Epidemiology of Yam Viruses in Guadeloupe: Role of Cropping Practices and Seed-Tuber Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Surveys and Collection of Leaf Samples
2.2. Virus Indexing
2.3. Cloning, Sequencing and Phylogenetic Analyses
2.4. Statistical Analyses of Field Survey Data
2.5. Assessment of Tuber Transmission of Yam Viruses
3. Results
3.1. Prevalence of Viruses Infecting Yam in Guadeloupe
3.2. Molecular Diversity of Macluraviruses
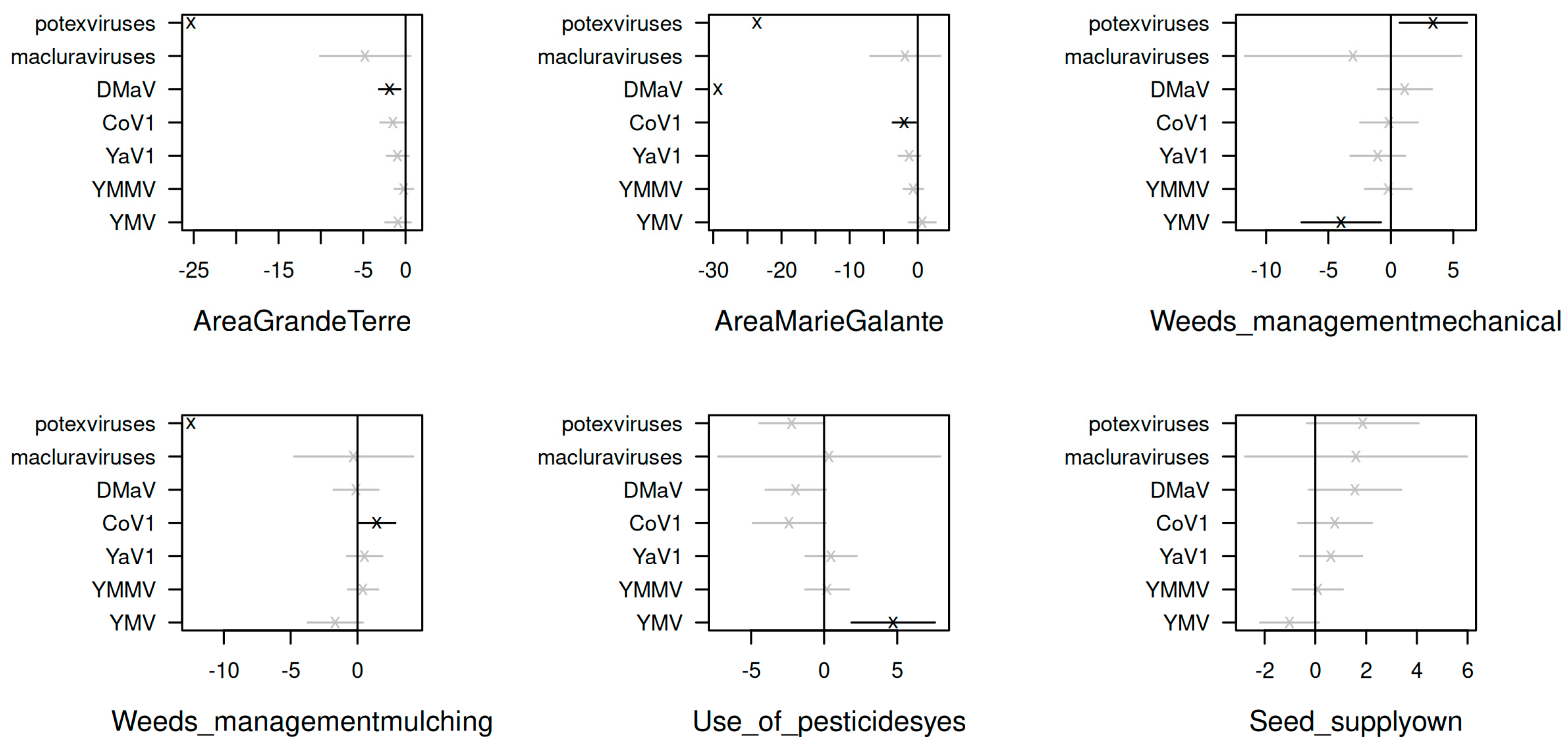
3.3. Correlation between Cropping-Related Factors and the Occurrence of Yam Viruses in Guadeloupe
3.4. Role of Weeds in the Epidemiology of Yam Viruses
3.5. Seed-Tuber Transmission of YMMV and YaV1 in D. trifida
3.6. Risk of Introduction of Yam Viruses through the Importation of Yam Tubers
4. Discussion
4.1. Prevalence and Diversity of Yam Viruses in Guadeloupe
4.2. Horizontal and Vertical Transmission of Yam Viruses
4.3. Role of Mixed Infection in the Etiology of Yam Viral Diseases
4.4. Role of Cropping Practices and Weeds in the Epidemiology of Yam Viruses in Guadeloupe
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 17 July 2022).
- Defèche, C. Diversité des Pratiques Agro-Techniques des Producteurs D’igname en Guadeloupe; Internal Report; INRA: Petit-Bourg, Guadeloupe, France, 2004. [Google Scholar]
- Agreste. Mémento de la Statistique Agricole. Available online: https://daaf.guadeloupe.agriculture.gouv.fr/memento-agricole-edition-2020-a1259.html (accessed on 10 October 2022).
- Bousalem, M.; Douzery, E.J.P.; Fargette, D. High Genetic Diversity, Distant Phylogenetic Relationships and Intraspecies Recombination Events among Natural Populations of Yam Mosaic Virus: A Contribution to Understanding Potyvirus Evolution. J. Gen. Virol. 2000, 81, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.R.; Margaria, P.; Nagata, T.; Winter, S.; Blawid, R. Characterization of Yam Mosaic Viruses from Brazil Reveals a New Phylogenetic Group and Possible Incursion from the African Continent. Virus Genes 2022, 58, 294–307. [Google Scholar] [CrossRef]
- Diouf, M.B.; Festus, R.; Silva, G.; Guyader, S.; Umber, M.; Seal, S.; Teycheney, P.Y. Viruses of Yams (Dioscorea Spp.): Current Gaps in Knowledge and Future Research Directions to Improve Disease Management. Viruses 2022, 14, 1884. [Google Scholar] [CrossRef] [PubMed]
- Adjei, E.A.; Esuma, W.; Alicai, T.; Chamba, E.B.; Edema, R.; Dramadri, I.O.; Ozimati, A.A.; Agaba, R.; Odong, T.L. Genotype-by-Environment Interaction of Yam (Dioscorea species) for Yam Mosaic Virus Resistance, Dry Matter Content and Yield in Uganda. Agronomy 2022, 12, 1984. [Google Scholar] [CrossRef]
- Bousalem, M.; Dallot, S.; Fuji, S.; Natsuaki, K.T. Origin, World-Wide Dispersion, Bio-Geographical Diversification, Radiation and Recombination: An Evolutionary History of Yam Mild Mosaic Virus (YMMV). Infect. Genet. Evol. 2003, 3, 189–206. [Google Scholar] [CrossRef]
- Acina-Mambole, I.; Bonheur, L.; Dumas, L.S.; Filloux, D.; Gomez, R.-M.; Faure, C.; Lange, D.; Anzala, F.; Pavis, C.; Marais, A.; et al. Molecular Characterization of Yam Virus X, a New Potexvirus Infecting Yams (Dioscorea spp.) and Evidence for the Existence of at Least Three Distinct Potexviruses Infecting Yams. Arch. Virol. 2014, 159, 3421–3426. [Google Scholar] [CrossRef]
- Umber, M.; Gomez, R.-M.; Gélabale, S.; Bonheur, L.; Pavis, C.; Teycheney, P.-Y. The Genome Sequence of Dioscorea Bacilliform TR Virus, a Member of the Genus Badnavirus Infecting Dioscorea spp., Sheds Light on the Possible Function of Endogenous Dioscorea Bacilliform Viruses. Arch. Virol. 2017, 162, 517–521. [Google Scholar] [CrossRef]
- Marais, A.; Umber, M.; Filloux, D.; Gomez, R.-M.; Faure, C.; Pavis, C.; Julian, C.; Roumagnac, P.; Acina-Mambole, I.; Bonheur, L.; et al. Yam Asymptomatic Virus 1, a Novel Virus Infecting Yams (Dioscorea spp.) with Significant Prevalence in a Germplasm Collection. Arch. Virol. 2020, 165, 2653–2657. [Google Scholar] [CrossRef]
- Umber, M.; Filloux, D.; Gélabale, S.; Gomez, R.-M.; Marais, A.; Gallet, S.; Gamiette, F.; Pavis, C.; Teycheney, P.-Y. Molecular Viral Diagnosis and Sanitation of Yam Genetic Resources: Implications for Safe Yam Germplasm Exchange. Viruses 2020, 12, 1101. [Google Scholar] [CrossRef]
- Umber, M.; Filloux, D.; Svanella-Dumas, L.; Bonheur, L.; Acina-Mambole, I.; Gomez, R.-M.; Faure, C.; Anzala, F.; Pavis, C.; Roumagnac, P.; et al. Host Range and Molecular Variability of the Sadwavirus Dioscorea Mosaic Associated Virus. Arch. Virol. 2022, 167, 917–922. [Google Scholar] [CrossRef]
- Diouf, M.B.; Gaspard, O.; Marais, A.; Filloux, D.; Gomez, R.; Faure, C.; Roumagnac, P.; Candresse, T.; Theil, S.; Contreras, S.; et al. Molecular Characterization of Cordyline Virus 1 Isolates Infecting Yam (Dioscorea spp.). Arch. Virol. 2022, 167, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Foissac, X.; Svanella-Dumas, L.; Gentit, P.; Dulucq, M.-J.; Marais, A.; Candresse, T. Polyvalent Degenerate Oligonucleotides Reverse Transcription-Polymerase Chain Reaction: A Polyvalent Detection and Characterization Tool for Trichoviruses, Capilloviruses, and Foveaviruses. Phytopathology 2005, 95, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.A.; Seal, S.E. Rapid Single-Tube Immunocapture RT-PCR for the Detection of Two Yam Potyviruses. J. Virol. Methods 1997, 69, 73–79. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Beule, L.; Karlovsky, P. Improved Normalization of Species Count Data in Ecology by Scaling with Ranked Subsampling (SRS): Application to Microbial Communities. PeerJ 2020, 8, e9593. [Google Scholar] [CrossRef]
- Niku, J.; Hui, F.K.C.; Taskinen, S.; Warton, D.I. Gllvm: Fast Analysis of Multivariate Abundance Data with Generalized Linear Latent Variable Models in r. Methods Ecol. Evol. 2019, 10, 2173–2182. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Filloux, D.; Fernandez, E.; Loire, E.; Claude, L.; Galzi, S.; Candresse, T.; Winter, S.; Jeeva, M.L.; Makeshkumar, T.; Martin, D.P.; et al. Nanopore-Based Detection and Characterization of Yam Viruses. Sci. Rep. 2018, 8, 17879. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Meng, Y.; Shen, P.; Li, R.; Ma, Y.; Tan, S.; Chen, H.; Cao, M.; Li, F. Complete Genome Sequence of Yam Chlorotic Necrosis Virus, a Novel Macluravirus Infecting Yam. Arch. Virol. 2018, 163, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Narina, S.S.; Buyyarapu, R.; Kottapalli, K.R.; Sartie, A.M.; Ali, M.I.; Robert, A.; Hodeba, M.J.D.; Sayre, B.L.; Scheffler, B.E. Generation and Analysis of Expressed Sequence Tags (ESTs) for Marker Development in Yam (Dioscorea alata L.). BMC Genom. 2011, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Penet, L.; Barthe, E.; Alleyne, A.; Blazy, J.M. Disease Risk Perception and Diversity of Management Strategies by Farmers: The Case of Anthracnose Caused by Colletotrichum Gloeosporioides on Water Yams (Dioscorea alata) in Guadeloupe. Crop Prot. 2016, 88, 7–17. [Google Scholar] [CrossRef]
- Barlagne, C.; Cornet, D.; Blazy, J.-M.; Diman, J.-L.; Ozier-Lafontaine, H. Consumers’ Preferences for Fresh Yam: A Focus Group Study. Food Sci. Nutr. 2017, 5, 54–66. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Palukaitis, P.; Choi, S.-K. CHAPTER 1: Host Range. In Cucumber Mosaic Virus; Virology; The American Phytopathological Society: St. Paul, MN, USA, 2019; pp. 15–18. ISBN 978-0-89054-610-9. [Google Scholar]
- Eni, A.O.; Kumar, P.L.; Asiedu, R.; Alabi, O.J.; Naidu, R.A.; Hughes, J.; Rey, M.E.C. First Report of Cucumber Mosaic Virus in Yams (Dioscorea Spp.) in Ghana, Togo, and Republic of Benin in West Africa. Plant Dis. 2008, 92, 833. [Google Scholar] [CrossRef][Green Version]
- Eni, A.O.; Kumar, P.L.; Asiedu, R.; Alabi, O.J.; Naidu, R.A.; Hughes, J.; Rey, M.E.C. Characterization of Cucumber Mosaic Virus Isolated from Yam (Dioscorea spp.) in West Africa. Afr. J. Biotechnol. 2013, 12, 3472–3480. [Google Scholar] [CrossRef]
- Messiaen, C.-M.; Blancard, D.; Rouxel, F.; Lafon, R. Les Maladies des Plantes Maraîchères; INRA: Paris, France, 1991; p. 552. [Google Scholar]
- Tropifruits—Virus de La Mosaïque Du Concombre Cucumber Mosaic Virus (CMV). Available online: https://ephytia.inra.fr/fr/C/26808/Tropifruits-Virus-de-la-mosaique-du-concombre-Cucumber-mosaic-virus-CMV (accessed on 7 October 2022).
- Étienne, J. Les Pucerons de Guadeloupe, des Grandes et Petites Antilles (Hemiptera, Aphididae). Bull. Société Entomol. Fr. 2005, 110, 455–462. [Google Scholar] [CrossRef]
- Migliori, A. Maladie A Virus De L’Igname (Dioscorea spp.). In Proceedings of the 14th Annual Meeting of the Caribbean Food Crops Society, Gosier, Guadeloupe, France, 28 June 1977. [Google Scholar]
- Dallot, S.; Guzmán, M.; Bousalem, M. Occurrence of Potyviruses on Yam (Dioscorea spp.) in Colombia and First Molecular Characterization of Yam Mild Mosaic Virus. Plant Dis. 2001, 85, 803. [Google Scholar] [CrossRef]
- Eni, A.O.; d’A Hughes, J.; Asiedu, R.; Rey, M.E.C. Survey of the Incidence and Distribution of Viruses Infecting Yam (Dioscorea Spp.) in Ghana and Togo. Ann. Appl. Biol. 2010, 156, 243–251. [Google Scholar] [CrossRef]
- Hayashi, E.A.I.; Blawid, R.; de Melo, F.L.; Andrade, M.S.; Pio-Ribeiro, G.; de Andrade, G.P.; Nagata, T. Complete Genome Sequence of a Putative New Secovirus Infecting Yam (Dioscorea) Plants. Arch. Virol. 2017, 162, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Azeteh, I.N.; Hanna, R.; Njukeng, A.P.; Oresanya, A.O.; Sakwe, P.N.; Lava Kumar, P. Distribution and Diversity of Viruses Infecting Yams (Dioscorea Spp.) in Cameroon. Virus Dis. 2019, 30, 526–537. [Google Scholar] [CrossRef]
- Luo, G.-F.; Podolyan, A.; Kidanemariam, D.B.; Pilotti, C.; Houliston, G.; Sukal, A.C. A Review of Viruses Infecting Yam (Dioscorea spp.). Viruses 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Bakayoko, Y.; Kouakou, A.M.; Kouassi, A.B.; Gomez, R.-M.; Dibi, K.E.B.; Essis, B.S.; N’Zué, B.; Adebola, P.; N’Guetta, A.S.-P.; Umber, M. Detection and Diversity of Viruses Infecting African Yam (Dioscorea rotundata) in a Collection and F1 Progenies in Côte d’Ivoire Shed Light to Plant-to-Plant Viral Transmission. Plant Pathol. 2021, 70, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Syller, J. Facilitative and Antagonistic Interactions between Plant Viruses in Mixed Infections. Mol. Plant Pathol. 2012, 13, 204–216. [Google Scholar] [CrossRef]
- Mascia, T.; Gallitelli, D. Synergies and Antagonisms in Virus Interactions. Plant Sci. 2016, 252, 176–192. [Google Scholar] [CrossRef]
- Dey, K.K.; Sugikawa, J.; Kerr, C.; Melzer, M.J. Air Potato (Dioscorea bulbifera) Plants Displaying Virus-like Symptoms Are Co-Infected with a Novel Potyvirus and a Novel Ampelovirus. Virus Genes 2019, 55, 117–121. [Google Scholar] [CrossRef]
- Thouvenel, J.-C.; Fauquet, C. Yam Mosaic, a New Potyvirus Infecting Dioscorea cayenensis in the Ivory Coast. Ann. Appl. Biol. 1979, 93, 279–283. [Google Scholar] [CrossRef]
- Odu, B.O.; d’A Hughes, J.; Shoyinka, S.A.; Dongo, L.N. Isolation, Characterisation and Identification of a Potyvirus from Dioscorea alata L. (Water Yam) in Nigeria. Ann. Appl. Biol. 1999, 134, 65–71. [Google Scholar] [CrossRef]
- Phillips, S.; Briddon, R.W.; Brunt, A.A.; Hull, R. The Partial Characterization of a Badnavirus Infecting the Greater Asiatic or Water Yam (Dioscorea alata). J. Phytopathol. 1999, 147, 265–269. [Google Scholar] [CrossRef]
- Bertschinger, L.; Bühler, L.; Dupuis, B.; Duffy, B.; Gessler, C.; Forbes, G.A.; Keller, E.R.; Scheidegger, U.C.; Struik, P.C. Incomplete Infection of Secondarily Infected Potato Plants—An Environment Dependent Underestimated Mechanism in Plant Virology. Front. Plant Sci. 2017, 8, 74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fargette, D.; Colon, L.T.; Bouveau, R.; Fauquet, C. Components of Resistance of Cassava to African Cassava Mosaic Virus. Eur. J. Plant Pathol. 1996, 102, 645–654. [Google Scholar] [CrossRef]
- Simmons, H.E.; Munkvold, G.P. Seed Transmission in the Potyviridae. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Gullino, M.L., Munkvold, G., Eds.; Plant Pathology in the 21st Century; Springer: Dordrecht, The Netherlands, 2014; pp. 3–15. ISBN 978-94-017-9389-6. [Google Scholar]
- Fuchs, M.; Bar-Joseph, M.; Candresse, T.; Maree, H.J.; Martelli, G.P.; Melzer, M.J.; Menzel, W.; Minafra, A.; Sabanadzovic, S.; Report Consortium, I. ICTV Virus Taxonomy Profile: Closteroviridae. J. Gen. Virol. 2020, 101, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Matile-Ferrero, D. Cochenilles des Antilles françaises et de quelques autres îles des Caraïbes [hemiptera, coccoidea]. Rev. Fr. Entomol. 2006, 28, 161–190. [Google Scholar]
- Di Mattia, J.; Ryckebusch, F.; Vernerey, M.-S.; Pirolles, E.; Sauvion, N.; Peterschmitt, M.; Zeddam, J.-L.; Blanc, S. Co-Acquired Nanovirus and Geminivirus Exhibit a Contrasted Localization within Their Common Aphid Vector. Viruses 2020, 12, 299. [Google Scholar] [CrossRef]
- Malpica, J.M.; Sacristán, S.; Fraile, A.; García-Arenal, F. Association and Host Selectivity in Multi-Host Pathogens. PLoS ONE 2006, 1, e41. [Google Scholar] [CrossRef]
- Bömer, M.; Rathnayake, A.I.; Visendi, P.; Silva, G.; Seal, S.E. Complete Genome Sequence of a New Member of the Genus Badnavirus, Dioscorea Bacilliform RT Virus 3, Reveals the First Evidence of Recombination in Yam Badnaviruses. Arch. Virol. 2018, 163, 533–538. [Google Scholar] [CrossRef]
- Zou, C.-W.; Meng, J.-R.; Yao, Z.-T.; Zhang, L.; Wang, Z.-Q.; Wei, B.-H.; Chen, B.-S. Genetic Diversity and Genome Recombination in Yam Mild Mosaic Virus Isolates. Phytopathol. Res. 2020, 2, 10. [Google Scholar] [CrossRef]
- Blanchard, A.; Rolland, M.; Lacroix, C.; Kerlan, C.; Jacquot, E. Potato Virus Y: A Century of Evolution. Curr. Top. Virol. 2008, 7, 21–32. [Google Scholar]
- Karasev, A.V.; Hu, X.; Brown, C.J.; Kerlan, C.; Nikolaeva, O.V.; Crosslin, J.M.; Gray, S.M. Genetic Diversity of the Ordinary Strain of Potato Virus Y (PVY) and Origin of Recombinant PVY Strains. Phytopathology 2011, 101, 778–785. [Google Scholar] [CrossRef]
- Quenouille, J.; Vassilakos, N.; Moury, B. Potato Virus Y: A Major Crop Pathogen That Has Provided Major Insights into the Evolution of Viral Pathogenicity. Mol. Plant Pathol. 2013, 14, 439–452. [Google Scholar] [CrossRef]
- Tian, Y.; Valkonen, J.P.T. Recombination of Strain O Segments to HC-pro-encoding Sequence of Strain N of Potato Virus Y Modulates Necrosis Induced in Tobacco and in Potatoes Carrying Resistance Genes Ny or Nc. Mol. Plant Pathol. 2015, 16, 735–747. [Google Scholar] [CrossRef]
- Green, K.J.; Quintero-Ferrer, A.; Chikh-Ali, M.; Jones, R.A.C.; Karasev, A.V. Genetic Diversity of Nine Non-Recombinant Potato Virus Y Isolates From Three Biological Strain Groups: Historical and Geographical Insights. Plant Dis. 2020, 104, 2317–2323. [Google Scholar] [CrossRef]
- Karyeija, R.F.; Kreuze, J.F.; Gibson, R.W.; Valkonen, J.P.T. Synergistic Interactions of a Potyvirus and a Phloem-Limited Crinivirus in Sweet Potato Plants. Virology 2000, 269, 26–36. [Google Scholar] [CrossRef]
- Mahuku, G.; Lockhart, B.E.; Wanjala, B.; Jones, M.W.; Kimunye, J.N.; Stewart, L.R.; Cassone, B.J.; Sevgan, S.; Nyasani, J.O.; Kusia, E.; et al. Maize Lethal Necrosis (MLN), an Emerging Threat to Maize-Based Food Security in Sub-Saharan Africa. Phytopathology 2015, 105, 956–965. [Google Scholar] [CrossRef]
- Moreno, A.B.; López-Moya, J.J. When Viruses Play Team Sports: Mixed Infections in Plants. Phytopathology 2020, 110, 29–48. [Google Scholar] [CrossRef]
- Jones, R.A.C. Disease Pandemics and Major Epidemics Arising from New Encounters between Indigenous Viruses and Introduced Crops. Viruses 2020, 12, 1388. [Google Scholar] [CrossRef]
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Kasschau, K.D.; Xie, Z.; Allen, E.; Llave, C.; Chapman, E.J.; Krizan, K.A.; Carrington, J.C. P1/HC-Pro, a Viral Suppressor of RNA Silencing, Interferes with Arabidopsis Development and MiRNA Function. Dev. Cell 2003, 4, 205–217. [Google Scholar] [CrossRef]
- González-Jara, P.; Atencio, F.A.; Martínez-García, B.; Barajas, D.; Tenllado, F.; Díaz-Ruíz, J.R. A Single Amino Acid Mutation in the Plum Pox Virus Helper Component-Proteinase Gene Abolishes Both Synergistic and RNA Silencing Suppression Activities. Phytopathology 2005, 95, 894–901. [Google Scholar] [CrossRef]
- Tatineni, S.; Alexander, J.; Gupta, A.K.; French, R. Asymmetry in Synergistic Interaction Between Wheat Streak Mosaic Virus and Triticum Mosaic Virus in Wheat. Mol. Plant-Microbe Interact. 2019, 32, 336–350. [Google Scholar] [CrossRef]
- Tomlinson, J.A. Epidemiology and Control of Virus Diseases of Vegetables. Ann. Appl. Biol. 1987, 110, 661–681. [Google Scholar] [CrossRef]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging Infectious Diseases of Plants: Pathogen Pollution, Climate Change and Agrotechnology Drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Asala, S.; Alegbejo, M.D.; Kashina, B.D.; Banwo, O.O.; Shinngu, C.P. Viruses in Weeds in Dioscorea Yam Fields in Nigeria. Afr. Crop Sci. J. 2014, 22, 109–115. [Google Scholar] [CrossRef]
- Aliyu, T.H.; Takim, F.O.; Olatinwo, L.K.; Arogundade, O.; Omotesho, K.F.; Oladoja, D.A. Severity of Viral Diseases and Types of Weeds as Alternative Viral Hosts in Dioscorea Fields in Southern Guinea Savannah Agroecology of Nigeria. J. Food Agric. 2021, 14, 1–16. [Google Scholar] [CrossRef]


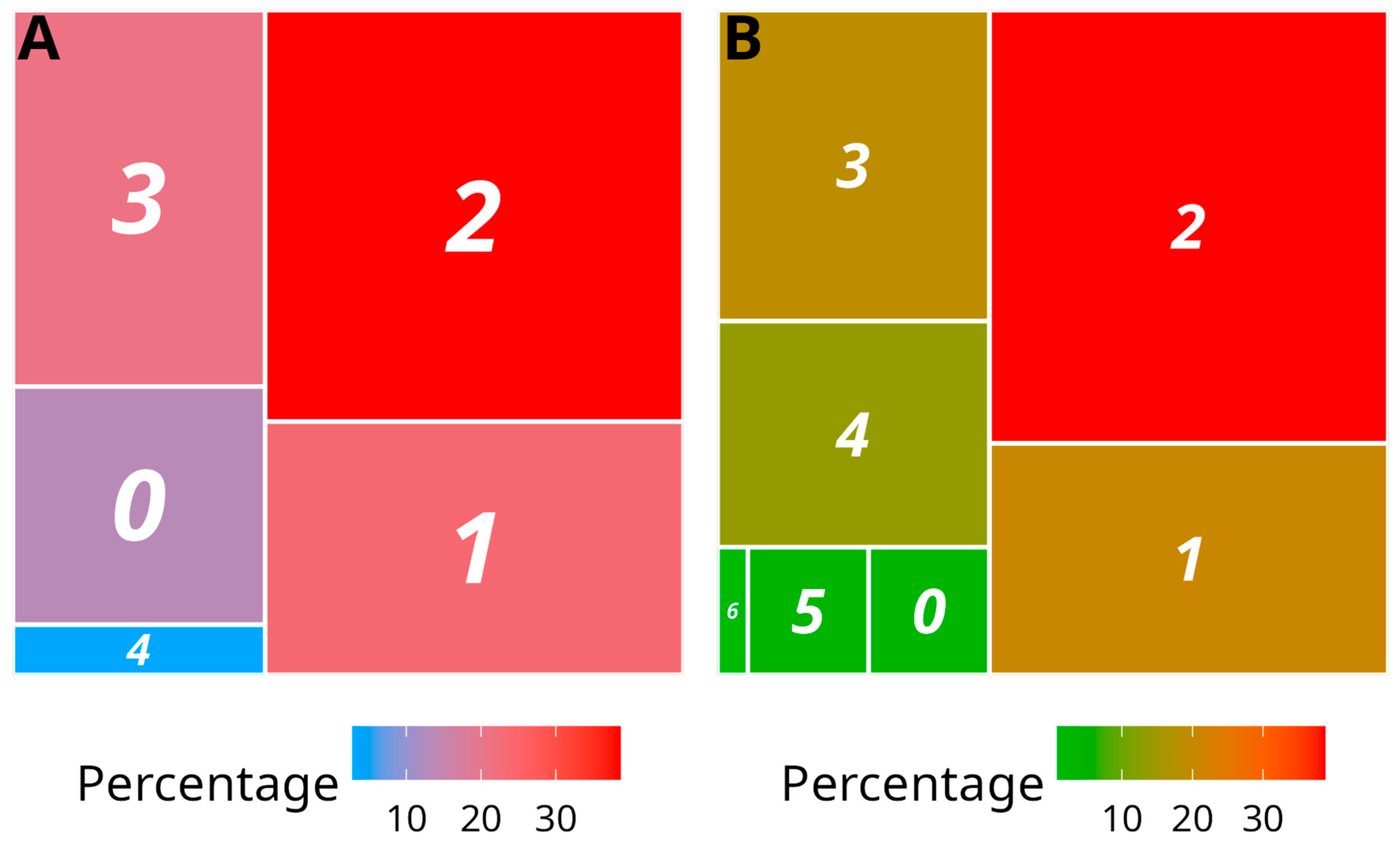



| Yam Species | Number of Indexed Samples | CMV | CoV1 | DMaV | YaV1 | YMV | YMMV | Macluraviruses | Potexviruses | Badnaviruses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| 2019 | |||||||||||||||||||
| Dioscorea alata | 604 | 0 | 0 | 96 | 15.9 | 72 | 11.9 | 398 | 65.9 | 59 | 9.8 | 451 | 74.7 | 28 | 4.6 | 2 | 0.3 | NA | NA |
| Dioscorea bulbifera | 16 | 0 | 0 | 2 | 12.5 | 8 | 50.0 | 0 | 0.0 | 1 | 6.3 | 13 | 81.3 | 0 | 0.0 | 1 | 6.3 | NA | NA |
| Dioscorea cayenensis | 26 | 0 | 0 | 0 | 0.0 | 14 | 53.8 | 0 | 0.0 | 5 | 19.2 | 15 | 57.7 | 0 | 0.0 | 7 | 26.9 | NA | NA |
| Dioscorea esculenta | 31 | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 9 | 29.0 | 9 | 29.0 | 29 | 93.5 | 0 | 0.0 | 1 | 3.2 | NA | NA |
| Dioscorea rotundata | 87 | 0 | 0 | 4 | 4.6 | 27 | 31.0 | 10 | 11.5 | 36 | 41.4 | 27 | 31.0 | 2 | 5.7 | 14 | 16.1 | NA | NA |
| Dioscorea trifida | 16 | 0 | 0 | 8 | 50.0 | 0 | 0.0 | 1 | 6.3 | 8 | 50.0 | 16 | 100.0 | 0 | 0.0 | 0 | 0.0 | NA | NA |
| Sub total | 780 | 0 | 0 | 110 | 14.1 | 121 | 15.5 | 418 | 53.6 | 118 | 15.1 | 551 | 70.6 | 30 | 3.8 | 25 | 3.2 | - | - |
| 2020 | |||||||||||||||||||
| Dioscorea alata | 116 | 0 | 0 | 34 | 29.3 | 34 | 29.3 | 83 | 71.6 | 0 | 0.0 | 86 | 74.1 | 13 | 11.2 | 0 | 0.0 | 20 | 17.2 |
| Total | 896 | 0 | 0 | 144 | 16.1 | 155 | 17.3 | 501 | 55.9 | 118 | 13.2 | 637 | 71.1 | 43 | 4.8 | 25 | 2.8 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diouf, M.B.; Guyader, S.; Gaspard, O.; Francius, E.; Teycheney, P.-Y.; Umber, M. Epidemiology of Yam Viruses in Guadeloupe: Role of Cropping Practices and Seed-Tuber Supply. Viruses 2022, 14, 2366. https://doi.org/10.3390/v14112366
Diouf MB, Guyader S, Gaspard O, Francius E, Teycheney P-Y, Umber M. Epidemiology of Yam Viruses in Guadeloupe: Role of Cropping Practices and Seed-Tuber Supply. Viruses. 2022; 14(11):2366. https://doi.org/10.3390/v14112366
Chicago/Turabian StyleDiouf, Mame Boucar, Sébastien Guyader, Olyvia Gaspard, Eric Francius, Pierre-Yves Teycheney, and Marie Umber. 2022. "Epidemiology of Yam Viruses in Guadeloupe: Role of Cropping Practices and Seed-Tuber Supply" Viruses 14, no. 11: 2366. https://doi.org/10.3390/v14112366
APA StyleDiouf, M. B., Guyader, S., Gaspard, O., Francius, E., Teycheney, P.-Y., & Umber, M. (2022). Epidemiology of Yam Viruses in Guadeloupe: Role of Cropping Practices and Seed-Tuber Supply. Viruses, 14(11), 2366. https://doi.org/10.3390/v14112366









