The Roles of Amphibian (Xenopus laevis) Macrophages during Chronic Frog Virus 3 Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Production of Recombinant CSF-1 and IL-34 Cytokines
2.3. FV3 Stocks, Frog-Macrophage Enrichments, and Viral Infections
2.4. Isolation of RNA and DNA from Kidney Tissues
2.5. Quantitative Analysis of Gene Expression and FV3 Viral Load Assessment
2.6. Plaque Assay Analyses of FV3-Infected Frog Kidneys
2.7. Histological Analyses of Kidney Tissues
2.8. Statistical Analyses
3. Results
3.1. CSF-1-MΦ-Enriched Frogs Succumb to FV3 Infections
3.2. FV3 Persistence and Replication in CSF-1- and IL-34-MΦ-Enriched Frogs
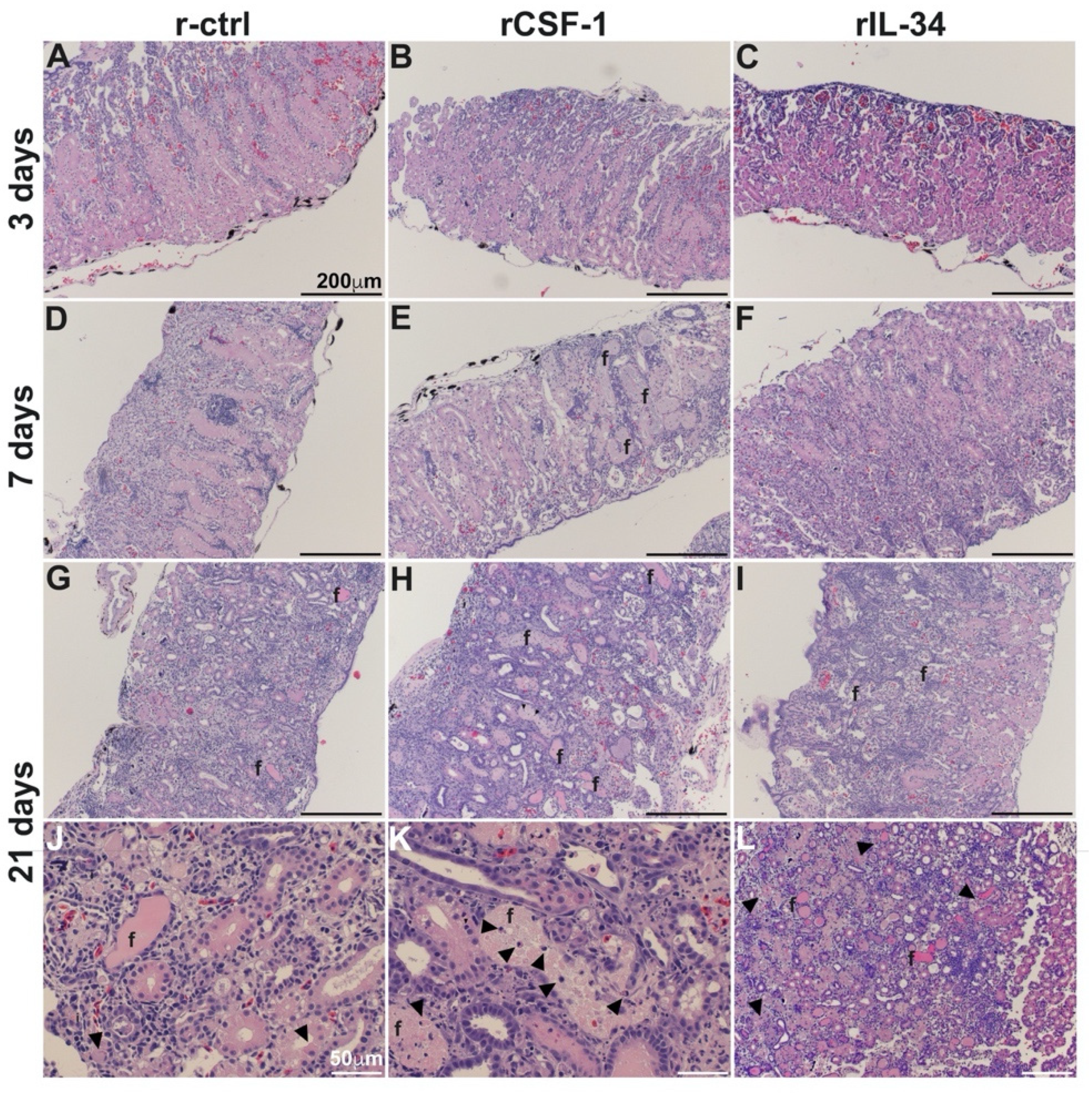
3.3. CSF-1-MΦ-Mediated Susceptibility to FV3 Is Marked by Kidney Fibrosis and Leukocyte Infiltration
3.4. CSF-1-MΦ-Enriched Frogs Exhibit Greater Kidney Expression of Chemokine Genes
3.5. CSF-1-MΦ-Enriched Frogs Possess Greater Kidney Infiltration by Granulocytes and Macrophages
3.6. Kidneys of CSF-1-MΦ-Enriched Frogs Possess Greater Expression of Genes Associated with Immune Suppression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, A.A.; Hyatt, A.D.; Russell, P.; Bennett, P.M. Emerging epidemic diseases of frogs in Britain are dependent on the source of ranavirus agent and the route of exposure. Epidemiol. Infect. 2007, 135, 1200–1212. [Google Scholar] [CrossRef]
- Greer, A.L.; Berrill, M.; Wilson, P.J. Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Dis. Aquat. Organ. 2005, 67, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Zupanovic, Z.; Lopez, G.; Hyatt, A.D.; Green, B.; Bartran, G.; Parkes, H.; Whittington, R.J.; Speare, R. Giant toads Bufo marinus in Australia and Venezuela have antibodies against ‘ranaviruses’. Dis. Aquat. Organ. 1998, 32, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Green, D.E.; Converse, K.A.; Schrader, A.K. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Ann. N. Y. Acad. Sci. 2002, 969, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Duffus, A.L.; Pauli, B.D.; Wozney, K.; Brunetti, C.R.; Berrill, M. Frog virus 3-like infections in aquatic amphibian communities. J. Wildl. Dis. 2008, 44, 109–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, S.F.; Greer, A.L.; Torres-Cervantes, R.; Collins, J.P. First case of ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Dis. Aquat. Organ. 2006, 72, 87–92. [Google Scholar] [CrossRef]
- Grayfer, L.; Robert, J. Distinct functional roles of amphibian (Xenopus laevis) colony-stimulating factor-1- and interleukin-34-derived macrophages. J. Leukoc. Biol. 2015, 98, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Grayfer, L.; Robert, J. Amphibian macrophage development and antiviral defenses. Dev. Comp. Immunol. 2016, 58, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Robert, J.; Abramowitz, L.; Gantress, J.; Morales, H.D. Xenopus laevis: A possible vector of Ranavirus infection? J. Wildl. Dis. 2007, 43, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Robert, J.; Grayfer, L.; Edholm, E.S.; Ward, B.; De Jesus Andino, F. Inflammation-induced reactivation of the ranavirus Frog Virus 3 in asymptomatic Xenopus laevis. PLoS ONE 2014, 9, e112904. [Google Scholar] [CrossRef] [Green Version]
- Samanta, M.; Yim, J.; De Jesus Andino, F.; Paiola, M.; Robert, J. TLR5-Mediated Reactivation of Quiescent Ranavirus FV3 in Xenopus Peritoneal Macrophages. J. Virol. 2021, 95, e00215-21. [Google Scholar] [CrossRef]
- Plüddemann, A.; Mukhopadhyay, S.; Gordon, S. Innate immunity to intracellular pathogens: Macrophage receptors and responses to microbial entry. Immunol. Rev. 2011, 240, 11–24. [Google Scholar] [CrossRef]
- de Vries, R.D.; Mesman, A.W.; Geijtenbeek, T.B.; Duprex, W.P.; de Swart, R.L. The pathogenesis of measles. Curr. Opin. Virol. 2012, 2, 248–255. [Google Scholar] [CrossRef]
- Duncan, C.J.; Sattentau, Q.J. Viral determinants of HIV-1 macrophage tropism. Viruses 2011, 3, 2255–2279. [Google Scholar] [CrossRef]
- Wang, T.; Hanington, P.C.; Belosevic, M.; Secombes, C.J. Two macrophage colony-stimulating factor genes exist in fish that differ in gene organization and are differentially expressed. J. Immunol. 2008, 181, 3310–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pixley, F.J.; Stanley, E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Hanington, P.C.; Wang, T.; Secombes, C.J.; Belosevic, M. Growth factors of lower vertebrates: Characterization of goldfish (Carassius auratus L.) macrophage colony-stimulating factor-1. J. Biol. Chem. 2007, 282, 31865–31872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, D.; Miller, J.; Merad, M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011, 35, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Lee, E.; Hestir, K.; Leo, C.; Huang, M.; Bosch, E.; Halenbeck, R.; Wu, G.; Zhou, A.; Behrens, D.; et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 2008, 320, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Yaparla, A.; Docter-Loeb, H.; Melnyk, M.L.S.; Batheja, A.; Grayfer, L. The amphibian (Xenopus laevis) colony-stimulating factor-1 and interleukin-34-derived macrophages possess disparate pathogen recognition capacities. Dev. Comp. Immunol. 2019, 98, 89–97. [Google Scholar] [CrossRef]
- Yaparla, A.; Koubourli, D.; Popovic, M.; Grayfer, L. Exploring the relationships between amphibian (Xenopus laevis) myeloid cell subsets. Dev. Comp. Immunol. 2020, 113, 103798. [Google Scholar] [CrossRef] [PubMed]
- Yaparla, A.; Popovic, M.; Grayfer, L. Differentiation-dependent antiviral capacities of amphibian (Xenopus laevis) macrophages. J. Biol. Chem. 2018, 293, 1736–1744. [Google Scholar] [CrossRef] [Green Version]
- Morales, H.D.; Abramowitz, L.; Gertz, J.; Sowa, J.; Vogel, A.; Robert, J. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J. Virol. 2010, 84, 4912–4922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayfer, L.; Robert, J. Divergent antiviral roles of amphibian (Xenopus laevis) macrophages elicited by colony-stimulating factor-1 and interleukin-34. J. Leukoc. Biol. 2014, 96, 1143–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayfer, L.; Robert, J. Colony-stimulating factor-1-responsive macrophage precursors reside in the amphibian (Xenopus laevis) bone marrow rather than the hematopoietic subcapsular liver. J. Innate. Immun. 2013, 5, 531–542. [Google Scholar] [CrossRef]
- Wendel, E.S.; Yaparla, A.; Koubourli, D.V.; Grayfer, L. Amphibian (Xenopus laevis) tadpoles and adult frogs mount distinct interferon responses to the Frog Virus 3 ranavirus. Virology 2017, 503, 12–20. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hauser, K.; Popovic, M.; Yaparla, A.; Koubourli, D.V.; Reeves, P.; Batheja, A.; Webb, R.; Forzán, M.J.; Grayfer, L.; Lesbarrères, D. Discovery of granulocyte-lineage cells in the skin of the amphibian Xenopus laevis. FACETS 2020, 5, 571–597. [Google Scholar] [CrossRef]
- Bayley, A.E.; Hill, B.J.; Feist, S.W. Susceptibility of the European common frog Rana temporaria to a panel of ranavirus isolates from fish and amphibian hosts. Dis. Aquat. Organ. 2013, 103, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Grayfer, L.; De Jesus Andino, F.; Robert, J. The amphibian (Xenopus laevis) type I interferon response to Frog Virus 3: New insight into ranavirus pathogenicity. J. Virol. 2014, 88, 5766–5777. [Google Scholar] [CrossRef] [Green Version]
- Hoverman, J.T.; Gray, M.J.; Miller, D.L. Anuran susceptibilities to ranaviruses: Role of species identity, exposure route, and a novel virus isolate. Dis. Aquat. Organ. 2010, 89, 97–107. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Kiryu, Y.; Tabuchi, M.; Waltzek, T.B.; Enge, K.M.; Reintjes-Tolen, S.; Preston, A.; Pessier, A.P. Co-infection by alveolate parasites and frog virus 3-like ranavirus during an amphibian larval mortality event in Florida, USA. Dis. Aquat. Organ. 2013, 105, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeve, B.C.; Crespi, E.J.; Whipps, C.M.; Brunner, J.L. Natural stressors and ranavirus susceptibility in larval wood frogs (Rana sylvatica). Ecohealth 2013, 10, 190–200. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Greaves, D.R.; Schall, T.J. Chemokines and myeloid cell recruitment. Microbes Infect. 2000, 2, 331–336. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Tsun, A.; Li, B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol. Immunol. 2015, 12, 558–565. [Google Scholar] [CrossRef]
- Nandi, S.; Akhter, M.P.; Seifert, M.F.; Dai, X.M.; Stanley, E.R. Developmental and functional significance of the CSF-1 proteoglycan chondroitin sulfate chain. Blood 2006, 107, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovic, M.; Yaparla, A.; Paquin-Proulx, D.; Koubourli, D.V.; Webb, R.; Firmani, M.; Grayfer, L. Colony-stimulating factor-1- and interleukin-34-derived macrophages differ in their susceptibility to Mycobacterium marinum. J. Leukoc. Biol. 2019, 106, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Melchjorsen, J.; Sørensen, L.N.; Paludan, S.R. Expression and function of chemokines during viral infections: From molecular mechanisms to in vivo function. J. Leukoc. Biol. 2003, 74, 331–343. [Google Scholar] [CrossRef]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef]
- Durr, C.; Pfeifer, D.; Claus, R.; Schmitt-Graeff, A.; Gerlach, U.V.; Graeser, R.; Kruger, S.; Gerbitz, A.; Negrin, R.S.; Finke, J.; et al. CXCL12 mediates immunosuppression in the lymphoma microenvironment after allogeneic transplantation of hematopoietic cells. Cancer Res. 2010, 70, 10170–10181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Bhattacharjee, S.; Paul Chowdhury, B.; Majumdar, S. CXCL10 is critical for the generation of protective CD8 T cell response induced by antigen pulsed CpG-ODN activated dendritic cells. PLoS ONE 2012, 7, e48727. [Google Scholar] [CrossRef] [Green Version]
- Koubourli, D.V.; Yaparla, A.; Popovic, M.; Grayfer, L. Amphibian (Xenopus laevis) Interleukin-8 (CXCL8): A Perspective on the Evolutionary Divergence of Granulocyte Chemotaxis. Front. Immunol. 2018, 9, 2058. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegelmeier, A.A.; van Vloten, J.P.; Mould, R.C.; Klafuric, E.M.; Minott, J.A.; Wootton, S.K.; Bridle, B.W.; Karimi, K. Myeloid Cells during Viral Infections and Inflammation. Viruses 2019, 11, 168. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef] [PubMed]
- Paquin-Proulx, D.; Greenspun, B.C.; Kitchen, S.M.; Saraiva Raposo, R.A.; Nixon, D.F.; Grayfer, L. Human interleukin-34-derived macrophages have increased resistance to HIV-1 infection. Cytokine 2018, 111, 272–277. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossainey, M.R.H.; Yaparla, A.; Hauser, K.A.; Moore, T.E.; Grayfer, L. The Roles of Amphibian (Xenopus laevis) Macrophages during Chronic Frog Virus 3 Infections. Viruses 2021, 13, 2299. https://doi.org/10.3390/v13112299
Hossainey MRH, Yaparla A, Hauser KA, Moore TE, Grayfer L. The Roles of Amphibian (Xenopus laevis) Macrophages during Chronic Frog Virus 3 Infections. Viruses. 2021; 13(11):2299. https://doi.org/10.3390/v13112299
Chicago/Turabian StyleHossainey, Muhammad Riadul Haque, Amulya Yaparla, Kelsey A. Hauser, Tyler E. Moore, and Leon Grayfer. 2021. "The Roles of Amphibian (Xenopus laevis) Macrophages during Chronic Frog Virus 3 Infections" Viruses 13, no. 11: 2299. https://doi.org/10.3390/v13112299
APA StyleHossainey, M. R. H., Yaparla, A., Hauser, K. A., Moore, T. E., & Grayfer, L. (2021). The Roles of Amphibian (Xenopus laevis) Macrophages during Chronic Frog Virus 3 Infections. Viruses, 13(11), 2299. https://doi.org/10.3390/v13112299




