreasing Latitude across the Northern Hemisphere. J. Biogeogr. 2017, 44, 2740–2751. [Google Scholar] [CrossRef]
- Bohonak, A.J. IBD (Isolation by Distance): A Program for Analyses of Isolation by Distance. J. Hered. 2002, 93, 153–154. [Google Scholar] [CrossRef]
- Westram, A.M.; Stankowski, S.; Surendranadh, P.; Barton, N. What Is Reproductive Isolation? J. Evol. Biol. 2022, 35, 1143–1164. [Google Scholar] [CrossRef]
- Liao, J.; Chen, S.; Liu, P.; Fontaneto, D.; Han, B. Environmental Selection and Gene Flow Jointly Determine the Population Genetic Diversity and Structure of Diaphanosoma dubium along a Watershed Elevation. Glob. Ecol. Conserv. 2024, 49, e02773. [Google Scholar] [CrossRef]
- Kling, M.M.; Ackerly, D.D. Global Wind Patterns Shape Genetic Differentiation, Asymmetric Gene Flow, and Genetic Diversity in Trees. Proc. Natl. Acad. Sci. USA 2021, 118, e2017317118. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wan, J.; Luo, F.; Yu, F. Relationships between Genetic Diversity of Vascular Plant Species and Climate Factors. J. Resour. Ecol. 2018, 9, 663–672. [Google Scholar] [CrossRef]
- Pauls, S.U.; Nowak, C.; Bálint, M.; Pfenninger, M. The Impact of Global Climate Change on Genetic Diversity within Populations and Species. Mol. Ecol. 2013, 22, 925–946. [Google Scholar] [CrossRef]
- Lu, M.; Krutovsky, K.V.; Loopstra, C.A. Predicting Adaptive Genetic Variation of Loblolly Pine (Pinus taeda L.) Populations under Projected Future Climates Based on Multivariate Models. J. Hered. 2019, 110, 857–865. [Google Scholar] [CrossRef]
- Ramírez-Aliaga, P.; Foyo-Moreno, I.; Cariñanos, P. Effects of Environmental Stress on the Pollen Viability of Ornamental Tree-Species in the City of Granada (South-Eastern Spain). Forests 2022, 13, 2131. [Google Scholar] [CrossRef]
- Myklestad, Å.; Birks, H.J.B. A Numerical Analysis of the Distribution Patterns of Salix L. Species in Europe. J. Biogeogr. 1993, 20, 1–32. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Wu, H.; Xue, X.; Ren, J. Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Salt Combined with Flooding Tolerance in Hybrid Willow (Salix matsudana × alba). Forests 2023, 14, 1858. [Google Scholar] [CrossRef]


| Group Number | Population | Number of Populations | Sample Size |
|---|---|---|---|
| 1 | Altay | 6 | 53 |
| 2 | Tacheng | 3 | 16 |
| 3 | Ili | 6 | 18 |
| 4 | Urumqi | 2 | 3 |
| 5 | Turpan | 1 | 11 |
| 6 | Hami | 2 | 46 |
| 7 | Bayingolin | 3 | 17 |
| 8 | Aksu | 7 | 63 |
| 9 | Kashgar | 8 | 73 |
| 10 | Hotan | 2 | 20 |
| Name of Primers | N Size | Na | Ne | h | I | PIC |
|---|---|---|---|---|---|---|
| SB1148 | 320 | 2 | 1.5127 | 0.3061 | 0.4593 | 0.6759 |
| SB1172 | 320 | 2 | 1.2017 | 0.1284 | 0.2196 | 0.7787 |
| SB1185 | 320 | 2 | 1.249 | 0.156 | 0.2613 | 0.5762 |
| SB24 | 320 | 2 | 1.4117 | 0.2333 | 0.3554 | 0.8102 |
| SB288 | 320 | 2 | 1.3271 | 0.2158 | 0.3467 | 0.8103 |
| SB38 | 320 | 2 | 1.2292 | 0.1499 | 0.2457 | 0.6993 |
| SB430 | 320 | 2 | 1.3184 | 0.1925 | 0.3051 | 0.8002 |
| SB565 | 320 | 2 | 1.3191 | 0.2032 | 0.3318 | 0.7418 |
| SB800 | 320 | 2 | 1.4831 | 0.2927 | 0.454 | 0.7587 |
| SB984 | 320 | 2 | 1.0238 | 0.0231 | 0.0613 | 0.05456 |
| Name of Primers | Forward Primer | Reverse Primer |
|---|---|---|
| SB1148 | TAGGATGTTTCTGAGGCTTTC | AGACTTGCTAGAGACTTGGCC |
| SB1172 | AGGACATTCATAACACACACA | CTGAAGCCATCTTCTCAAGGA |
| SB1185 | TGAGGTCATGGTTGAGTTATG | ATGGTGCCTGCAATCTTTAAC |
| SB24 | ACTTCAATCTCTCTGTATTCT | CTATTTATGGGTTGGTCGATC |
| SB288 | AGGCTTCACTGTCTCCTCTAG | TCATCACAGCATCTTATCAGG |
| SB38 | CCACTTGAGGAGTGTAAGGAT | CTTAAATGTAAAACTGAATCT |
| SB430 | CACCCTCATAACAAAAATGGC | CAAATCAGAAAAGAAGTTAAC |
| SB565 | GAAAATATAATGCCCAGGAAG | ACAGAACACAGCGACATGAAC |
| SB800 | TAATGGAGTTCACAGTCCTCC | ATACAGAGCCCATTTCATCAC |
| SB984 | ACAATCACACTTCGCATATCA | GGATGGAAAGATTCAAGGATT |
| Areas | N | Na | Ne | h | I | Ht | Gst | Nm |
|---|---|---|---|---|---|---|---|---|
| Altay | 53 | 1.4807 | 1.1442 | 0.0918 | 0.1498 | 0.0851 | 0.1596 | 2.6322 |
| Tacheng | 16 | 1.2543 | 1.1357 | 0.082 | 0.1254 | 0.0819 | 0.1356 | 3.1866 |
| Ili | 18 | 1.5656 | 1.3706 | 0.2095 | 0.3088 | 0.1841 | 0.5119 | 0.4767 |
| Urumqi | 3 | 1.366 | 1.2928 | 0.1626 | 0.233 | 0.1221 | 0.4033 | 0.7398 |
| Turpan | 11 | 1.5674 | 1.131 | 0.1024 | 0.1831 | 0.1988 | 0.9569 | 0.0225 |
| Hami | 46 | 1.5599 | 1.0502 | 0.045 | 0.0947 | 0.088 | 0.1129 | 3.9306 |
| Bayingolin | 17 | 1.2824 | 1.2005 | 0.1173 | 0.1711 | 0.1332 | 0.75 | 0.1667 |
| Aksu | 63 | 1.6397 | 1.1717 | 0.1072 | 0.1744 | 0.1144 | 0.4518 | 0.6066 |
| Kashgar | 73 | 1.4466 | 1.178 | 0.108 | 0.1656 | 0.1186 | 0.4802 | 0.5413 |
| Hotan | 20 | 1.3297 | 1.0484 | 0.0394 | 0.0762 | 0.0493 | 0.5577 | 0.3956 |
| Pop | Altay | Tacheng | Ili | Urumqi | Turpan | Hami | Bayingolin | Aksu | Kashgar | Hotan |
|---|---|---|---|---|---|---|---|---|---|---|
| Altay | 0.9846 | 0.9425 | 0.9461 | 0.8436 | 0.8284 | 0.8837 | 0.8605 | 0.8446 | 0.8099 | |
| Tacheng | 0.0155 | 0.9383 | 0.9465 | 0.8365 | 0.8225 | 0.8864 | 0.8596 | 0.8446 | 0.808 | |
| Ili | 0.0592 | 0.0637 | 0.9752 | 0.8623 | 0.8402 | 0.8919 | 0.8741 | 0.8562 | 0.8238 | |
| Urumqi | 0.0554 | 0.0549 | 0.0251 | 0.8606 | 0.8379 | 0.8879 | 0.8681 | 0.8497 | 0.8188 | |
| Turpan | 0.17 | 0.1785 | 0.1482 | 0.1501 | 0.9927 | 0.9681 | 0.9787 | 0.9717 | 0.9838 | |
| Hami | 0.1882 | 0.1954 | 0.1741 | 0.1768 | 0.0074 | 0.9769 | 0.9826 | 0.9767 | 0.9946 | |
| Bayingolin | 0.1237 | 0.1206 | 0.1144 | 0.1189 | 0.0324 | 0.0234 | 0.9816 | 0.9748 | 0.9715 | |
| Aksu | 0.1503 | 0.1513 | 0.1345 | 0.1414 | 0.0215 | 0.0176 | 0.0186 | 0.9959 | 0.9739 | |
| Kashgar | 0.1689 | 0.1689 | 0.1552 | 0.1629 | 0.0287 | 0.0235 | 0.0256 | 0.0041 | 0.9684 | |
| Hotan | 0.2109 | 0.2131 | 0.1938 | 0.1999 | 0.0163 | 0.0054 | 0.029 | 0.0264 | 0.0321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Dong, H.; Yang, X.; Liu, T. Cold-Season Precipitation and Latitudinal Differences Are Key Drivers of Salix alba Genetic Diversity in Arid Zones. Forests 2025, 16, 725. https://doi.org/10.3390/f16050725
He J, Dong H, Yang X, Liu T. Cold-Season Precipitation and Latitudinal Differences Are Key Drivers of Salix alba Genetic Diversity in Arid Zones. Forests. 2025; 16(5):725. https://doi.org/10.3390/f16050725
Chicago/Turabian StyleHe, Jiajing, Hegan Dong, Xiaopeng Yang, and Tong Liu. 2025. "Cold-Season Precipitation and Latitudinal Differences Are Key Drivers of Salix alba Genetic Diversity in Arid Zones" Forests 16, no. 5: 725. https://doi.org/10.3390/f16050725
APA StyleHe, J., Dong, H., Yang, X., & Liu, T. (2025). Cold-Season Precipitation and Latitudinal Differences Are Key Drivers of Salix alba Genetic Diversity in Arid Zones. Forests, 16(5), 725. https://doi.org/10.3390/f16050725





