The Role of HLA and KIR Immunogenetics in BK Virus Infection after Kidney Transplantation
Abstract
:1. Introduction
2. An Overview of BKV Infection and BKVAN in Kidney Transplantation
2.1. Risk Factors, Screening and Treatment of BKV Infection and BKVAN
2.2. Prognosis of BKV Infection and BKVAN
3. Association of HLA with BKV Infection and BKVAN
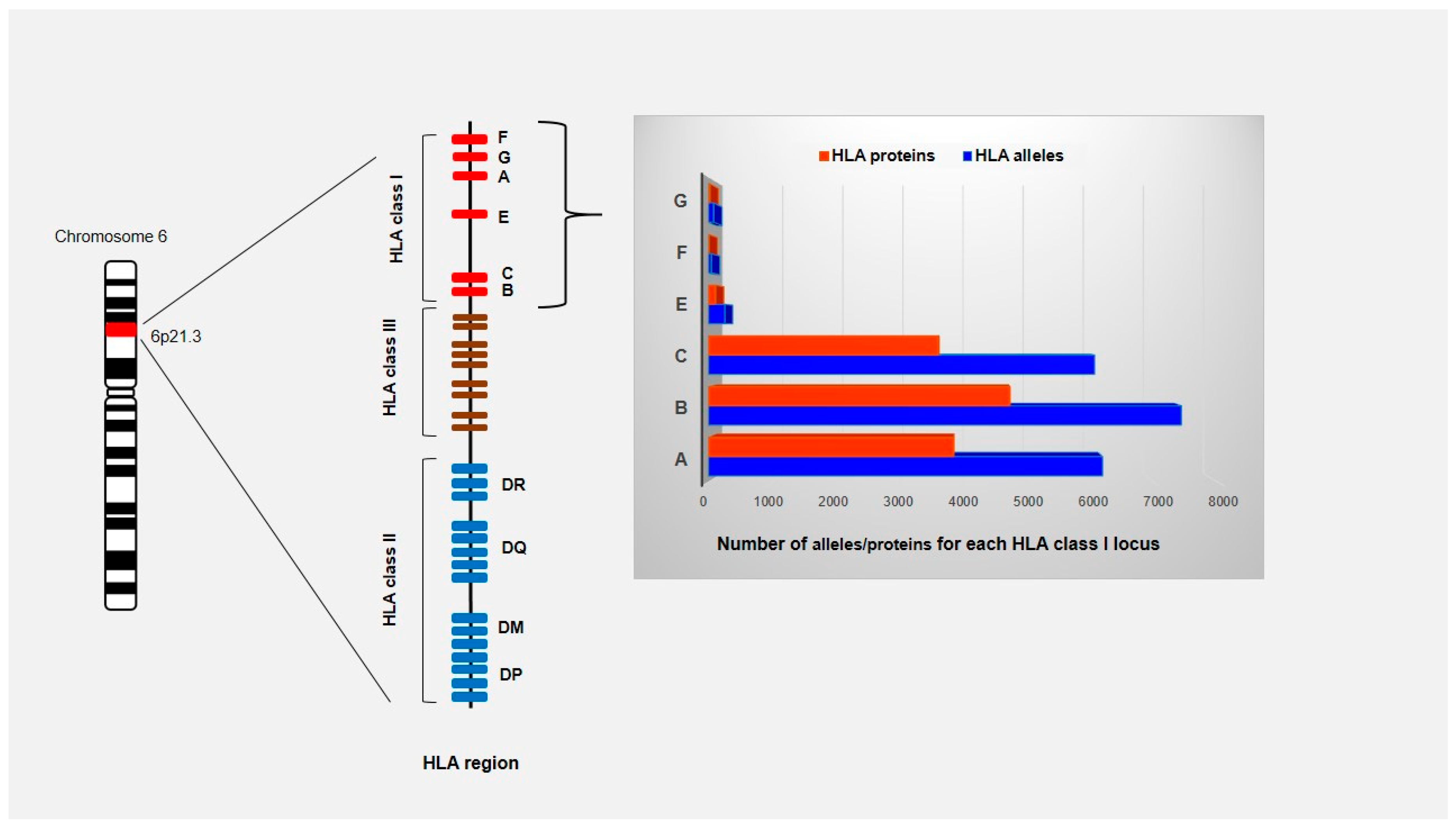
3.1. HLA Alleles
3.2. HLA Mismatch, HLA and ABO Blood Group System Incompatibility and Desensitization
3.3. HLA Panel Reactive Antibodies
3.4. HLA Donor-Specific Antibodies
4. Duality of NK Cells in Kidney Transplantation
4.1. Role of NK Cells in ABMR Mediated through Fc Receptor (CD16)
4.2. Role of NK Cells in Viral Infections Mediated through KIRs
4.3. Role of KIR-HLA Interactions on NK Cell Responsiveness in ABMR
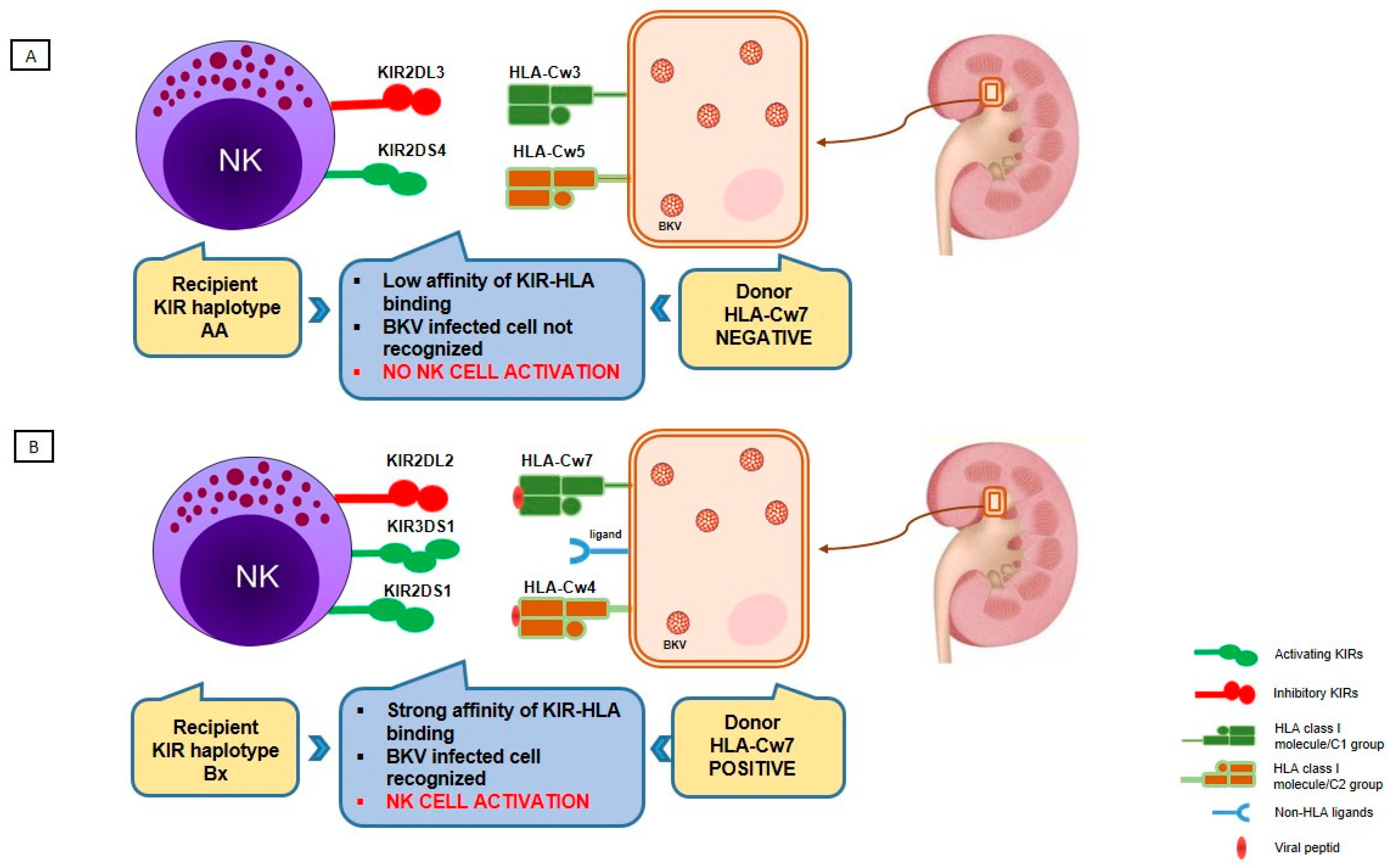
5. Role of KIR and Prognostic Implications of KIR Type in BKV and BKVAN
6. HLA, KIR and BKV
7. Future Directions of Research
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gardner, S.; Field, A.; Coleman, D.; Hulme, B. New Human Papovavirus (b.k.) Isolated from Urine after Renal Transplantation. Lancet 1971, 297, 1253–1257. [Google Scholar] [CrossRef]
- Helle, F.; Brochot, E.; Handala, L.; Martin, E.; Castelain, S.; François, C.; Duverlie, G. Biology of the BKPyV: An Update. Viruses 2017, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongprayoon, C.; Khoury, N.J.; Bathini, T.; Aeddula, N.R.; Boonpheng, B.; Leeaphorn, N.; Ungprasert, P.; Bruminhent, J.; Lertjitbanjong, P.; Watthanasuntorn, K.; et al. BK polyomavirus genotypes in renal transplant recipients in the United States: A meta-analysis. J. Evid. Based Med. 2019, 12, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Infanti, L.; Dumoulin, A.; Buser, A.; Samaridis, J.; Stebler, C.; Gosert, R.; Hirsch, H.H. Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors. J. Infect. Dis. 2009, 199, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanchiere, J.A.; Abudayyeh, S.; Copeland, C.M.; Lu, L.B.; Graham, D.Y.; Butel, J.S. Polyomavirus Shedding in the Stool of Healthy Adults. J. Clin. Microbiol. 2009, 47, 2388–2391. [Google Scholar] [CrossRef] [Green Version]
- Sundsfjord, A.; Spein, A.R.; Lucht, E.; Flaegstad, T.; Seternes, O.M.; Traavik, T. Detection of BK virus DNA in nasopharyngeal aspirates from children with respiratory infections but not in saliva from immunodeficient and immunocompetent adult patients. J. Clin. Microbiol. 1994, 32, 1390–1394. [Google Scholar] [CrossRef] [Green Version]
- Knowles, W.A.; Pipkin, P.; Andrews, N.J.; Vyse, A.; Minor, P.; Brown, D.W.G.; Miller, E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 2003, 71, 115–123. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Steiger, J. Polyomavirus BK. Lancet Infect. Dis. 2003, 3, 611–623. [Google Scholar] [CrossRef]
- Bohl, D.L.; Brennan, D.C. BK Virus Nephropathy and Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2007, 2, S36–S46. [Google Scholar] [CrossRef]
- Wunderink, H.F.; De Brouwer, C.S.; Gard, L.; De Fijter, J.W.; Kroes, A.C.M.; Rotmans, J.I.; Feltkamp, M.C.W. Source and Relevance of the BK Polyomavirus Genotype for Infection After Kidney Transplantation. Open Forum Infect. Dis. 2019, 6, ofz078. [Google Scholar] [CrossRef] [Green Version]
- Nankivell, B.J.; Renthawa, J.; Sharma, R.N.; Kable, K.; O’Connell, P.J.; Chapman, J.R. BK Virus Nephropathy: Histological Evolution by Sequential Pathology. Arab. Archaeol. Epigr. 2017, 17, 2065–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yooprasert, P.; Rotjanapan, P. BK Virus–Associated Nephropathy: Current Situation in a Resource-Limited Country. Transplant. Proc. 2018, 50, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tsuchiya, T.; Inagaki, I.; Seishima, M.; Deguchi, T. Prediction of Early BK Virus Infection in Kidney Transplant Recipients by the Number of Cells With Intranuclear Inclusion Bodies (Decoy Cells). Transplant. Direct 2018, 4, e340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Portier, B.P.; Hu, B.; Chiesa-Vottero, A.; Myles, J.; Procop, G.W.; Tubbs, R.R. Diagnosis of BK Viral Nephropathy in the Renal Allograft Biopsy. J. Mol. Diagn. 2012, 14, 494–500. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K.; Randhawa, P.; Drachenberg, C.B.; Bhatnagar, R.; Bracamonte, E.; Chang, A.; Chon, W.J.; Dadhania, D.; Davis, V.G.; et al. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic Definitions and Clinical Correlations. J. Am. Soc. Nephrol. 2017, 29, 680–693. [Google Scholar] [CrossRef] [Green Version]
- Hässig, A.; Roos, M.; Etter, A.; Bossart, W.; Müller, N.; Schiesser, M.; Wüthrich, R.; Fehr, T. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl. Infect. Dis. 2013, 16, 44–54. [Google Scholar] [CrossRef]
- Dogan, S.; Celebi, Z.; Akturk, S.; Kutlay, S.; Tuzuner, A.; Keven, K.; Sengul, S. Prevalence and Risk Factors of BK Viremia in Patients With Kidney Transplantation: A Single-Center Experience From Turkey. Transplant. Proc. 2017, 49, 532–536. [Google Scholar] [CrossRef]
- Masutani, K.; Ninomiya, T.; Randhawa, P.S. HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol. Dial. Transplant. 2013, 28, 3119–3126. [Google Scholar] [CrossRef]
- Demey, B.; Tinez, C.; François, C.; Helle, F.; Choukroun, G.; Duverlie, G.; Castelain, S.; Brochot, E. Risk factors for BK virus viremia and nephropathy after kidney transplantation: A systematic review. J. Clin. Virol. 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Mallat, S.G.; Tanios, B.Y.; Itani, H.S.; Lotfi, T.; McMullan, C.; Gabardi, S.; Akl, E.A.; Azzi, J.A. CMV and BKPyV Infections in Renal Transplant Recipients Receiving an mTOR Inhibitor–Based Regimen versus a CNI-Based Regimen: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Clin. J. Am. Soc. Nephrol. 2017, 12, 1321–1336. [Google Scholar] [CrossRef]
- Sawinski, D.; Goral, S. BK virus infection: An update on diagnosis and treatment. Nephrol. Dial. Transplant. 2014, 30, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plafkin, C.; Singh, T.; Astor, B.C.; Parajuli, S.; Bhutani, G.; Safdar, N.; Panzer, S.E. Kidney transplant recipients with polycystic kidney disease have a lower risk of post-transplant BK infection than those with end-stage renal disease due to other causes. Transpl. Infect. Dis. 2018, 20, e12974. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, L.; Liang, X.; Qiu, J.; Deng, R.; Li, J.; Chen, G.; Dong, Y.; Chen, L. Risk Factors for BK Virus Infection and BK Virus–Associated Nephropathy Under the Impact of Intensive Monitoring and Pre-emptive Immunosuppression Reduction. Transplant. Proc. 2014, 46, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Alachkar, N.; Bagnasco, S.; Geetha, D.; Gupta, G.; Womer, K.; Arend, L.; Racusen, L.; Montgomery, R.; Kraus, E. Incidence and Outcomes of BK Virus Allograft Nephropathy among ABO- and HLA-Incompatible Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 1320–1327. [Google Scholar] [CrossRef]
- Mannon, R.B.; Hoffmann, S.C.; Kampen, R.L.; Cheng, O.C.; Kleiner, D.E.; Ryschkewitsch, C.; Curfman, B.; Major, E.; Hale, D.A.; Kirk, A.D. Molecular Evaluation of BK Polyomavirus Nephropathy. Arab. Archaeol. Epigr. 2005, 5, 2883–2893. [Google Scholar] [CrossRef]
- Ito, Y.; Nishi, S.; Imai, N.; Yoshita, K.; Saito, K.; Nakagawa, Y.; Takahashi, K.; Narita, I. The case of BK virus infection in which it was difficult to differentiate from acute rejection. Clin. Transplant. 2011, 25, 44–48. [Google Scholar] [CrossRef]
- Menter, T.; Mayr, M.; Schaub, S.; Mihatsch, M.J.; Hirsch, H.H.; Hopfer, H. Pathology of Resolving Polyomavirus-Associated Nephropathy. Arab. Archaeol. Epigr. 2013, 13, 1474–1483. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Renthawa, J.; Shingde, M.; Khan, A. The Importance of Kidney Medullary Tissue for the Accurate Diagnosis of BK Virus Allograft Nephropathy. Clin. J. Am. Soc. Nephrol. 2020, 15, 1015–1023. [Google Scholar] [CrossRef]
- Vu, D.; Shah, T.; Ansari, J.; Naraghi, R.; Min, D. Efficacy of Intravenous Immunoglobulin in the Treatment of Persistent BK Viremia and BK Virus Nephropathy in Renal Transplant Recipients. Transplant. Proc. 2015, 47, 394–398. [Google Scholar] [CrossRef]
- Kable, K.; Davies, C.D.; OʼconnellP, J.; Chapman, J.R.; Nankivell, B.J. Clearance of BK Virus Nephropathy by Combination Antiviral Therapy With Intravenous Immunoglobulin. Transplant. Direct 2017, 3, e142. [Google Scholar] [CrossRef]
- Josephson, M.A.; Gillen, D.; Javaid, B.; Kadambi, P.; Meehan, S.; Foster, P.; Harland, R.; Thistlethwaite, R.J.; Garfinkel, M.; Atwood, W.; et al. Treatment of Renal Allograft Polyoma BK Virus Infection with Leflunomide. Transplantation 2006, 81, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Krisl, J.C.; Taber, D.J.; Pilch, N.; Chavin, K.; Bratton, C.; Thomas, B.; McGillicuddy, J.; Baliga, P. Leflunomide Efficacy and Pharmacodynamics for the Treatment of BK Viral Infection. Clin. J. Am. Soc. Nephrol. 2012, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faguer, S.; Hirsch, H.H.; Kamar, N.; Guilbeau-Frugier, C.; Ribes, D.; Guitard, J.; Esposito, L.; Cointault, O.; Modesto, A.; Lavit, M.; et al. Leflunomide treatment for polyomavirus BK-associated nephropathy after kidney transplantation. Transpl. Int. 2007, 20, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.; Vandooren, A.-K.; Lerut, E.; Evenepoel, P.; Claes, K.; Snoeck, R.; Naesens, L.; Vanrenterghem, Y. Adjuvant Low-Dose Cidofovir Therapy for BK Polyomavirus Interstitial Nephritis in Renal Transplant Recipients. Arab. Archaeol. Epigr. 2005, 5, 1997–2004. [Google Scholar] [CrossRef]
- Vandercam, B.; Goffin, E.; Marot, J.C.; Cosyns, J.P.; Moreau, M.; Jadoul, M. Cidofovir-Induced End-Stage Renal Failure. Clin. Infect. Dis. 1999, 29, 948–949. [Google Scholar] [CrossRef]
- Josephson, M.A.; Williams, J.W.; Chandraker, A.; Randhawa, P.S. Polyomavirus-associated nephropathy: Update on antiviral strategies. Transpl. Infect. Dis. 2006, 8, 95–101. [Google Scholar] [CrossRef]
- Leung, A.Y.H.; Chan, M.T.L.; Yuen, K.-Y.; Cheng, V.C.C.; Chan, K.-H.; Wong, C.L.P.; Liang, R.; Lie, A.K.W.; Kwong, Y.-L. Ciprofloxacin Decreased Polyoma BK Virus Load in Patients Who Underwent Allogeneic Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2005, 40, 528–537. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.T.; Gabardi, S.; Grafals, M.; Hofmann, R.M.; Akalin, E.; Aljanabi, A.; Mandelbrot, D.A.; Adey, D.B.; Heher, E.; Fan, P.-Y.; et al. Efficacy of Levofloxacin in the Treatment of BK Viremia: A Multicenter, Double-Blinded, Randomized, Placebo-Controlled Trial. Clin. J. Am. Soc. Nephrol. 2014, 9, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Knoll, G.A.; Humar, A.; Fergusson, D.; Johnston, O.; House, A.A.; Kim, S.J.; Ramsay, T.; Chassé, M.; Pang, X.L.; Zaltzman, J.; et al. Levofloxacin for BK Virus Prophylaxis Following Kidney Transplantation. JAMA 2014, 312, 2106–2114. [Google Scholar] [CrossRef]
- Tohme, F.; Kalil, R.; Thomas, C.P. Conversion to a sirolimus-based regimen is associated with lower incidence of BK viremia in low-risk kidney transplant recipients. Transpl. Infect. Dis. 2015, 17, 66–72. [Google Scholar] [CrossRef]
- Hodowanec, A.C.; Simon, D.M. BK virus screening and management practices among US renal transplant programs: A survey. Transpl. Int. 2015, 28, 1339–1341. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.; Tönshoff, B.; Hirsch, H.H. Perception, diagnosis and management of BK polyomavirus replication and disease in paediatric kidney transplant recipients in Europe. Nephrol. Dial. Transplant. 2016, 31, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Bischof, N.; Hirsch, H.H.; Wehmeier, C.; Amico, P.; Dickenmann, M.; Hirt-Minkowski, P.; Steiger, J.; Menter, T.; Helmut, H.; Schaub, S. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: Long-term outcomes. Nephrol. Dial. Transplant. 2019, 34, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Höcker, B.; Schneble, L.; Murer, L.; Carraro, A.; Pape, L.; Kranz, B.; Oh, J.; Zirngibl, M.; Strologo, L.D.; Büscher, A.; et al. Epidemiology of and Risk Factors for BK Polyomavirus Replication and Nephropathy in Pediatric Renal Transplant Recipients. Transplantation 2019, 103, 1224–1233. [Google Scholar] [CrossRef]
- Schachtner, T.; Babel, N.; Reinke, P. Different risk factor profiles distinguish early-onset from late-onset BKV-replication. Transpl. Int. 2015, 28, 1081–1091. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Arab. Archaeol. Epigr. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Randhawa, P.S. AST Infectious Diseases Community of Practice BKpolyomavirus in solid organ transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Renthawa, J.; Jeoffreys, N.; Kable, K.; O’Connell, P.J.; Chapman, J.R.; Wong, G.; Sharma, R.N. Clinical Utility of Urinary Cytology to Detect BK Viral Nephropathy. Transplantation 2015, 99, 1715–1722. [Google Scholar] [CrossRef]
- Comoli, P.; Cioni, M.; Basso, S.; Gagliardone, C.; Potenza, L.; Verrina, E.; Luppi, M.; Zecca, M.; Ghiggeri, G.M.; Ginevri, F. Immunity to Polyomavirus BK Infection: Immune Monitoring to Regulate the Balance between Risk of BKV Nephropathy and Induction of Alloimmunity. Clin. Dev. Immunol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Vasudev, B.; Hariharan, S.; Hussain, S.A.; Zhu, Y.-R.; Bresnahan, B.A.; Cohen, E.P. BK virus nephritis: Risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005, 68, 1834–1839. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Chen, L.-Z.; Qiu, J.; Wang, C.-X.; Fei, J.-G.; Deng, S.-X.; Li, J.; Chen, G.-D.; Zhang, L.; Fu, Q.; et al. Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: A single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin. Transplant. 2009, 24, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Cho, J.-H.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, Y.-L.; Kim, H.-K.; Huh, S.; Kim, C.-D. Clinical Impact of BK Virus Surveillance on Outcomes in Kidney Transplant Recipients. Transplant. Proc. 2015, 47, 660–665. [Google Scholar] [CrossRef]
- Park, W.Y.; Kang, S.S.; Jin, K.; Park, S.B.; Choe, M.; Han, S. Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients. Kidney Res. Clin. Prac. 2018, 37, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfadawy, N.; Flechner, S.M.; Schold, J.D.; Srinivas, T.R.; Poggio, E.; Fatica, R.; Avery, R.; Mossad, S.B. Transient versus Persistent BK Viremia and Long-Term Outcomes after Kidney and Kidney–Pancreas Transplantation. Clin. J. Am. Soc. Nephrol. 2014, 9, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-T.; Yang, S.-C.; Li, J.; Deng, R.-H.; Chen, W.-F.; Qiu, J.; Chen, L.-Z.; Wang, C.-X.; Huang, Y. Prognosis of BK polyomavirus nephropathy. Chin. Med. J. 2019, 132, 388–394. [Google Scholar] [CrossRef]
- Mohamed, M.; Parajuli, S.; Muth, B.; Astor, B.; Panzer, S.; Mandelbrot, D.; Zhong, W.; Djamali, A. In kidney transplant recipients with BK polyomavirus infection, early BK nephropathy, microvascular inflammation, and serum creatinine are risk factors for graft loss. Transpl. Infect. Dis. 2016, 18, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Leeaphorn, N.; Thongprayoon, C.; Chon, W.J.; Cummings, L.S.; Mao, M.A.; Cheungpasitporn, W. Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Arab. Archaeol. Epigr. 2019, 20, 1334–1340. [Google Scholar] [CrossRef]
- Alelign, T.; Ahmed, M.M.; Bobosha, K.; Tadesse, Y.; Howe, R.; Petros, B. Kidney Transplantation: The Challenge of Human Leukocyte Antigen and Its Therapeutic Strategies. J. Immunol. Res. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Trowsdale, J.; Knight, J.C. Major Histocompatibility Complex Genomics and Human Disease. Annu. Rev. Genom. Hum. Genet. 2013, 14, 301–323. [Google Scholar] [CrossRef] [Green Version]
- De Wit, J.; Borghans, J.A.M.; Kesmir, C.; Van Baarle, D. Editorial: Role of HLA and KIR in Viral Infections. Front. Immunol. 2016, 7, 286. [Google Scholar] [CrossRef] [Green Version]
- Mina, M.M.; Luciani, F.; Cameron, B.; Bull, R.A.; Beard, M.R.; Booth, D.; Lloyd, A.R. Resistance to hepatitis C virus: Potential genetic and immunological determinants. Lancet Infect. Dis. 2015, 15, 451–460. [Google Scholar] [CrossRef]
- Rao, X.; Hoof, I.; Van Baarle, D.; Kesmir, C.; Textor, J. HLA Preferences for Conserved Epitopes: A Potential Mechanism for Hepatitis C Clearance. Front. Immunol. 2015, 6, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunderink, H.F.; Haasnoot, G.W.; De Brouwer, C.S.; Van Zwet, E.W.; Kroes, A.C.M.; De Fijter, J.W.; Rotmans, J.I.; Claas, F.H.J.; Feltkamp, M.C. Reduced Risk of BK Polyomavirus Infection in HLA-B51–positive Kidney Transplant Recipients. Transplantation 2019, 103, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Bohl, D.L.; Storch, G.A.; Ryschkewitsch, C.; Gaudreault-Keener, M.; Schnitzler, M.A.; Major, E.O.; Brennan, D.C. Donor Origin of BK Virus in Renal Transplantation and Role of HLA C7 in Susceptibility to Sustained BK Viremia. Arab. Archaeol. Epigr. 2005, 5, 2213–2221. [Google Scholar] [CrossRef]
- Teutsch, K.; Schweitzer, F.; Knops, E.; Kaiser, R.; Pfister, H.; Verheyen, J.; Gobel, H.; Cingöz, T.; Di Cristanziano, V. Early identification of renal transplant recipients with high risk of polyomavirus-associated nephropathy. Med. Microbiol. Immunol. 2015, 204, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Gheith, O.; Al-Otaibi, T.; Zakaria, Z.; Halim, M.; Nampoory, N. Human leukocyte antigen Cw7-mediated protection against polyoma BK virus in renal transplant recipients who received grafts from antigen-positive donors. Exp. Clin. Transplant. 2015, 13, 383–387. [Google Scholar] [PubMed]
- Vojtusek, I.K.; Kamenaric-Burek, M.; Ivkovic, V.; Bulimbasic, S.; Marekovic, I.; Coric, M.; Bosnjak, Z.; Grubic, Z.; Zunec, R. Combined association of recipient killer cell immunoglobulin-like haplotype AA and donor HLA-C*07 gene with BK virus associated nephropathy in kidney transplant patients. HLA 2019, 94, 4–10. [Google Scholar] [CrossRef]
- El-Husseini, A.; Hassan, W.; Yaseen, M.; Suleiman, B.; Saleh, S.; Malik, O.; Ashqar, H.; Maibam, A.; Mei, X.; Castellanos, A.L.; et al. Impact of human leukocyte antigen and calculated panel reactive antibody on BK viremia in kidney transplant recipients: A single-center experience and literature review. Transpl. Infect. Dis. 2019, 21, e13071. [Google Scholar] [CrossRef]
- Kavuzlu, M.; Baştürk, B.; Ataç, F.B.; Alışkan, H.E.; Kantaroğlu, B. Investigation of the Relationship between BK Virus and Human Leukocyte Antigens in Kidney Transplant Recipients. Exp. Clin. Transplant. 2020, 18, 51–54. [Google Scholar] [CrossRef]
- Roark, C.; Shah, P.; Wiseman, A.; Anderson, K.; Aubrey, M.; Freed, B. P100 Resistance to BK Viremia in renal transplant patients is associated with HLA-DQ5 and HLA-DQ6. Hum. Immunol. 2016, 77, 112. [Google Scholar] [CrossRef]
- Shah, P.; Anderson, K.; Aubrey, M.; Roark, C.; Freed, B.W.A. HLA Class II and BK Virus Reactivation in Renal Transplant Recipients. Am. J. Transpl. 2016, 3, 16. [Google Scholar]
- Rohn, H.; Michita, R.T.; Schramm, S.; Dolff, S.; Gäckler, A.; Korth, J.; Heinemann, F.M.; Wilde, B.; Trilling, M.; Horn, P.A.; et al. HLA-E Polymorphism Determines Susceptibility to BK Virus Nephropathy after Living-Donor Kidney Transplant. Cells 2019, 8, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohn, H.; Schwich, E.; Michita, R.T.; Schramm, S.; Dolff, S.; Gäckler, A.; Korth, J.; Heinemann, F.M.; Wilde, B.; Trilling, M.; et al. HLA-G 3′ untranslated region gene variants are promising prognostic factors for BK polyomavirus replication and acute rejection after living-donor kidney transplant. Hum. Immunol. 2020, 81, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tonnerre, P.; Gérard, N.; Gavlovsky, P.-J.; Mazalrey, S.; Hourmant, M.; Cheneau, M.-L.; Gautier, A.C.; Renaudin, K.; Bressollette-Bodin, C.; Charreau, B. MICA mutant A5.1 influences BK Polyomavirus Reactivation and Associated Nephropathy after Kidney Transplantation. J. Infect. Dis. 2016, 214, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, H.H.; Knowles, W.; Dickenmann, M.; Passweg, J.; Klimkait, T.; Mihatsch, M.J.; Steiger, J. Prospective Study of Polyomavirus Type BK Replication and Nephropathy in Renal-Transplant Recipients. N. Engl. J. Med. 2002, 347, 488–496. [Google Scholar] [CrossRef]
- Awadalla, Y.; Randhawa, P.; Ruppert, K.; Zeevi, A.; Duquesnoy, R.J. HLA Mismatching Increases the Risk of BK Virus Nephropathy in Renal Transplant Recipients. Arab. Archaeol. Epigr. 2004, 4, 1691–1696. [Google Scholar] [CrossRef]
- Suleiman, B.; Saleh, S.; Hassan, W.; Wang, K.; Maibam, A.; Karim, A.; Hines, A.; Mei, X.; El-Husseini, A.; Yaseen, M.; et al. HLA Mismatching and CPRA Do Not Affect BK Viremia and Nephropathy Risk in Kidney Transplantation: A Retrospective Analysis. Am. J. Transplant. 2017, 17, C188. [Google Scholar]
- Favi, E.; Puliatti, C.; Sivaprakasam, R.; Ferraresso, M.; Ambrogi, F.; Delbue, S.; Gervasi, F.; Salzillo, I.; Raison, N.; Cacciola, R. Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J. Clin. Cases 2019, 7, 270–290. [Google Scholar] [CrossRef]
- Borni-Duval, C.; Caillard, S.; Olagne, J.; Perrin, P.; Braun-Parvez, L.; Heibel, F.; Moulin, B. Risk Factors for BK Virus Infection in the Era of Therapeutic Drug Monitoring. Transplantation 2013, 95, 1498–1505. [Google Scholar] [CrossRef]
- Dieplinger, G.; Everly, M.J.; Briley, K.P.; Haisch, C.E.; Bolin, P.; Maldonado, A.; Kendrick, W.; Kendrick, S.; Morgan, C.L.; Terasaki, P.I.; et al. Onset and progression ofde novodonor-specific anti-human leukocyte antigen antibodies after BK polyomavirus and preemptive immunosuppression reduction. Transpl. Infect. Dis. 2015, 17, 848–858. [Google Scholar] [CrossRef]
- Sawinski, D.; Forde, K.A.; Trofe-Clark, J.; Patel, P.; Olivera, B.; Goral, S.; Bloom, R.D. Persistent BK Viremia Does Not Increase Intermediate-Term Graft Loss but Is Associated with De Novo Donor-Specific Antibodies. J. Am. Soc. Nephrol. 2014, 26, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.J.; Kuten, S.A.; Knight, R.J.; Graviss, E.A.; Nguyen, D.; Gaber, A.O. Incidence and Factors Associated with De Novo DSA After BK Viremia in Renal Transplant Recipients. Clin. Transpl. 2016, 32, 103–109. [Google Scholar] [PubMed]
- Everly, M.J.; Briley, K.P.; Haisch, C.E.; Dieplinger, G.; Bolin, P.; Kendrick, S.A.; Morgan, C.; Maldonado, A.Q.; Rebellato, L.M. Racial Differences in Incident De Novo Donor-Specific Anti-HLA Antibody among Primary Renal Allograft Recipients. Transpl. Int. 2017, 30, 566–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosten, S.A.; Sullivan, L.C.; Ottenhoff, T.H.M. Characteristics of HLA-E Restricted T-Cell Responses and Their Role in Infectious Diseases. J. Immunol. Res. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romagnani, C.; Pietra, G.; Falco, M.; Mazzarino, P.; Moretta, L.; Mingari, M.C. HLA-E–restricted recognition of human cytomegalovirus by a subset of cytolytic T lymphocytes. Hum. Immunol. 2004, 65, 437–445. [Google Scholar] [CrossRef]
- Guberina, H.; Nardi, F.D.S.; Michita, R.T.; Dolff, S.; Bienholz, A.; Heinemann, F.M.; Wilde, B.; Trilling, M.; Horn, P.A.; Kribben, A.; et al. Susceptibility of HLA-E*01:03 Allele Carriers to Develop Cytomegalovirus Replication After Living-Donor Kidney Transplantation. J. Infect. Dis. 2018, 217, 1918–1922. [Google Scholar] [CrossRef] [Green Version]
- Helanterä, I.; Salmela, K.; Kyllönen, L.; Räisänen-Sokolowski, A.; Auvinen, E.; Mannonen, L.; Koskinen, P.; Lautenschlager, I. BK virus viremia in a well-HLA-matched kidney transplant population mainly on low-dose cyclosporine-based immunosuppression. Clin. Transplant. 2012, 26, E596–E601. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, J.Y.; Kim, D.H.; Ko, Y.; Choi, J.Y.; Shin, S.; Jung, J.H.; Kim, Y.H.; Han, D.J. Effect of simultaneous presence of anti-blood group A/B and -HLA antibodies on clinical outcomes in kidney transplantation across positive crossmatch: A nationwide cohort study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Fernández, C.; Calvo, M.; Leite, N.; López, A.; Ferreiro, T.; Ribera, R.; Seijo, R.; Alonso, Á. Trasplante renal procedente de donante vivo HLA incompatible: Eficacia y pronóstico en 32 pacientes tras desensibilización. Nefrología 2017, 37, 638–645. [Google Scholar] [CrossRef]
- Kauke, T.; Klimaschewski, S.; Schoenermarck, U.; Fischereder, M.; Dick, A.; Guba, M.; Stangl, M.; Werner, J.; Meiser, B.; Habicht, A. Outcome after Desensitization in HLA or ABO-Incompatible Kidney Transplant Recipients: A Single Center Experience. PLoS ONE 2016, 11, e0146075. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, S.; Muth, B.L.; Turk, J.A.; Astor, B.C.; Mohammed, M.; Mandelbrot, D.A.; Djamali, A. In Kidney Transplant Recipients With a Positive Virtual Crossmatch, High PRA was Associated With Lower Incidence of Viral Infections. Transplantation 2016, 100, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Callemeyn, J.; Gazut, S.; Lerut, E.; De Loor, H.; Wevers, M.; Heylen, L.; Saison, C.; Koenig, A.; Thaunat, O.; et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 2019, 95, 188–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontrelli, P.; Rascio, F.; Castellano, G.; Grandaliano, G.; Gesualdo, L.; Stallone, G. The Role of Natural Killer Cells in the Immune Response in Kidney Transplantation. Front. Immunol. 2020, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Van Huyen, J.-P.D.; Hidalgo, L.; Reeve, J.; Racapé, M.; Aubert, O.; Venner, J.M.; Falmuski, K.; Bories, M.C.; Beuscart, T.; et al. Gene Expression Profiling for the Identification and Classification of Antibody-Mediated Heart Rejection. Circulation 2017, 135, 917–935. [Google Scholar] [CrossRef] [Green Version]
- Fildes, J.E.; Yonan, N.; Tunstall, K.; Walker, A.H.; Griffiths-Davies, L.; Bishop, P.; Leonard, C.T. Natural Killer Cells in Peripheral Blood and Lung Tissue Are Associated With Chronic Rejection After Lung Transplantation. J. Heart Lung Transplant. 2008, 27, 203–207. [Google Scholar] [CrossRef]
- Navarro, F.; Portalès, P.; Candon, S.; Pruvot, F.R.; Pageaux, G.; Fabre, J.M.; Domergue, J.; Clot, J. Natural killer cell and αβ and γδ lymphocyte traffic into the liver graft immediately after liver transplantation. Transplantation 2000, 69, 633–639. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Moretta, L.; Biassoni, R.; Bottino, C.; Mingari, M.C.; Moretta, A. Human NK-cell receptors. Immunol. Today 2000, 21, 420–422. [Google Scholar] [CrossRef]
- Rajalingam, R. Overview of the Killer Cell Immunoglobulin-Like Receptor System. Adv. Struct. Saf. Stud. 2012, 882, 391–414. [Google Scholar] [CrossRef]
- Hadad, U.; Martinez, O.; Krams, S.M. NK cells after transplantation: Friend or foe. Immunol. Res. 2014, 58, 259–267. [Google Scholar] [CrossRef]
- Hidalgo, L.G.; Sis, B.; Sellares, J.; Campbell, P.M.; Mengel, M.; Einecke, G.; Chang, J.; Halloran, P.F. NK Cell Transcripts and NK Cells in Kidney Biopsies from Patients with Donor-Specific Antibodies: Evidence for NK Cell Involvement in Antibody-Mediated Rejection. Arab. Archaeol. Epigr. 2010, 10, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.-E.; Rickassel, C.; Healy, H.; Kassianos, A.J. Natural Killer Cells in Kidney Health and Disease. Front. Immunol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hens, J.; Jennes, W.; Kestens, L. The role of NK cells in HIV-1 protection: Autologous, allogeneic or both? AIDS Res. Ther. 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.P.; Gao, X.; Lee, J.-H.; Nelson, G.W.; Detels, R.; Goedert, J.J.; Buchbinder, S.; Hoots, K.; Vlahov, D.; Trowsdale, J.; et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 2002, 31, 429–434. [Google Scholar] [CrossRef]
- Hölzemer, A.; Garcia-Beltran, W.F.; Altfeld, M. Natural Killer Cell Interactions with Classical and Non-Classical Human Leukocyte Antigen Class I in HIV-1 Infection. Front. Immunol. 2017, 8, 1496. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Elsässer, H.; Hönger, G.; Steiger, J.; Schaub, S.; Hess, C. The Number of Activating KIR Genes Inversely Correlates with the Rate of CMV Infection/Reactivation in Kidney Transplant Recipients. Arab. Archaeol. Epigr. 2008, 8, 1312–1317. [Google Scholar] [CrossRef]
- Stern, M.; Hadaya, K.; Hönger, G.; Martin, P.-Y.; Steiger, J.; Hess, C.; Villard, J. Telomeric Rather than Centromeric Activating KIR Genes Protect from Cytomegalovirus Infection after Kidney Transplantation. Arab. Archaeol. Epigr. 2011, 11, 1302–1307. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.C.; Peacock, S.J.; Hughes, D.A.; Traherne, J.A.; Allen, R.L.; Barnardo, M.C.N.M.; Friend, P.J.; Taylor, C.J.; Fuggle, S.V.; Trowsdale, J.; et al. Killer immunoglobulin-like receptor gene repertoire influences viral load of primary human cytomegalovirus infection in renal transplant patients. Genes Immun. 2014, 15, 562–568. [Google Scholar] [CrossRef]
- De Rham, C.; Hadaya, K.; Bandelier, C.; Ferrari-Lacraz, S.; Villard, J. Expression of killer cell immunoglobulin-like receptors (KIRs) by natural killer cells during acute CMV infection after kidney transplantation. Transpl. Immunol. 2014, 31, 157–164. [Google Scholar] [CrossRef]
- Gonzalez, A.; Schmitter, K.; Hirsch, H.H.; Garzoni, C.; Van Delden, C.; Boggian, K.; Mueller, N.J.; Berger, C.; Villard, J. KIR-associated protection from CMV replication requires pre-existing immunity: A prospective study in solid organ transplant recipients. Genes Immun. 2014, 15, 495–499. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, B.; Chen, S.; Gai, Z.; Feng, Z.; Liu, X.; Liu, Y.; Wen, X.; Li, L.; Jiao, Y.; et al. Association of KIR Genotypes and Haplotypes with Susceptibility to Chronic Hepatitis B Virus Infection in Chinese Han Population. Cell. Mol. Immunol. 2008, 5, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, S.I. HLA and NK Cell Inhibitory Receptor Genes in Resolving Hepatitis C Virus Infection. Science 2004, 305, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Romo, S.; García-Sepúlveda, C.A.; Comas-García, A.; Lovato-Salas, F.; Salgado-Bustamante, M.; Gómez-Gómez, A.; Noyola, D.E. Killer-cell immunoglobulin-like receptors (KIR) in severe A (H1N1) 2009 influenza infections. Immunogenetics 2012, 64, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, N.; Padilla, C.; Becquart, P.; Leroy, E.M.; Vieillard, V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics 2010, 62, 767–771. [Google Scholar] [CrossRef] [Green Version]
- Schmied, L.; Terszowski, G.; Gonzalez, A.; Schmitter, K.; Hirsch, H.H.; Garzoni, C.; Van Delden, C.; Boggian, K.; Mueller, N.; Berger, C.; et al. Protection From Varicella Zoster in Solid Organ Transplant Recipients Carrying Killer Cell Immunoglobulin-Like Receptor B Haplotypes. Transplantation 2015, 99, 2651–2655. [Google Scholar] [CrossRef] [Green Version]
- Moraru, M.; Cisneros, E.; Gómez-Lozano, N.; De Pablo, R.; Portero, F.; Cañizares, M.; Vaquero, M.; Roustán, G.; Millán, I.; López-Botet, M.; et al. Host Genetic Factors in Susceptibility to Herpes Simplex Type 1 Virus Infection: Contribution of Polymorphic Genes at the Interface of Innate and Adaptive Immunity. J. Immunol. 2012, 188, 4412–4420. [Google Scholar] [CrossRef] [Green Version]
- Chijioke, O.; Müller, A.; Feederle, R.; Barros, M.H.M.; Krieg, C.; Emmel, V.; Marcenaro, E.; Leung, C.S.; Antsiferova, O.; Landtwing, V.; et al. Human Natural Killer Cells Prevent Infectious Mononucleosis Features by Targeting Lytic Epstein-Barr Virus Infection. Cell Rep. 2013, 5, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Bonagura, V.; Du, Z.; Ashouri, E.; Luo, L.; Hatam, L.J.; DeVoti, J.A.; Rosenthal, D.W.; Steinberg, B.M.; Abramson, A.L.; Gjertson, D.W.; et al. Activating killer cell immunoglobulin-like receptors 3DS1 and 2DS1 protect against developing the severe form of recurrent respiratory papillomatosis. Hum. Immunol. 2010, 71, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Anfossi, N.; André, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.-J.; Yang, L.; French, A.R.; Sunwoo, J.B.; Lemieux, S.; Hansen, T.H.; et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nat. Cell Biol. 2005, 436, 709–713. [Google Scholar] [CrossRef]
- Rajalingam, R. The Impact of HLA Class I-Specific Killer Cell Immunoglobulin-Like Receptors on Antibody-Dependent Natural Killer Cell-Mediated Cytotoxicity and Organ Allograft Rejection. Front. Immunol. 2016, 7, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, M.S.; Zipperlen, K.; Gallant, M.; Grant, M.D. Killer cell immunoglobulin-like receptor 3DL1 licenses CD16-mediated effector functions of natural killer cells. J. Leukoc. Biol. 2010, 88, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.S.; Wren, L.; Isitman, G.; Navis, M.; Stratov, I.; Bernard, N.F.; Kent, S.J. HIV Infection Abrogates the Functional Advantage of Natural Killer Cells Educated through KIR3DL1/HLA-Bw4 Interactions to Mediate Anti-HIV Antibody-Dependent Cellular Cytotoxicity. J. Virol. 2012, 86, 4488–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.; Parham, P.; Miller, J.S. Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation. Blood 2018, 131, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Trydzenskaya, H.; Juerchott, K.; Lachmann, N.; Kotsch, K.; Kunert, K.; Weist, B.; Schönemann, C.; Schindler, R.; Nickel, P.; Melzig, M.F.; et al. The genetic predisposition of natural killer cell to BK virus–associated nephropathy in renal transplant patients. Kidney Int. 2013, 84, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotter, P.; Padley, J.; Crick, K.; Jayaraman, J.; Traherne, J.; Trowsdale, J.; Clatworthy, M. The Role of KIR2DS4 Polymorphisms in BK Virus and Acute Rejection Following Renal Transplantation. Transplantation 2018, 102, S224. [Google Scholar] [CrossRef]
- Brochot, E.; Desoutter, J.; Presne, C.; De Araujo, I.; Flahaut, G.; Castelain, S.; Westeel, P.-F.; Choukroun, G.; Guillaume, N. The association between killer-cell immunoglobulin-like receptor (KIR) and KIR ligand genotypes and the likelihood of BK virus replication after kidney transplantation. Transpl. Int. 2016, 29, 1168–1175. [Google Scholar] [CrossRef]
- Domingo-Calap, P.; Schubert, B.; Joly, M.; Solis, M.; Untrau, M.; Carapito, R.; Georgel, P.; Caillard, S.; Fafi-Kremer, S.; Paul, N.; et al. An unusually high substitution rate in transplant-associated BK polyomavirus in vivo is further concentrated in HLA-C-bound viral peptides. PLoS Pathog. 2018, 14, e1007368. [Google Scholar] [CrossRef]

| References | Factor | n | Clinical Effect |
|---|---|---|---|
| HLA Class I Allele (Classical) | |||
| Bohl et al., 2005 [64] | Cw7 neg | 195 | In D; in R; in both D and R— ↑ BK viremia and BKVAN |
| Masutani et al., 2013 [18] | A2 pos B44 pos DR15 pos | 998 | In R—↓ BK viremia In R—↓ BK viremia In R—↓ BK viremia |
| Teutsch et al., 2015 [65] | A9 pos A2 pos A28/A68 pos | 329 | In D—↑ BKVAN In R—↑ BKVAN In R—↑ BK viremia |
| Gheith et al., 2015 [66] | Cw7 neg | 5 | In D; in R—↑ BK viremia |
| Dogan et al., 2017 [17] | A24 pos B55 pos | 183 | R and D matched—↑ BK viremia R and D matched—↑ BK viremia |
| Wunderink et al., 2018 [63] | B51 pos | 407 | In R—↓ BK viremia and BKVAN |
| Kovacevic Vojtusek et al., 2019 [67] | C*07 pos | 23 | In D—↓ BKVAN |
| El Husseini et al., 2019 [68] | HLA-A, -B, -C | No association | |
| Kavuzlu et al., 2020 [69] | DRB1*03 pos B*13 pos | 232 | In R—↑ BKV infection In R—↓ BKV infection |
| HLA Class II Allele | |||
| Roark et al., 2016 [70] | DQ5/DQ6 pos | 102 | In R—↓ BKV viremia In D—no association |
| Shah et al., 2016 [71] | DQ2/DQ3/DQ4 pos DQ5/DQ6 pos | 433 | In R—↑ BK viremia In R—↓ BK viremia |
| HLA Class I Allele (Non-Classical) | |||
| Rohn et al., 2019 [72] |
HLA-E*01:01 pos HLA-E*01:01 homozygote | 278 | In R—↓ BKVAN In R—↓ BKVAN |
| Rohn et al., 2019 [73] | HLA-G 3’UTR-4 haplotype pos | 251 | In R and D— ↑ viremia and BKVAN |
| Tonnerre et al., 2016 [74] | MICA A5.1 pos | 144 | In D—↓ BKVAN D/R MM ↑ BKVAN |
| HLA MM | |||
| Hirsch et al., 2002 [75] | MM 3–6 | 78 | ↑ viremia and BKVAN |
| Awadalla et al., 2004 [76] | MM > 4 | 40 | ↑ BKVAN |
| Bohl et al., 2005 [64] | MM 0–6 | 195 | No association for BK viremia |
| Hässig et al., 2014 [16] | MM > 4 | 152 | ↑ BK viremia |
| Suleiman 2017 [77] | MM 0–2; 3–4 or 5–6 | 537 | No association for BK viremia |
| Favi et al., 2019 [78] | MM > 4 | 629 | ↑ BK viremia |
| El Husseini 2019 [68] | MM 0–2; 3–4 or 5–6 | 649 | No association for BK viremia |
| Kavuzlu et al., 2020 [69] | MM 0–6 | 232 | No association for BK viremia |
| cPRA | |||
| Awadalla 2004 [76] | cPRAneg; cPRA > 0% | 40 | No association |
| Borni-Duval et al., 2013 [79] | Cpra > 0% | 240 | ↑ BK viremia and BKVAN |
| Masutani et al., 2013 [18] | cPRAneg; cPRA > 0% | 998 | No association |
| Suleiman 2017 [77] | cPRAneg; cPRA > 0% | 537 | No association |
| El-Husseini 2019 [68] | cPRAneg; cPRA > 0% | 649 | No association |
| HLA DSA | |||
| Dieplinger et al., 2015 [80] | 174 | BK viremia associated with higher rate de novo DSA | |
| Sawinski et al., 2015 [81] | 785 | persistent BK viremia associated with class II de novo DSA | |
| Patel et al., 2016 [82] | 1019 | No association of de novo DSA and BK viremia | |
| Everly et al., 2017 [83] | 341 | BK viremia associated with higher rate of de novo DSA |
| References | Factor | n | Clinical Effect |
|---|---|---|---|
| Trydzenskaya et al., 2013 [125] | Low number of aKIR KIR3DS1 neg Tel B KIR haplotype | 48 | In R—↑ BKVAN In R—↑ BKVAN In R—↓ BK viremia and BKVAN |
| Brochot et al., 2016 [127] | KIR haplotype aKIR number R:KIR2DS1/C1C2 + D:C2 R:KIR2DS2/C1C2 + D:C1 | 103 | No association with BK viremia No association with BK viremia ↑ BKV reactivation ↑ BKV reactivation |
| Trotter et al., 2018 [126] | KIR2DS4 | 1010 | No association with BK viremia |
| Kovacevic Vojtusek et al., 2019 [67] | Lacking aKIR KIR haplotype AA R:KIR AA pos + D:Cw7 neg | 23 | In R—↑ BKVAN ↑ BKVAN ↑ BKVAN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burek Kamenaric, M.; Ivkovic, V.; Kovacevic Vojtusek, I.; Zunec, R. The Role of HLA and KIR Immunogenetics in BK Virus Infection after Kidney Transplantation. Viruses 2020, 12, 1417. https://doi.org/10.3390/v12121417
Burek Kamenaric M, Ivkovic V, Kovacevic Vojtusek I, Zunec R. The Role of HLA and KIR Immunogenetics in BK Virus Infection after Kidney Transplantation. Viruses. 2020; 12(12):1417. https://doi.org/10.3390/v12121417
Chicago/Turabian StyleBurek Kamenaric, Marija, Vanja Ivkovic, Ivana Kovacevic Vojtusek, and Renata Zunec. 2020. "The Role of HLA and KIR Immunogenetics in BK Virus Infection after Kidney Transplantation" Viruses 12, no. 12: 1417. https://doi.org/10.3390/v12121417
APA StyleBurek Kamenaric, M., Ivkovic, V., Kovacevic Vojtusek, I., & Zunec, R. (2020). The Role of HLA and KIR Immunogenetics in BK Virus Infection after Kidney Transplantation. Viruses, 12(12), 1417. https://doi.org/10.3390/v12121417






