Differences in the Response to Acute Drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design and Inoculation
2.3. Parameters Measured
2.4. Pathogen Isolation
2.5. Data Analysis
3. Results
3.1. Plant and Root Symptoms
3.2. Water Status
3.3. Photosynthesis
3.4. Growth and Biomass Allocation
4. Discussion
General Symptoms and Stress Indicators
Physiological Changes
Biomass allocation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Rigo, D.; Cadullo, G. Quercus ilex in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; pp. 152–153. [Google Scholar]
- Quercus, L.; Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks Physiological Ecology. Exploring the Functional Diversity of Genus; Tree Physiology; Springer International Publishing: Cham, Switzerland, 2017; Volume 7, ISBN 978-3-319-69098-8. [Google Scholar]
- Guzmán Álvarez, J.R. The image of a tamed landscape: Dehesa through history in Spain. Cult. Hist. Digit. J. 2016, 5, 003. [Google Scholar] [CrossRef]
- Pinto-Correia, T.; Azeda, C. Public policies creating tensions in Montado management models: Insights from farmers’ representations. Land Use Policy 2017, 64, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Consejería de Agricultura y Pesca. Caracterización Socioeconómica de la Dehesa en Andalucía; Secretaría General Técnica, Servicio de Publicaciones y Divulgación; Junta de Andalucía Consejería de Agricultura y Pesca: Sevilla, Spain, 2008. [Google Scholar]
- Gea-Izquierdo, G.; Fernández-de-Uña, L.; Cañellas, I. Growth projections reveal local vulnerability of Mediterranean oaks with rising temperatures. For. Ecol. Manag. 2013, 305, 282–293. [Google Scholar] [CrossRef]
- Swiecki, T.J.; Berndhart, E.A. Testing and implementing methods for managing Phytophthora root diseases in California native habitats and restoration sites. In Proceedings of the Sudden Oak Death Sixth Science Symposium, San Francisco, CA, USA, 20–23 June 2016; p. 53. [Google Scholar]
- Brasier, C.M.; Robredo, F.; Ferraz, J.F.P. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993, 42, 140–145. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant. Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Moralejo, E.; García-Muñoz, J.A.; Descals, E. Susceptibility of Iberian trees to Phytophthora ramorum and P. cinnamomi. Plant Pathol. 2009, 58, 271–283. [Google Scholar] [CrossRef]
- Ruiz Gómez, F.J.; Sanchez-Cuesta, R.; Navarro-Cerrillo, R.M.; Perez-de-Luque, A. A method to quantify infection and colonization of holm oak (Quercus ilex) roots by Phytophthora cinnamomi. Plant Methods 2012, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Gómez, F.J.; Navarro-Cerrillo, R.M.; Sánchez-Cuesta, R.; Pérez-de-Luque, A. Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathol. 2015, 64, 605–616. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric. For. Meteorol. 2014, 192–193, 1–8. [Google Scholar] [CrossRef]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Horta Jung, M.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant–Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Sghaier-Hammami, B.; Valero-Galvàn, J.; Romero-Rodríguez, M.C.; Navarro-Cerrillo, R.M.; Abdelly, C.; Jorrín-Novo, J. Physiological and proteomics analyses of Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) responses to Phytophthora cinnamomi. Plant Physiol. Biochem. 2013, 71, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Corcobado, T.; Cubera, E.; Moreno, G.; Solla, A. Quercus ilex forests are influenced by annual variations in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi. Agric. For. Meteorol. 2013, 169, 92–99. [Google Scholar] [CrossRef]
- León, I.; García, J.; Fernández, M.; Vázquez-Piqué, J.; Tapias, R. Differences in root growth of Quercus ilex and Quercus suber seedlings infected with Phytophthora cinnamomi. Silva Fenn. 2017, 51. [Google Scholar] [CrossRef]
- Maurel, M.; Robin, C.; Capron, G.; Desprez-Loustau, M.-L. Effects of root damage associated with Phytophthora cinnamomi on water relations, biomass accumulation, mineral nutrition and vulnerability to water deficit of five oak and chestnut species. For. Pathol. 2001, 31, 353–369. [Google Scholar] [CrossRef]
- Turco, E.; Close, T.J.; Fenton, R.D.; Ragazzi, A. Synthesis of dehydrin-like proteins in Quercus ilex L. and Quercus cerris L. seedlings subjected to water stress and infection with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2004, 65, 137–144. [Google Scholar] [CrossRef]
- Bongers, F.J.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.P.R.; Villar, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control: Tansley review. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, Y.; Xu, T.; Wang, Y.; Wang, H.; Duan, B. Root order-dependent seasonal dynamics in the carbon and nitrogen chemistry of poplar fine roots. New For. 2017, 48, 587–607. [Google Scholar] [CrossRef]
- Lopez-Iglesias, B.; Villar, R.; Poorter, L. Functional traits predict drought performance and distribution of Mediterranean woody species. Acta Oecol. 2014, 56, 10–18. [Google Scholar] [CrossRef]
- Olmo, M.; Lopez-Iglesias, B.; Villar, R. Drought changes the structure and elemental composition of very fine roots in seedlings of ten woody tree species. Implications for a drier climate. Plant Soil 2014, 384, 113–129. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Navarro Cerrillo, R.M.; Ruiz Gómez, F.J.; Cabrera-Puerto, R.J.; Sánchez-Cuesta, R.; Palacios Rodriguez, G.; Quero Pérez, J.L. Growth and physiological sapling responses of eleven Quercus ilex ecotypes under identical environmental conditions. For. Ecol. Manag. 2018, 415, 58–69. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R. Interactions of drought and shade effects on seedlings of four Quercus species: Physiological and structural leaf responses. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, R.; Longobucco, A.; Miglietta, F.; Raschi, A. Transpiration and stomatal behaviour of Quercus ilex plants during the summer in a Mediterranean carbon dioxide spring. Plant Cell Environ. 1998, 21, 613–622. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Available online: https://www.nature.com/articles/nmeth.2089 (accessed on 14 March 2018).
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Jeffers, S.N. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 1986, 70, 1038–1043. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, NY, USA, 1996; ISBN 978-0-89054-212-5. [Google Scholar]
- Valladares, F.; Sánchez-Gómez, D. Ecophysiological traits associated with drought in Mediterranean tree seedlings: Individual responses versus interspecific trends in eleven species. Plant Biol. 2006, 8, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Milton, J.S.; Tsokos, J.O. Statistical Methods in the Biological and Health Sciences; McGraw-Hill: New York, NY, USA, 1983; ISBN 978-0-07-042359-6. [Google Scholar]
- Canny, M. Tyloses and the maintenance of transpiration. Ann. Bot. 1997, 80, 565–570. [Google Scholar] [CrossRef]
- Borghetti, M.; Grace, J.; Raschi, A. Water transport in plants under climatic stress. In Proceedings of the International Workshop, Firenze, Italy, 29–31 May 1990. [Google Scholar]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.L.; Sterck, F.J.; Martínez-Vilalta, J.; Villar, R. Water-use strategies of six co-existing Mediterranean woody species during a summer drought. Oecologia 2011, 166, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Aigbe, S.O.; Remison, S.U. The Influence of root rot on dry matter partition of three cassava cultivars planted in different agro-ecological environments. Asian J. Plant Pathol. 2010, 4, 82–89. [Google Scholar] [CrossRef]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Tuset, J.J.; Hinarejos, C.; Mira, J.L.; Cobos, J.M. Implicación de Phytophthora cinnamomi Rands en la enfermedad de la «seca» de encinas y alcornoques. Bol. Sanid. Veg. Plagas 1996, 22, 491–499. [Google Scholar]
- Robin, C.; Capron, G.; Desprez-Loustau, M.L. Root infection by Phytophthora cinnamomi in seedlings of three oak species. Plant Pathol. 2001, 50, 708–716. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.P.; Romero-Rodríguez, M.C.; Sánchez-Lucas, R.; Navarro-Cerrillo, R.M.; Medina-Aunon, J.A.; Jorrín-Novo, J.V. 2-DE proteomics analysis of drought treated seedlings of Quercus ilex supports a root active strategy for metabolic adaptation in response to water shortage. Front. Plant Sci. 2015, 6, 627. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.A.; Perez-Sierra, A.; Abad-Campos, P.; Torres, L.; Solla, A.; Reig-Arminana, J.; Garcia-Breijo, F. Histology of Quercus ilex roots during infection by Phytophthora cinnamomi. Trees-Struct. Funct. 2015, 29, 1943–1957. [Google Scholar] [CrossRef]
- Blaschke, H. Decline symptoms on roots of Quercus robur. For. Pathol. 1994, 24, 386–398. [Google Scholar] [CrossRef]
- Manes, F.; Vitale, M.; Donato, E.; Giannini, M.; Puppi, G. Different ability of three Mediterranean oak species to tolerate progressive water stress. Photosynthetica 2006, 44, 387–393. [Google Scholar] [CrossRef]
- Leiva, M.J.; Fernández-Alés, R. Variability in seedling water status during drought within a Quercus ilex subsp. ballota population, and its relation to seedling morphology. For. Ecol. Manag. 1998, 111, 147–156. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Enrı́quez, E.; Rubira, J.P. Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. For. Ecol. Manag. 2004, 196, 257–266. [Google Scholar] [CrossRef]
- Jönsson, U. Phytophthora species and oak decline—Can a weak competitor cause significant root damage in a nonsterilized acidic forest soil? New Phytol. 2004, 162, 211–222. [Google Scholar] [CrossRef]
- Cahill, D. Cellular and histological changes induced by Phytophthora cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology 1989, 79, 417–424. [Google Scholar] [CrossRef]
- Sánchez, M.; Caetano, P.; Ferraz, J.; Trapero, A. Phytophthora disease of Quercus ilex in south-western Spain. For. Pathol. 2002, 32, 5–18. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Andicoberry, S.; Trapero, A. Pathogenicity of three Phytophthora spp. causing late seedling rot of Quercus ilex ssp. ballota. For. Pathol. 2005, 35, 115–125. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Belisario, A.; Maccaroni, M.; Vannini, A. Evaluation of root damage to English walnut caused by five Phytophthora species. Plant Pathol. 2003, 52, 491–495. [Google Scholar] [CrossRef]



| Variable | Abv. | Units | Description |
|---|---|---|---|
| Water status | |||
| Volumetric Water content | ϴ | cm3 (cm)−3 | Relative humidity of pot substrate |
| Midday Water potential | Ψm | MPa | Water potential of leaves at midday (at solar noon) |
| Dry Matter of root | DMr | g (100 g)−1 | Dry matter of root relative to 100 g of fresh weight |
| Plant growth and biomass allocation | |||
| Net Photosynthesis | A | μmol CO2 m−2 s−1 | Mean estimate of photosynthetic rate of leaves |
| Stomatal Conductance | Gs | mmol H2O m−2 s−1 | Mean estimate leaf stomatal conductance |
| Photosynthetic Efficiency | QY | … | Max. quantum efficiency of photosystem II (Fv/Fm) |
| Root Dry Weight | RDW | g | Weight of root system after oven drying (root biomass) |
| Stem Dry Weight | SDW | g | Weight of stem after oven drying (stem biomass) |
| Leaf Dry Weight | LDW | g | Weight of leaves after oven drying (leaves biomass) |
| Root Mass Fraction | RMF | g (g)−1 | Root biomass relative to total plant biomass |
| Stem Mass Fraction | SMF | g (g)−1 | Stem biomass relative to total plant biomass |
| Leaf Mass Fraction | LMF | g (g)−1 | Leaves biomass relative to total plant biomass |
| Factors | df | Experimental Variable | |||
|---|---|---|---|---|---|
| A | Gs | QY | ϴ | ||
| Between-subjects source | |||||
| Inoculation (I) | 1 | 31.531 *** | 0.016 ** | 0.183 ** | 0.047 * |
| Drought (D) | 1 | 86.041 *** | 0.010 * | 0.001 | 0.660 ** |
| I × D | 1 | 0.394 | 0.000 | 0.351 *** | 0.002 |
| Error | 16 | 31.698 | 0.023 | 0.304 | 0.058 |
| Within-subjects source | |||||
| Days (dpi) | 4 | 22.821 | 0.007 | 0.262 *** | 0.901 *** |
| dpi × I | 4 | 3.254 | 0.003 | 0.015 | 0.002 |
| dpi × D | 4 | 77.014 *** | 0.007 | 0.162 ** | 0.147 *** |
| dpi × I × D | 4 | 8.279 | 0.001 | 0.051 | 0.001 |
| Error | 64 | 139.518 | 0.051 | 0.472 | 0.02 |
| Factors | ||||
|---|---|---|---|---|
| Variable | Inoculation (I) | Drought (D) | I × D | R2 |
| Ψm | 2.4 | 6763.7 *** (−) | 2.4 | 0.99 *** |
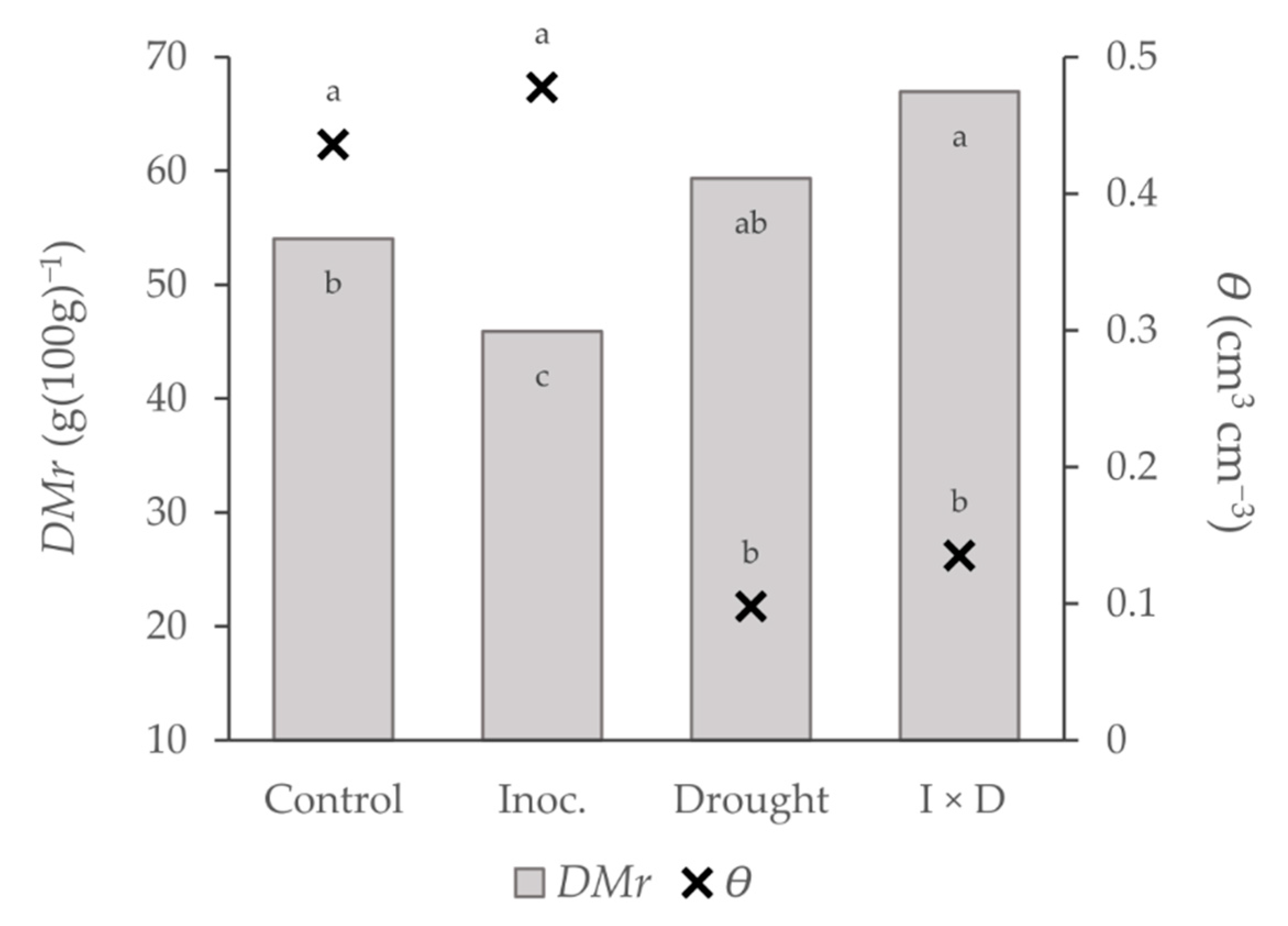
| DMr | 0.0 | 25.1 *** (+) | 8.0 ** | 0.61 *** |
| RDW | 0.7 | 4.3 * (+) | 0.0 | 0.24 ** |
| SDW | 5.0 * (−) | 3.3 | 5.5 * | 0.46 *** |
| RMF | 10 ** (−) | 41.7 *** (+) | 0.0 n/s | 0.76 *** |
| SMF | 5.0 * (−) | 12.4 ** (−) | 7.6 ** | 0.61 *** |
| LMF | 18.8 *** (+) | 8.8 ** (−) | 3.9 n/s | 0.66 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Gómez, F.J.; Pérez-de-Luque, A.; Sánchez-Cuesta, R.; Quero, J.L.; Navarro Cerrillo, R.M. Differences in the Response to Acute Drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. Seedlings. Forests 2018, 9, 634. https://doi.org/10.3390/f9100634
Ruiz Gómez FJ, Pérez-de-Luque A, Sánchez-Cuesta R, Quero JL, Navarro Cerrillo RM. Differences in the Response to Acute Drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. Seedlings. Forests. 2018; 9(10):634. https://doi.org/10.3390/f9100634
Chicago/Turabian StyleRuiz Gómez, Francisco J., Alejandro Pérez-de-Luque, Rafael Sánchez-Cuesta, José L. Quero, and Rafael M. Navarro Cerrillo. 2018. "Differences in the Response to Acute Drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. Seedlings" Forests 9, no. 10: 634. https://doi.org/10.3390/f9100634
APA StyleRuiz Gómez, F. J., Pérez-de-Luque, A., Sánchez-Cuesta, R., Quero, J. L., & Navarro Cerrillo, R. M. (2018). Differences in the Response to Acute Drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. Seedlings. Forests, 9(10), 634. https://doi.org/10.3390/f9100634






