Abstract
The study of treefall and its after-effects is a common theme in studies of forest structure and local dynamics, yet its value as descriptor of broader-scale ecological dynamics is rarely explored. Here we synthesize the most highly cited literature on treefalls, from 1985 to 2016 (in three-year blocks), highlighting the importance of the causes, characteristics and consequences of such events. We then ask how this knowledge might contribute to the broader conceptual model of forest dynamics, and develop two conceptual models, which we use to illustrate both the classic and alternative views of how forests ‘work’. Treefalls are one of the few ‘integrating’ attributes of forests, because of their ubiquity and longevity, and therefore can inform a variety of processes (e.g., tree mortality, turnover rates, structural impacts, recruitment, and fire frequency) due to their impacts occurring simultaneously over space (patterns), and time (legacy effects). The substantial knowledge that already exists on localized treefall dynamics should be combined with more integrative approaches to studying forest ecosystems, to investigate landscape-scale patterns of treefall and reconstruct past disturbance events.
1. Introduction
As threats to global biodiversity from land-use change and other anthropogenic influences (e.g., climate change) mount, the future of the world’s forests has become progressively more uncertain. As a consequence, studies focussing on the impact and sustainability of activities associated with human development on forest biomes (e.g., logging, cultivation), and their interaction with the agents of global change (e.g., climate change, fire regimes, non-native species), have become prolific over the last two decades [1,2,3,4]. However, to forecast future forest distribution and biodiversity, it is also essential to have a comprehensive understanding of the eco-evolutionary forces that shape the structural features and dynamic processes that occur within forests (such as mortality, turnover rates, rate of treefall, gap-phase regeneration, recruitment, nutrient cycling), as well as feedbacks between ecological and biophysical attributes. Forest community composition and turnover are influenced by many ecological processes [5,6,7]. While some factors are consistently important and ubiquitous (e.g., climate, plant-plant interactions, mortality rates), others are spatially heterogeneous in effect and can be highly context dependent (e.g., disturbance) [8,9]. However, forests are inherently complex systems [10], and strong interactions among processes can lead to reinforcing or diminishing feedbacks that are difficult to detect unless measurements over multiple spatial scales or temporal snap-shots are combined. These dynamic mechanisms cannot be studied effectively in isolation; moreover, the further back in-time we try to reach with our inferences, the more indiscernible the imprints of past processes become (e.g., legacy treefalls) [11].
Much of the focus of the forest-ecology literature has been on the position, size and species identity of growing and mature trees, and the consequences of their removal (gap dynamics). Additionally, it is well known that trees can die standing, and remain in this ‘state’ for years as stags or stumps. As a forest attribute, stags and stumps are important as they provide critical habitat for fauna (e.g., Leadbeater’s possum, Gymnobelideus leadbeateri) and constitute an integral component of the forest structure [12]. However, unless the wood is harvested, the tree will eventually fall to the forest floor, either due to biotic (pathogens, competition) or abiotic factors (e.g., wind, fire).
This now-dead residue of the once-living forest is usually called ‘coarse woody debris’ (CWD), or treefall when the fallen log is still relatively intact. The age and volume of the dead wood contains signatures of past tree mortality, and so opens a temporal window through which we might perceive forest turnover rates, disturbance frequency, die-off events, past recruitment pulses and species-trait responses. For example, the presence of heliophilous species in an old-growth forest may be indicative of a past disturbance event that enhanced light availability by opening canopy gaps [13]. In systems where decay rates are slow (e.g., cool-temperate or boreal forests) or regions where disturbances such as fire are rare, the fallen wood can persist for decades to centuries [14], thus providing a long-term record of change in the forest.
Yet there remains ambiguity about the structural effects of treefalls on the spatial distribution of the living components of forests at different scales [15]. Is treefall a forest attribute worth studying for its intrinsic ecological value, or in the overall context of forest dynamics, is its importance defined by how it opens canopy gaps for the recruitment, growth and competition of new living trees? The current definition of a treefall typically relates to the size, frequency and purported causation of the fallen wood (e.g., windthrow or blowdown, forest or canopy gap, or average size and density of the CWD). However, ecologically, treefalls might equally refer to both structural characteristics and temporal features simultaneously, including the dead (but still standing) trees, the act and consequences of a tree falling, the fallen log on the forest floor, and the legacy effects (e.g., past physical displacement of large trees, root pits and mounds) that persist as an imprint after the dead wood has decayed.
Here we present a systematic overview of the last three decades of literature on treefalls and dead wood, and show that although treefalls have been repeatedly demonstrated as important facilitators of forest structure and process, their relationship to the living components is usually overlooked or implicitly downplayed. Here, we use the term ‘treefall’ to refer to not only act of the tree falling, but also the physical consequences of the fallen tree, and the gap-phase regeneration that it triggers. In this context, treefall is not only an event, but also a legacy record of past forest dynamics, and a driver of turnover processes. Specifically, we sought to: (i) examine the causes, characteristics and important consequences of treefalls, drawing attention to current gaps in our knowledge of treefall events; (ii) critically evaluate the importance of treefalls as key components of forest ecological processes; (iii) highlight areas for future study, including a re-evaluation of the conceptual model of forests when treefall is given explicit priority (and measured regularly and systematically, alongside attributes of the living forest). In pursuit of our final aim, we compare an example of a classic model of forest dynamics (traditionally focused on the life cycle of a tree) with an alternative approach, where tree death and treefall are seen as complementary windows into hidden underlying ecological processes.
2. Methodology
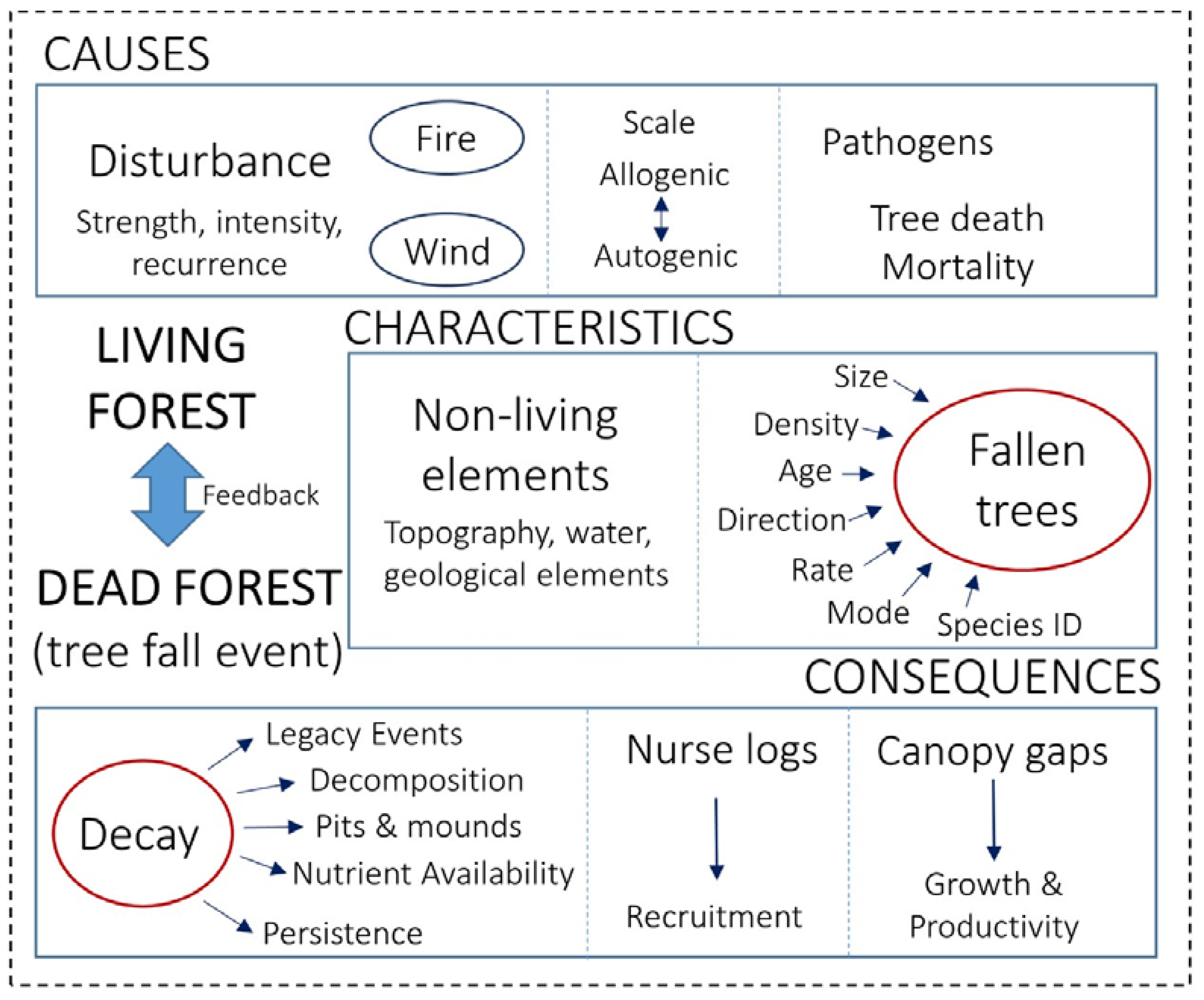
To sample the literature representatively, we undertook a series of searches using different combinations of key words relevant to treefall, disturbance, woody debris and forests (for example: TS = (treefall AND log AND forest); TS = (“fallen tree” AND log AND forest); TS = forest AND (“coarse woody debris” OR CWD); TS = (forest AND disturbance AND dead trees AND stumps, etc.)). The subsequent references and citations in the most highly cited of these papers were also scrutinised. We then combined the results and cross-referenced across the searches to remove duplicates, leaving a useable tally of ~2500 papers. To ensure a comprehensive yet tractable synthesis of this literature, we then created two summary tables, one that listed the most highly cited literature from 1985 to 2016 (separated as sequential three-year blocks; Table S1; 73 papers), and another (Table 1; 25 papers) that focused on four examples (not duplicated in Table S1), each representing a classic, well-cited, review/meta-analysis, and recent study (published within the last two years). Our choice of categories for grouping the selected papers was dictated by the most common themes that were covered in the literature. These were: (i) causes of treefall; (ii) consequences of a treefall and; (iii) characteristics of the fallen tree and the landscape, which is typically influenced by (i) and has an effect on (ii) (Figure 1). Within this ecological context, for Table S1 we broke the studies into six categories within each of the three-year blocks, being: (a) canopy gaps; (b) decay and nurse logs; (c) extreme-weather events and disturbance; (d) modelling and forest management; (e) non-living and structural effects; and (f) tree mortality and standing dead.

Table 1.
Contextualisation of the treefall, dead wood, and gap-phase regeneration literature, categorised into four major research themes: Causes of treefall, characteristics contributing to propensity of a tree to fall, consequences of a treefall event, and management or modelling applications. The papers that that were included in each category were chosen to represent the following four criteria: (i) a ‘classic’ study (for historical grounding); (ii) a highly cited example; (iii) the most recent published review; and (iv) a recently published study based on primary research.
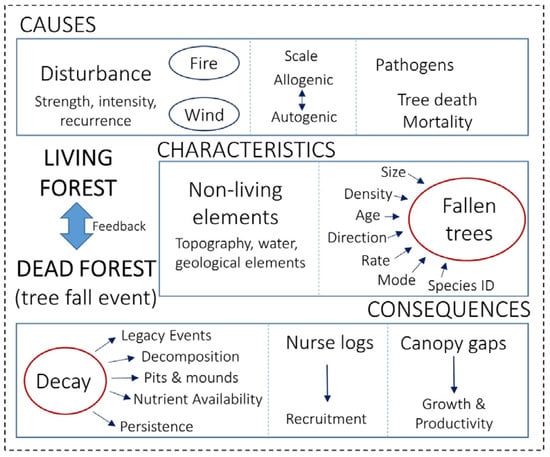
Figure 1.
Summary of the most commonly studied research themes in the treefall literature (broken down by the categories shown in Table 1). Each theme relates to whether the study predominately explored the causes, consequences, or characteristics of the treefall.
3. Treefall Literature: Current Knowledge
The most common terms used in the literature across all groups included ‘gap(s)’, ‘coarse woody debris’ and ‘treefall’ (Table 1). These terms were often used interchangeably and were chosen/defined at the researcher’s discretion, depending on the question or the main finding e.g., [16,17]. Providing clear definitions is crucial to the understanding of treefall and its impact (e.g., at what point does a fallen tree cease to exist as its’ components decay and are incorporated into the soil), but is beyond the scope of this study. Field measurements and observations were the most common type of study, and these were predominately done at an individual- to community-level (Table 1). For the papers that were included in this research synthesis, ecosystem and landscape-scale investigations included mainly reviews, meta-analyses or syntheses as these were the most likely to be heavily cited (Table 1).
3.1. Causes
Mortality of trees was a central focus of the literature across all categories (Table 1) and disturbance events were considered the main drivers of treefall [24,38]. While plant senescence leads trees to be more susceptible to biotic and abiotic factors, the death of the entire individual does not occur often without an external disturbing agent [12]. Fire, extreme wind events and knock-on effects to neighbouring living trees by a treefall event are common examples. It follows that the characteristics of treefall events are strongly correlated with type, magnitude and frequency of disturbance [39]. For instance, the severity of wind damage can vary from the death of a single tree to extensive windthrow [23], depending on storm intensity, timing, and its interaction with local conditions, tree size, and species involved [40,41]. Similarly, the interplay of fire regimes (frequency and intensity) and topography—which affects fire behaviour and fuel load—determines the extent of tree damage and recovery time [26]. Disturbance characteristics also influence the spatio-temporal distribution of standing and fallen dead wood [27,32] and consequently, treefall analysis can be a noninvasive technique for reconstruction of disturbance history and tree death.
3.2. Characteristics
Depending on tree size, treefall can occur through trunk snap or tree uprooting, the latter of which determines the formation of pit-and-mound microtopography [42]. At a fine scale, the common view is that pits and mounds inhibit soil development. For instance, Ulanova [13] found that microsites characterised by pit-and-mound topography differ pedogenically from undisturbed soil, and the time required for soil profile to recover was directly related to uprooting depth. Microsites can differ in light, soil moisture and temperature [43], and their extent is directly related to tree size [44]. However, at the scale of a forest ecosystem, the impact of tree uprooting on soil spatial variability is still poorly understood and more quantitative data are required to fully comprehend the ecological consequences of this phenomenon [25].
Dead trees themselves also provide, through accumulation of coarse woody debris, a sizeable fraction of a mature forest’s stored carbon (biomass), and nutrient budget [12]. Consequently, CWD quantity, quality, and decomposition rates have a crucial influence on nutrient cycling, because large amounts of organic matter are transferred in the soil and/or in the atmosphere [32]. That said, the total amount of CWD in a given forest varies greatly with species composition, stand age, tree size, temperature, and humidity [29,33]. Moreover, landscape features such as slopes and valleys affect CWD spatial distribution and decomposition, with logs tending to move downhill where they are also susceptible to more rapid decay [34,45].
3.3. Consequences
The creation of a canopy gap is arguably the most obvious consequence of treefall in a closed forest. Accordingly, the most common and highly cited research category in the literature on treefall was canopy-gaps and gap-dynamics (Table 1 and Table S1), with 50% of papers focussing on this topic alone e.g., [18,46,47,48]. Depending on their characteristics—particularly size, shape, distribution, and age—canopy gaps introduce environmental heterogeneity locally, determining changes in light levels, soil nutrient availability, litter depth, belowground competition and spatial patterns in regeneration at a landscape level [21,49]. These effects have been recorded in both temperate and tropical forest environments [46], although with exceptions, which were predominantly focused on single-treefall gaps in any forest e.g., [20]. The microhabitats generated by canopy gaps enhance plant regeneration, with the magnitude of this effect depending on forest type, gap characteristics, local conditions and plant functional traits [22]. Gaps can result in: (i) increases biodiversity by facilitating the establishment of pioneer, shade-intolerant species; (ii) rejuvenation of the gene pool, since gaps are mostly colonised by seeds and spores; and (iii) enhanced structural complexity, as species are represented by individuals at different life stages [13,18,21,24]. Gaps can be more or less important depending on the regeneration regime and forest type. For example, in continuously regenerating tropical forests, light is extremely limiting, and gaps here provide regeneration ‘pulses’ that would not otherwise occur without the gap [18]. Conversely, Australian tall eucalypt forests predominately regenerate via a stand-replacing disturbance event and rely less on the continuous availability of gaps [50], although gap size have been correlated with regeneration success in Eucalyptus regnans stands [51]. Despite the influence of canopy gaps on local conditions, both terminology and field work protocols are still inconsistent between studies and therefore results can be difficult to interpret and compare [36].
Other than creating a gap in the canopy, the physical presence of fallen logs also facilitates plant establishment for some tree species, particularly at advanced stages of decay when stored nutrients are more readily accessible [32]. Nutrients and water are released slowly from CWD and hence, when CWD is not removed, they are retained in the ecosystems until plant productivity recovers [52]. However, these dynamics are still poorly understood and results from different studies can be contradictory, or relate to very different process such as seedling establishment versus nutrient dynamics. For example, mounds and decaying wood are important substrates for the germination of the coniferous species Picea abies [13,53], but a study on meso-eutrophic forests found that only one of nine species investigated displayed higher seedling density on logs, suggesting that differences amongst species (in trait characteristics, presence or absence of mycorrhizal associations, for instance) might also play an important role [54]. Furthermore, Laiho and Prescott [55] inferred only a limited role for CWD in the nutrient cycle of north coniferous forests. The positive effect of fallen logs on seedling establishment could then be due to the lower competition with herbs and mosses occurring on CWD compared with soil, and only partially to enhanced nutrient availability [56]. The presence of decaying wood is also crucial for organisms other than plants, such as bryophytes and saproxylic fungi and invertebrates, which rely on spatio-temporal continuity of suitable host trees for their persistence in the forest community [1].
4. Living-Forest Dynamics
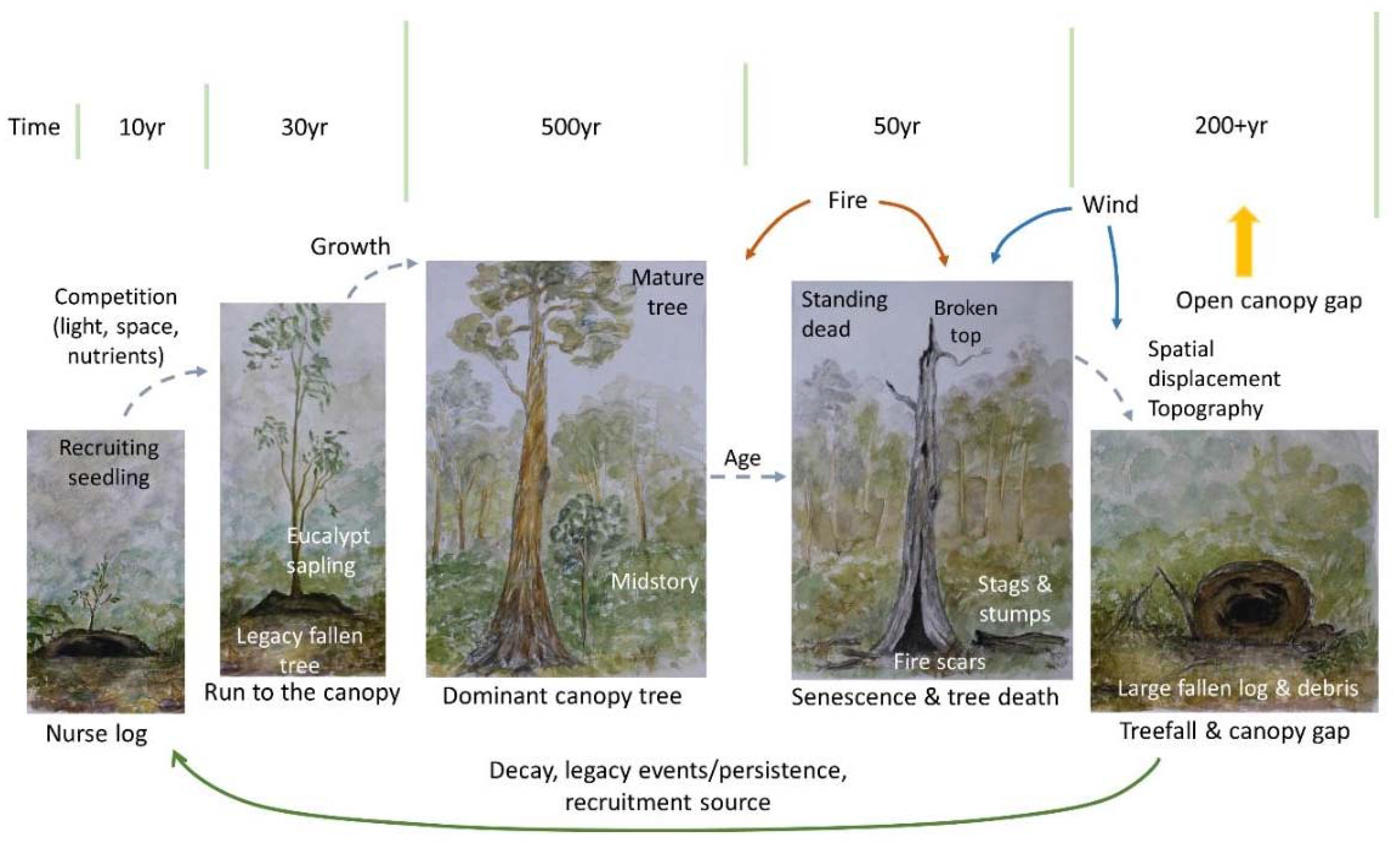
In models the dynamics of a forest classically begins with recruitment and seedling establishment, through growth, maturity and reproduction of the canopy tree, and ends with its death and eventual fall [12]. Over time, the fallen log decays, with this process in turn facilitating many important ecosystem services across space and time, including recruitment (as a nurse log, or via gap-dynamics), decomposition (nutrient turnover, microbial community growth and diversity), habitat for animals or bryophytes, and structural influences on the pattern and growth of living trees; so the cycle begins again (Figure 2).
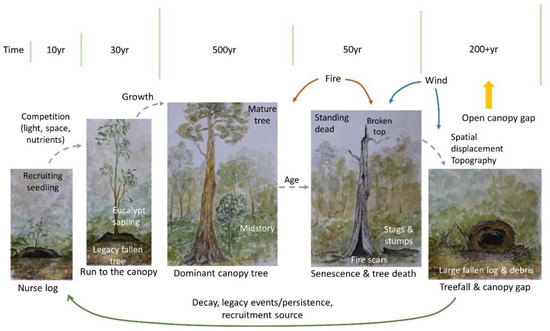
Figure 2.
Example of a classical model of forest dynamics, in the context of canopy trees. It begins with recruitment and growth, and ends with treefall and log decay. The direction of the arrows show movement between states, and where key forest processes may be occurring. The green bars indicate duration of stages—in the case of a stand replacing event, the forest may transition from a living tree, or a stag, directly to regeneration following fire. In these circumstances however, it is unlikely that the fire will result in 100% removal of coarse woody debris (CWD).
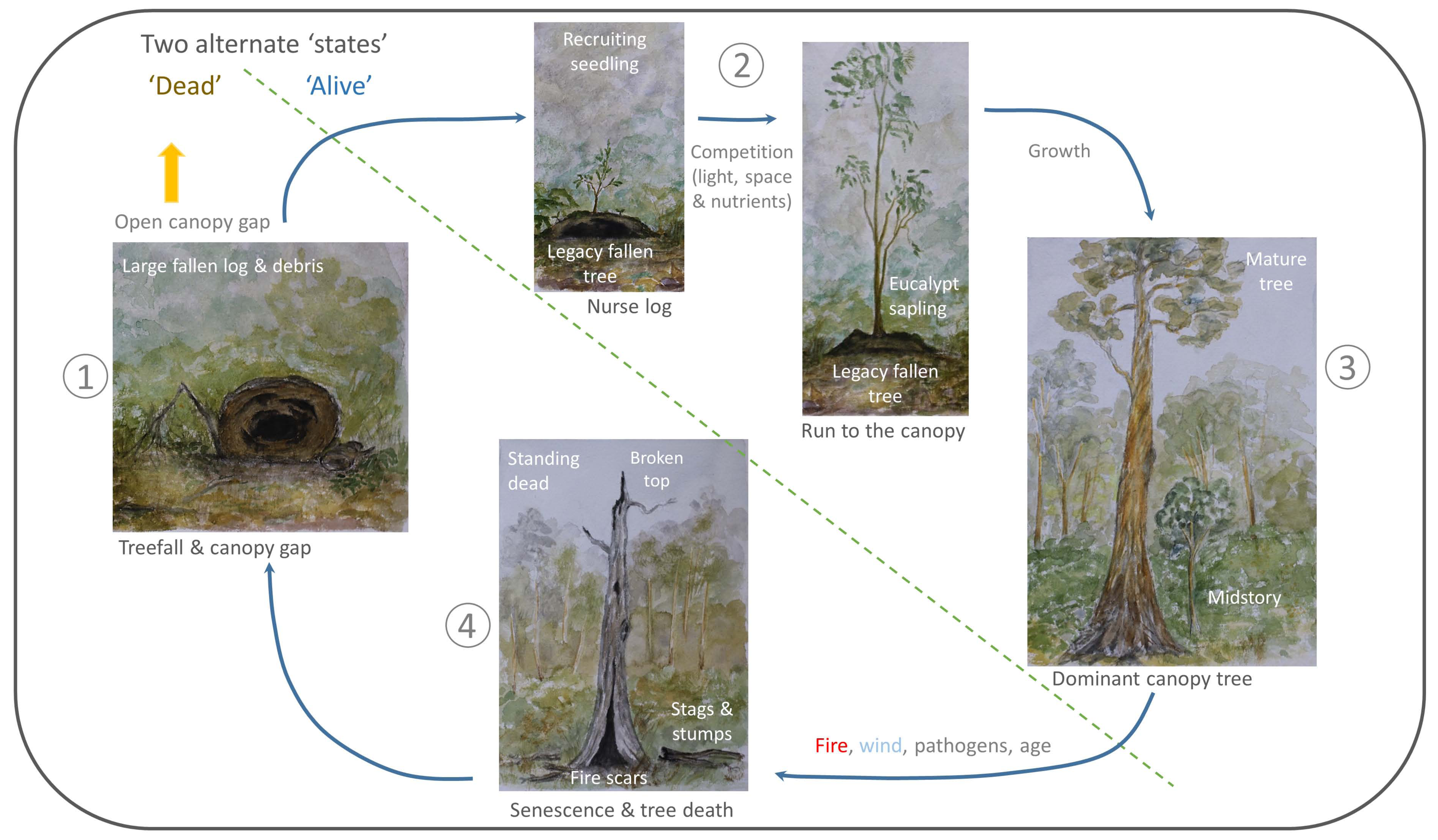
Additionally, treefalls are obvious indicators of disturbance events e.g., [57]. In this context, it is apparent that an important driver of change and structure in this classical tree-focused conceptual model of a forest is the ‘dead’ component—and the dynamical processes that it facilitates. We recognise two defining and inseparable features of forests; the ‘living’ (seedlings, saplings, mature trees) and the ‘dead’ (stags, fallen logs, etc.). The transition between these states needs a stimulus, making a disturbance event (e.g., wind, fire, pathogens) and time, the key to maintaining this dynamic flux (Figure 3).
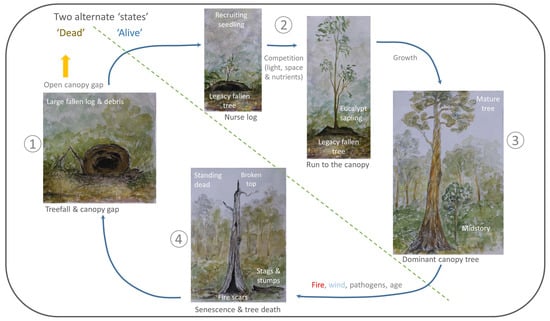
Figure 3.
Example of an alternative conceptual model of forest turnover (cf. Figure 2), with large fallen logs (the ‘dead’ components of forests) as the central focus. The two alternative states of forest turnover are separated by the dotted line, and includes the ‘living’ (with the mature tree as the ‘end’ point of the ‘living’ state) and the ‘dead’ states. The direction of the arrows show movement from one state to the next (with the key processes involved in the movement between states written above the arrows where required). This image is conceptually similar to Figure 2, but depicts both stages being equally as important to the structure and dynamics of an example forest. Disturbances such as fire, wind, pathogens and age are key to the transition into the ‘dead’ state, where canopy gaps, for example in tropical forests, are the key to unlocking continual regeneration of the living state. Note that gap processes can often commence around stags, before the treefall has occurred, due to de-foliation, and stem breakage.
5. A Treefall’s Eye-View of a FOREST—What Is Next?
When looked at from the perspective of treefall, the prevailing state of a forest centres on dead components; standing-dead trees, coarse-woody debris and fallen logs, which typically persist for much longer than it takes for a seed to establish, compete, and race to the canopy. From the perspective of the dead tree (fallen log), the living forest is arguably more unstable and in a constant flux, depending on inputs, environmental conditions (determining decay rates) and disturbance (fire, wind). Observations and measurements of the dead states (e.g., the size, spatial position, decay state and fire scars on a fallen log), can provide a powerful tool for inferring deeper-time ecoevolutionary processes—reaching much further back than the relatively evanescent information provided by observations of only the living components would allow [58,59]. As such, the analysis of treefall allows us to look back into the past using snap-shot patterns and log dating, and so measuring the attributes of the dead forest offers a crucial augmentation to measurements of the sizes, identities and positions of the living trees. Yet the measurement and use of the spatial locations of treefalls remains underexploited in plot-based studies, based on our survey of the literature (Table 1 and Supplementary Materials Table S1). A more explicit focus on the causes and consequences of treefall as more than just ‘an opener of canopy gaps’ might also be useful for improving pattern-oriented models (POM) of forests [60]. This is because analysis of the patterns of the fallen wood should permit an explicit filtering and verification of the adequacy of structural forest models that seek to characterise the interplay between living and dead forest components, and broader community dynamics.
6. Future Directions
The relative stability of the dead component of a forest is largely context-dependent. For example, the persistence of dead wood can, on average, last much longer in cool compared warm rainforests. Indeed, in the tropics, logs tend to decay at faster rates, and the trees often lack distinct growth rings due to a continuous growing season. In cool rainforests, by contrast, the dead wood of some species can persist on the forest floor for decades, and trees show marked growth rings due to seasonal growth periods. However, the general unresponsiveness of the ‘dead forest’ to short-term fluctuations in environmental conditions, allows information on past events and dynamics to be preserved through time.
To properly identify and contextualise the importance of treefall as a key forest process, integrative modelling (e.g., POM) is a necessary approach [61] because it allows for an explicit mechanistic view of functions and feedbacks, as well as permitting sensitivity analysis of key parameters and scenario testing. For instance, a forest represented in silico (e.g., the BEFORE model; [62]) can be used to manipulate treefall frequency, density and occurrence patterns, and assess the role of treefalls in determining equilibrium dynamics, disturbance and the spatial positions and/or growth of living trees, via a simulation that encompasses anything from a cohort of canopy trees through to a model of the entire forest community or ecosystem. Further, ‘bottom-up’ model verification, based on pattern-oriented approaches, can be used to test the influence of multiple predictors on observations (e.g., treefall, in combination with other biotic and abiotic processes such as competition, facilitation, fire, humans). Using POM filters in this way could allow for testing the sensitivity of processes and centrality of treefall in shaping the character and definition of a forest, such as the probability of a phase transition into an alternative state (e.g., degradation into open vegetation, or continued thickening into a heavily closed, continuously regenerating, and gap-dependent system). This type of modelling approach might also help underpin decisions on the resolution and ecological basis of the structural thresholds currently used to define and characterise what a forest is, i.e., what is the biological basis of current thresholds of >10% canopy cover at 5 m in height and covering an area of at least half a hectare [63]?
Two of the key advantages to characterising the metrics of treefall in forest-plot protocols are: (i) the literature already contains ample information on the importance and function of treefalls in forest communities (Table S1, Supplementary Materials); and (ii) because a treefall is relatively easy to observe (it can be readily seen and measured) and persists (in the absence of fire) in the landscape, researchers can take advantage of mensurative experiments (e.g., patchy landscape fires) to infer temporal dynamics of a system based on ‘snap-shot’ patterns. For example, studies comparing different forest types with matched pairs that are either undisturbed or selectively logged (i.e., living trees remain intact but fallen logs are removed), or treefall-frequent (continuously disturbed) versus treefall-infrequent forests, can reveal the importance of fallen and legacy wood in shaping the structure and dynamics of a forest [31]. Additionally, uncovering which forest species benefit most from treefalls, and how treefalls fit in systems that are heavily reliant on mass disturbance and regeneration, could also be a key direction.
Of course, measuring and modelling the dynamic components of forests (e.g., treefalls) will, in some cases, be infeasible. For instance, mapping the size and position of potentially hundreds of fallen logs per hectare is a significant logistical undertaking. Furthermore, the reliability of LiDAR and remote sensing in the spatial analysis of treefall is, although promising, yet to be fully developed, particularly for what concerns logs [64]. The information that can be gained from treefall in any given forest will depend on a variety of factors, like climate, fire frequency, decay rates, and so on. Such factors will influence the rate of transition between the states. For instance, in warmer, drier forests, the frequency of fire and activity of termites will typically be high, removing any lasting legacy of the fallen trees (reaching an extreme in the tropical savannas). This contrasts strongly with cool, wet rain forests, where ancient logs on the forest floor are among the most persistent feature of the ecosystem, shaping its dynamics across time scales that last much longer than a typical plant lifespan [65,66].
The relative importance of treefall to a given forest’s dynamics might also wax and wane over time, and in situations where the forests of a given region switch repeatedly between different states. For instance, in an old-growth forest, ecologically influential treefall events would be rare, because the mature, canopy-forming individuals are long-lived and the mid-storey trees are typically too small to cause consistent disturbance effects [67]. However, when a treefall does occur in such an ecosystem (i.e., after tree death or major disturbance), the magnitude and cascading after-effects of the event can be profound. Another case is forests in which stand-replacing events occur, such as after a rare but intense wildfire or catastrophic storm. This can lead to a persistent unimodal size distribution of trees, with common ages [68]. In such a situation, large individual treefalls might not constitute an important component of the system for decades or centuries; perhaps never, if the return interval of the disturbance is sufficiently frequent. Yet even in these cases, the process of succession might lead to multiple peaks in the frequency distribution of treefalls, derived first from the shortestlived, fastest growing colonist species, and eventually as a result of the stochastic deaths within the climax community of canopy trees.
7. Conclusions
Systematically incorporating dynamic components of a forest like treefall (dead wood) as legacy components into forest-plot measurements and studies of forest processes should encourage researchers to consider and apply more active and standardised approaches to exploring the ways in which patterns link to underlying processes. For instance, snap-shot observations of living trees in forest plots are collected largely because they are thought to capture a suite of deeper-time ecological and evolutionary processes [69]; here we emphasize that patterns in the dead forest are just as important in realising this goal. Forests should thus be envisaged as not just a static landscape type, but as a complex system that can be theorised, observed, experimented and modelled in a consistent way. This sentiment was echoed centuries ago by the French explorer, Bruni D’Entrecasteaux, who upon seeing the majestic tall forests of Tasmania wrote: “nature in all her vigour, and yet in a state of decay seems to offer to the imagination something more picturesque and more imposing than the sight of this same nature bedecked by the hand of civilised man.” [70]. The science of forest ecology ought to capture the vigour of these systems that so impressed D’Entrecasteaux, and this begins by progressing and enhancing our understanding of forests, both vital (living) and decaying (dead), into more of an integrative framework.
Supplementary Materials
The following is available online at www.mdpi.com/1999-4907/8/4/123/s1. Table S1: Synthesis of the global literature on dead-wood forest components.
Acknowledgments
Author Contributions
J.C.B. and B.W.B. conceived the ideas; J.C.B. and S.O. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siitonen, J. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Fridman, J.; Walheim, M. Amount, structure, and dynamics of dead wood on managed forestland in Sweden. For. Ecol. Manag. 2000, 131, 23–36. [Google Scholar] [CrossRef]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Ganey, J.L.; Bird, B.J.; Baggett, L.S.; Jenness, J.S. Density of large snags and logs in northern Arizona mixed-conifer and ponderosa pine forests. For. Sci. 2015, 61, 353–362. [Google Scholar] [CrossRef]
- Rogers, P. Disturbance Ecology and for. Management: A Review of the Literature; US Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1996.
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities: Positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. BioScience 2001, 51, 235–246. [Google Scholar]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.; Anthelme, F. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of temperate forest productivity to insect and pathogen disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Orwin, K.H.; Wardle, D.A.; Greenfield, L.G. Context-dependent changes in the resistance and resilience of soil microbes to an experimental disturbance for three primary plant chronosequences. Oikos 2006, 112, 196–208. [Google Scholar] [CrossRef]
- Filotas, E.; Parrott, L.; Burton, P.J.; Chazdon, R.L.; Coates, K.D.; Coll, L.; Haeussler, S.; Martin, K.; Nocentini, S.; Puettmann, K.J. Viewing forests through the lens of complex systems science. Ecosphere 2014, 5, 1–23. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Fajardo, A. Beyond description: The active and effective way to infer processes from spatial patterns. Ecology 2009, 90, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.F.; Shugart, H.H.; Harmon, M.E. Tree death as an ecological process. BioScience 1987, 37, 550–556. [Google Scholar] [CrossRef]
- Ulanova, N.G. The effects of windthrow on forests at different spatial scales: A review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Siitonen, J.; Martikainen, P.; Punttila, P.; Rauh, J. Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. For. Ecol. Manag. 2000, 128, 211–225. [Google Scholar] [CrossRef]
- You, H.; He, D.; You, W.; Xiao, S.; Hong, W. Spatial distribution pattern of coarse woody debris (cwd) in two typical forest types in Tianbaoyan National Nature Reserve. Proceedings of 2012 World Automation Congress (Wac), Puerto Vallarta, Mexico, 24–28 June 2012; pp. 1–8. [Google Scholar]
- Soderberg, U.; Wulff, S.; Stahl, G. The choice of definition has a large effect on reported quantities of dead wood in boreal forest. Scand. J. For. Res. 2014, 29, 252–258. [Google Scholar] [CrossRef]
- Guby, N.A.B.; Dobbertin, M. Quantitative estimates of coarse woody debris and standing dead trees in selected Swiss forests. Glob. Ecol. Biogeogr. Lett. 1996, 5, 327–341. [Google Scholar] [CrossRef]
- Brokaw, N.V.L. Gap-phase regeneration in a tropical forest. Ecology 1985, 66, 682–687. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef]
- Uhl, C.; Clark, K.; Dezzeo, N.; Maquirino, P. Vegetation dynamics in Amazonian treefall gaps. Ecology 1988, 69, 751–763. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, D.; Zhang, W. Effects of gaps on regeneration of woody plants: A meta-analysis. J. For. Res. 2014, 25, 501–510. [Google Scholar] [CrossRef]
- Canham, C.D.; Loucks, O.L. Catastrophic windthrow in the presettlement forests of Wisconsin. Ecology 1984, 65, 803–809. [Google Scholar] [CrossRef]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Šamonil, P.; Král, K.; Hort, L. The role of tree uprooting in soil formation: A critical literature review. Geoderma 2010, 157, 65–79. [Google Scholar] [CrossRef]
- Bassett, M.; Chia, E.K.; Leonard, S.W.J.; Nimmo, D.G.; Holland, G.J.; Ritchie, E.G.; Clarke, M.F.; Bennett, A.F. The effects of topographic variation and the fire regime on coarse woody debris: Insights from a large wildfire. For. Ecol. Manag. 2015, 340, 126–134. [Google Scholar] [CrossRef]
- Lugo, A.E.; Scatena, F.N. Background and catastrophic tree mortality in tropical moist, wet, and rain forests. Biotropica 1996, 28, 585–599. [Google Scholar] [CrossRef]
- Sollins, P. Input and decay of coarse woody debris in coniferous stands in western Oregon and Washington. Can. J. For. Res. 1982, 12, 18–28. [Google Scholar] [CrossRef]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.; Zanne, A.E.; Wirth, C.; Coomes, D.A. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.J.M.; Battles, J.J.; Sanders, J.E.; York, R.A. Decay patterns and carbon density of standing dead trees in California mixed conifer forests. For. Ecol. Manag. 2015, 353, 136–147. [Google Scholar] [CrossRef]
- Maser, C. The Seen and Unseen World of the Fallen Tree; General Technical Report; USDA Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1984; p. 56.
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.; Lattin, J.; Anderson, N.; Cline, S.; Aumen, N.; Sedell, J. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 302. [Google Scholar]
- Woldendorp, G.; Keenan, R.J. Coarse woody debris in Australian forest ecosystems: A review. Aust. Ecol. 2005, 30, 834–843. [Google Scholar] [CrossRef]
- Oberle, B.; Milo, A.; Myers, J.A.; Young, D.F.; Walton, M.L.; Zanne, A.E. Direct estimates of downslope deadwood movement over 30 years in a temperature forest illustrate impacts of treefall on forest ecosystem dynamics. Cana. J. For. Res. 2015. [CrossRef]
- Lorimer, C.G. Methodological considerations in the analysis of forest disturbance history. Can. J. For. Res. 1985, 15, 200–213. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bockheim, J.G. Methods for studying treefall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Fisher, B.L.; Howe, H.F.; Wright, S.J. Survival and growth of Virola surinamensis yearlings—Water augmentation in gap and understory. Oecologia 1991, 86, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Dead wood in European beech (Fagus sylvatica) forest reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Jonsson, B.G.; Dynesius, M. Uprooting in boreal spruce forests: Long-term variation in disturbance rate. Can. J. For. Res. 1993, 23, 2383–2388. [Google Scholar] [CrossRef]
- Everham, E.M.; Brokaw, N.V.L. Forest damage and recovery from catastrophic wind. Bot. Rev. 1996, 62, 113–185. [Google Scholar] [CrossRef]
- Rich, R.L.; Frelich, L.E.; Reich, P.B. Wind-throw mortality in the southern boreal forest: Effects of species, diameter and stand age. J. Ecol. 2007, 95, 1261–1273. [Google Scholar] [CrossRef]
- Peterson, C.J.; Pickett, S.T.A. Treefall and resprouting following catastrophic windthrow in an old-growth hemlock-hardwoods forest. For. Ecol. Manag. 1991, 42, 205–217. [Google Scholar] [CrossRef]
- Peterson, C.J.; Carson, W.P.; McCarthy, B.C.; Pickett, S.T.A. Microsite variation and soil dynamics within newly created treefall pits and mounds. Oikos 1990, 58, 39–46. [Google Scholar] [CrossRef]
- Sobhani, V.M.; Barrett, M.; Peterson, C.J. Robust prediction of treefall pit and mound sizes from tree size across 10 forest blowdowns in eastern north America. Ecosystems 2014, 17, 837–850. [Google Scholar] [CrossRef]
- Zanne, A.E.; Oberle, B.; Dunham, K.M.; Milo, A.M.; Walton, M.L.; Young, D.F. A deteriorating state of affairs: How endogenous and exogenous factors determine plant decay rates. J. Ecol. 2015, 103, 1421–1431. [Google Scholar] [CrossRef]
- Canham, C.D.; Denslow, J.S.; Platt, W.J.; Runkle, J.R.; Spies, T.A.; White, P.S. Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can. J. For. Res. 1990, 20, 620–631. [Google Scholar] [CrossRef]
- Whitmore, T. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Arevalo, J.R.; Fernandez-Palacios, J.M. Treefall gap characteristics and regeneration in the laurel forest of Tenerife. J. Veg. Sci. 1998, 9, 297–306. [Google Scholar] [CrossRef]
- Bowman, D.; Kirkpatrick, J. Establishment, suppression and growth of Eucalyptus delegatensis R.T. Baker in multiaged forests. iii. Intraspecific allelopathy, competition between adult and juvenile for moisture and nutrients, and frost damage to seedlings. Aust. J. Bot. 1986, 34, 81–94. [Google Scholar] [CrossRef]
- Attiwill, P.M. Ecological disturbance and the conservative management of eucalypt forests in Australia. For. Ecol. Manag. 1994, 63, 301–346. [Google Scholar] [CrossRef]
- Van Der Meer, P.J.; Dignan, P.; Saveneh, A.G. Effect of gap size on seedling establishment, growth and survival at three years in mountain ash (Eucalyptus regnans F. Muell.) forest in Victoria, Australia. For. Ecol. Manag. 1999, 117, 33–42. [Google Scholar] [CrossRef]
- Harmon, M.E.; Hua, C. Coarse woody debris dynamics in two old-growth ecosystems. BioScience 1991, 41, 604–610. [Google Scholar] [CrossRef]
- Zielonka, T. When does dead wood turn into a substrate for spruce replacement? J. Veg. Sci. 2006, 17, 739–746. [Google Scholar] [CrossRef]
- Chećko, E.; Jaroszewicz, B.; Olejniczak, K.; Kwiatkowska-Falińska, A.J. The importance of coarse woody debris for vascular plants in temperate mixed deciduous forests 1. Can. J. For. Res. 2015, 45, 1154–1163. [Google Scholar] [CrossRef]
- Laiho, R.; Prescott, C.E. Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: A synthesis. Can. J. For. Res. 2004, 34, 763–777. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F. Tree seedlings on logs in picae-tsuga forests of Oregon and Washington. Ecology 1989, 70, 48–59. [Google Scholar] [CrossRef]
- Van Der Meer, P.J.; Bongers, F. Patterns of tree-fall and branch-fall in a tropical rain forest in French Guiana. J. Ecol. 1996, 84, 19–29. [Google Scholar] [CrossRef]
- Boswijk, G.; Fowler, A.; Palmer, J.; Fenwick, P.; Hogg, A.; Lorrey, A.; Wunder, J. The late Holocene kauri chronology: Assessing the potential of a 4500-year record for palaeoclimate reconstruction. Quat. Sci. Rev. 2014, 90, 128–142. [Google Scholar] [CrossRef]
- Swetnam, T.W. Fire history and climate change in giant sequoia groves. Science 1993, 262, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, T.; Jeltsch, F.; Hanski, I.; Grimm, V. Using pattern-oriented modeling for revealing hidden information: A key for reconciling ecological theory and application. Oikos 2003, 100, 209–222. [Google Scholar] [CrossRef]
- Grimm, V.; Revilla, E.; Berger, U.; Jeltsch, F.; Mooij, W.M.; Railsback, S.F.; Thulke, H.-H.; Weiner, J.; Wiegand, T.; DeAngelis, D.L. Pattern-oriented modeling of agent-based complex systems: Lessons from ecology. Science 2005, 310, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, C.; Neuert, C.; Grundmann, V.; Wissel, C.; Grimm, V. Reconstructing spatiotemporal dynamics of central European natural beech forests: The rule-based forest model before. For. Ecol. Manag. 2004, 194, 349–368. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation (FAO) of the United Nations. Forest Resources Assessment: Terms and Definitions of Forests; FAO Forestry Department: Rome, Italy, 2012. [Google Scholar]
- Wing, B.M.; Ritchie, M.W.; Boston, K.; Cohen, W.B.; Gitelman, A.; Olsen, M.J. Prediction of understory vegetation cover with airborne lidar in an interior ponderosa pine forest. Remote Sens. Environ. 2012, 124, 730–741. [Google Scholar] [CrossRef]
- Vanderwel, M.C.; Malcolm, J.R.; Smith, S.M. An integrated model for snag and downed woody debris decay class transitions. For. Ecol. Manag. 2006, 234, 48–59. [Google Scholar] [CrossRef]
- Sollins, P.; Cline, S.P.; Verhoeven, T.; Sachs, D.; Spycher, G. Patterns of log decay in old-growth douglas-fir forests. Cana. J. For. Res. 1987, 17, 1585–1595. [Google Scholar] [CrossRef]
- Larson, A.J.; Lutz, J.A.; Donato, D.C.; Freund, J.A.; Swanson, F.J.; HilleRisLambers, J.; Sprugel, D.G.; Franklin, J.F. Spatial aspects of tree mortality strongly differ between young and old-growth forests. Ecology 2015, 11, 2855–2861. [Google Scholar] [CrossRef]
- Muir, P.S. Disturbance effects on structure and tree species composition of Pinus contorta forests in western Montana. Can. J. For. Res. 1993, 23, 1617–1625. [Google Scholar] [CrossRef]
- Aakala, T.; Kuuluvainen, T.; De Grandpre, L.; Gauthier, S. Trees dying standing in the northeastern boreal old-growth forests of Quebec: Spatial patterns, rates, and temporal variation. Can. J. For. Res. 2007, 37, 50–61. [Google Scholar] [CrossRef]
- Duyker, E.; Duyker, M. Bruny D’entrecasteaux: Voyage to Australia and the Pacific 1791–1793; Miegunyah/Melbourne University Press: Melbourne, Australia, 2006; p. 392. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).