Dear Professor Remi N. Charrel and Professor Jerome Depaquit, we thank you for your interest in our research and for your kind suggestions. We agree that the suggested approach for host identification, namely, PCR + NGS (or amplicon-based NGS), which reveals detailed taxonomic compositions from a pooled sample, is more powerful and informative in comparison to the one we had used (i.e., PCR + Sanger sequencing). Despite that, we carried out further analyses (described below), which confirmed that the species identification here is reliable, such that Phlebotomus chinensis is the most probable vector for Heidi virus (HEDV).
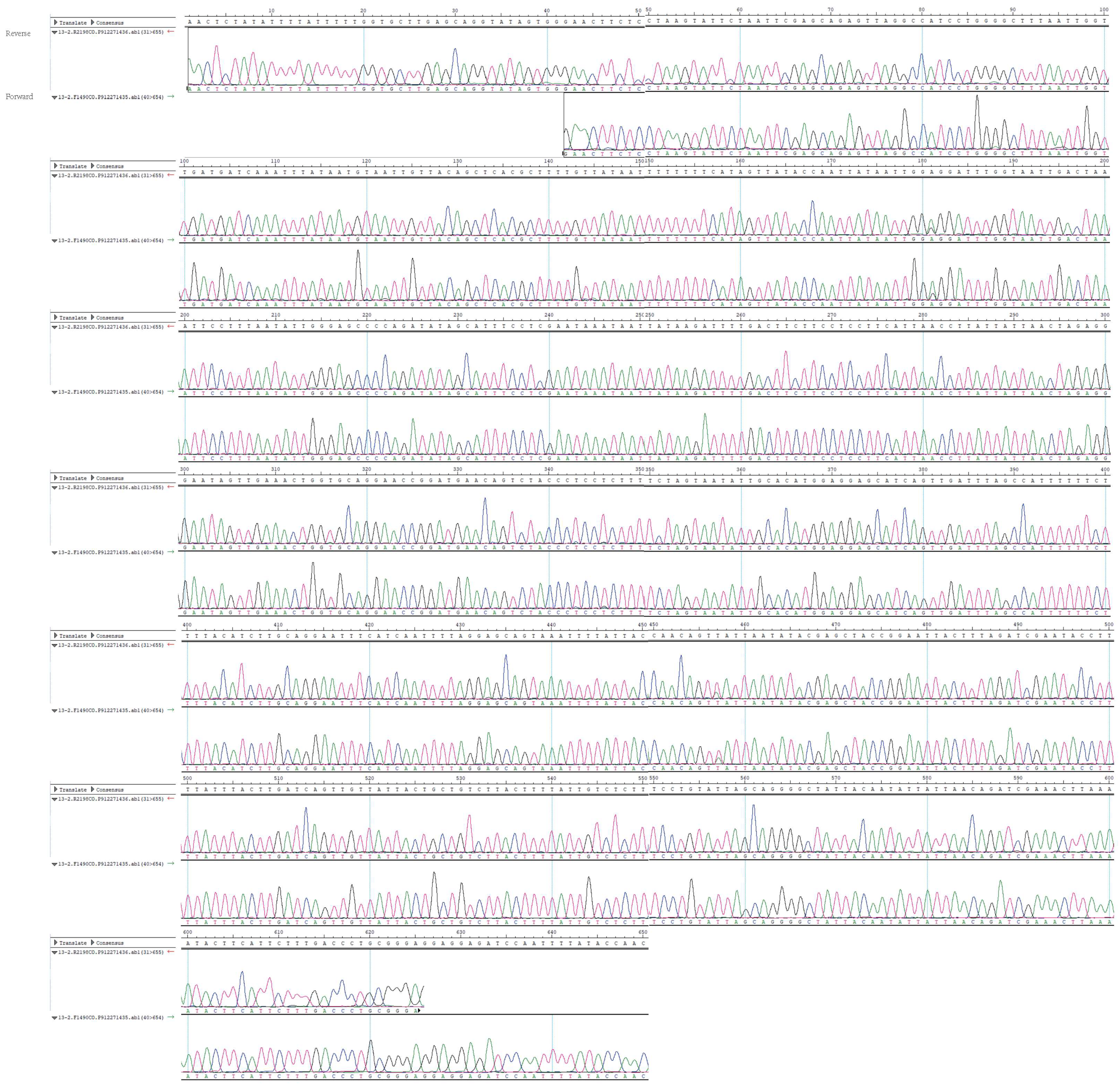
On the one hand, we carefully examined the DNA sequence trace chromatogram obtained from the Sanger sequencing performed in our study, which showed clear and unambiguous signals throughout except for a single position (position 457), which had a mixture of “A” and “T” (Figure 1). Since multiple mixed chromatography trace signals are expected with the presence of more than two species of sandflies, because they shared less than 90% nucleotide identity in the COI gene, we are highly confident to suggest that the majority, if not all, sandflies in the sample belonged to Ph. chinensis.

Figure 1.
The trace chromatogram for Sanger sequencing of cytochrome c oxidase I (COI) gene within the HEDV positive sample. The amplicon is around 650 nucleotides in length. For each position, both forward and reverse base callings and chromatograms are presented, and their positions and consensus sequences are shown above the chromatograms.
On the other hand, we performed taxonomy profiling of a (different) pooled sample containing HEDV, which was analyzed based on meta-transcriptomic NGS analysis (SRA accession: SRR16970469). Ph. chinensis samples were captured in Jul 2020 at Yuncheng of Shanxi Province. The sample collection was performed with light traps placed near a livestock shed that housed dogs and chickens. Species identification was carried out by an experienced field biologist, and subsequently, Ph. chinensis samples (n = 50) were pooled for total RNA sequencing. Although the sample characterized here was not the same as the one used in our previous study (none were left after the initial virus isolation procedures), it contained HEDV whose abundance level reached 56.4 Reads per million, or RPM, based on read mapping using bowtie 2 program version 2.3.5 [1], suggesting a relatively high viral load within the sample. Reads from meta-transcriptomics sequencing were then de novo assembled using megahit program version 1.2.8 [2] and compared against COI sequence databases using the BLAST program, which revealed three COI-related contigs associated with Ph. chinensis (1444.5 RPM), Bradysia spp. (8.6 RPM), and an unidentified member of the Order Diptera (45.1 RPM), respectively. Given the high abundance level of the HEDV, it is more likely to be associated with Ph. chinensis, the dominant species, than the other two species. Furthermore, an analysis of minor variants using Geneious software package version 11.04 [3], based on reads mapped to COI genes, did not suggest the presence of a second species within the sample. Although polymorphism was identified in 24 positions (Table 1), it is too low to imply the presence of another species. Collectively, Ph. chinensis is the dominant species within the HEDV-positive samples and the only sandfly species identified here.

Table 1.
Minor nucleotide variants of Ph. chinensis COI gene in the sample containing HEDV.
Nevertheless, the meta-transcriptomics result here did indicate that the species composition of a pooled sample can be complex, as correctly pointed out by you. To resolve this issue, it requires species composition analyses from more HEDV positive samples, such that a more definite vector–virus relationship can be established. We are currently screening more sandfly pools with meta-transcriptomics as well as PCR + NGS approaches, and hopefully, this matter can be clarified in the future.
Author Contributions
Conceptualization, G.L. and M.S.; methodology, Z.X., S.F. and J.S.; software, Z.X., X.H. and M.S.; validation, Z.X., N.F. and S.F.; formal analysis, Z.X., N.F., X.H. and M.S.; investigation, Z.X., N.F. and J.W.; resources, S.F.; data curation, Z.X. and X.H.; writing—original draft preparation, M.S.; writing—review and editing, Z.X. and G.L.; visualization, Z.X. and X.H.; supervision, G.L.; project administration, S.F.; funding acquisition, M.S. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Natural Science Foundation of China (81290342), the Development Grant of the State Key Laboratory of Infectious Disease Prevention and Control (2014SKLID103), Guangdong Province “Pearl River Talent Plan” Innovation and Entrepreneurship Team Project (2019ZT08Y464), and the United States National Institutes of Health U01 (AI151810).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the materials and data that were used or generated in this study are described and available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).