A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Animals
2.3. Laryngoscopy
2.4. Vocal Fold Abrasion
2.5. MmuPV1 Infection
2.6. Animal Health
2.7. Oral Swabs for Longitudinal MmuPV1 DNA Detection
2.8. Endoscopic Assessment of Lesions
2.9. Tissue Collection and Histology
2.10. Detection of MmuPV1 DNA in Larygneal Lavage, Extralaryngeal Tissue, and FFPE Slides
2.11. Pathology Grading
2.12. RNAscope ISH for MmuPV1 RNA
2.13. MmuPV1 L1 and K14 Dual Immunofluorescence
2.14. Image Acquisition
2.15. Infectivity of Laryngeal Lavage
2.16. Statistical Analysis
3. Results
3.1. Animal Health
3.2. MmuPV1 DNA in Oral Swabs and Laryngeal Lavage
3.3. Endoscopic Assessment of Lesions
3.4. Pathology Grading
3.5. MmuPV1 RNA and Capsid Protein in Larynx
3.6. MmuPV1 RNA and Capsid Protein in Extralaryngeal Head and Neck Tissues
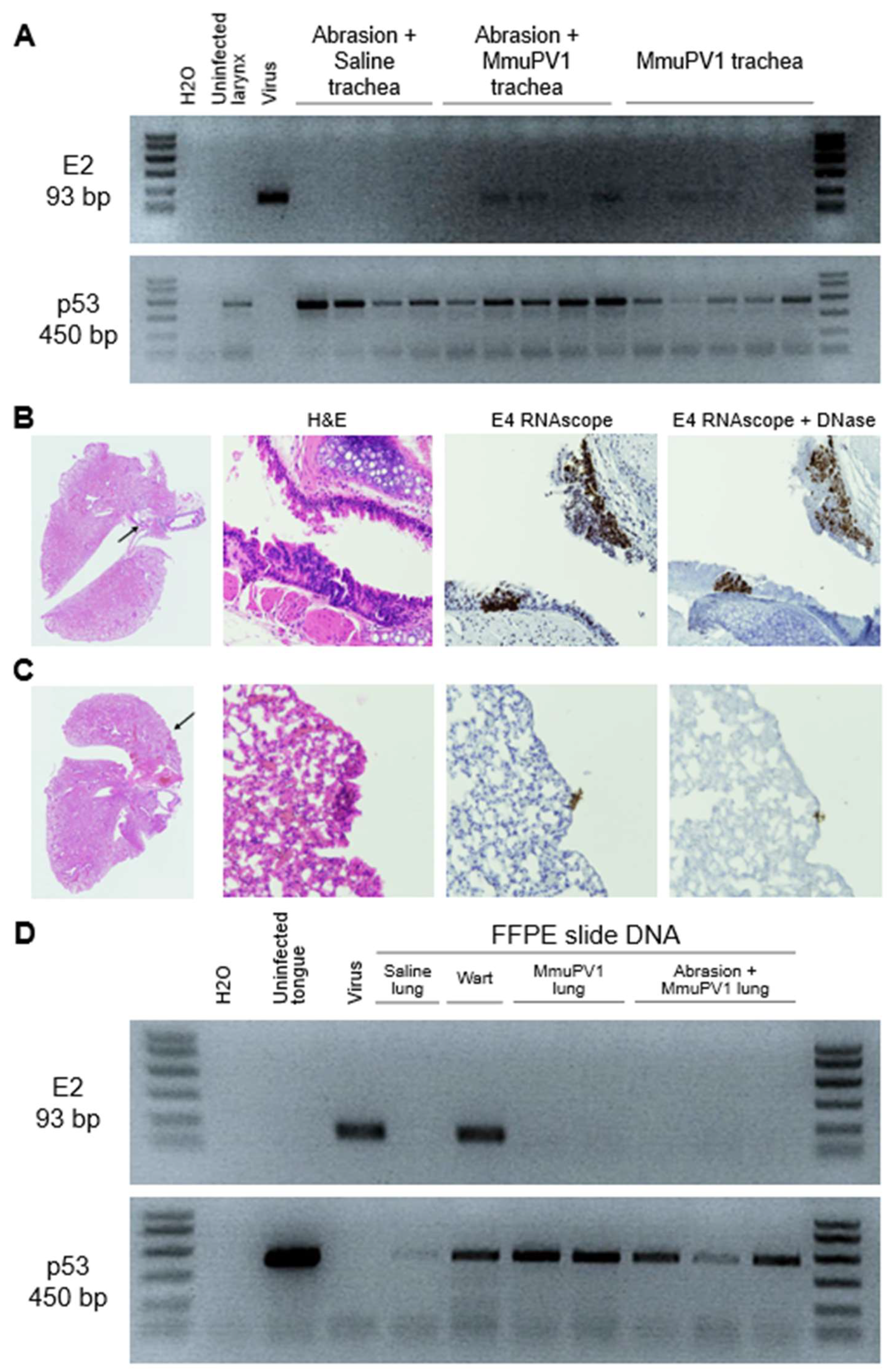
3.7. MmuPV1 Spread to Lower Airways and Esophagus
3.8. Infectivity of Laryngeal Lavage and Secondary Laryngeal Infections in Nude Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larson, D.A.; Derkay, C.S. Epidemiology of Recurrent Respiratory Papillomatosis. APMIS 2010, 118, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Araujo Neto, C.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent Respiratory Papillomatosis: A State-of-the-Art Review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human Papillomavirus Molecular Biology and Disease Association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Syrjänen, S.; Syrjänen, K. HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential. Viruses 2021, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Benedict, P.A.; Ruiz, R.; Yoo, M.; Verma, A.; Ahmed, O.H.; Wang, B.; Dion, G.R.; Voigt, A.; Merati, A.; Rosen, C.A.; et al. Laryngeal Distribution of Recurrent Respiratory Papillomatosis in a Previously Untreated Cohort. Laryngoscope 2018, 128, 138–143. [Google Scholar] [CrossRef]
- Tse, J.R.; Zhang, Z.; Long, J.L. Effects of Vocal Fold Epithelium Removal on Vibration in an Excised Human Larynx Model. J. Acoust. Soc. Am. 2015, 138, EL60–EL64. [Google Scholar] [CrossRef] [Green Version]
- Derkay, C.S.; Bluher, A. Recurrent Respiratory Papillomatosis: Update 2018. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 421–425. [Google Scholar] [CrossRef]
- Chadha, N.K.; Allegro, J.; Barton, M.; Hawkes, M.; Harlock, H.; Campisi, P. The Quality of Life and Health Utility Burden of Recurrent Respiratory Papillomatosis in Children. Otolaryngol. Head Neck Surg. 2010, 143, 685–690. [Google Scholar] [CrossRef]
- San Giorgi, M.R.M.; Aaltonen, L.-M.; Rihkanen, H.; Tjon Pian Gi, R.E.A.; van der Laan, B.F.A.M.; Hoekstra-Weebers, J.E.H.M.; Dikkers, F.G. Quality of Life of Patients with Recurrent Respiratory Papillomatosis. Laryngoscope 2017, 127, 1826–1831. [Google Scholar] [CrossRef]
- Loizou, C.; Laurell, G.; Lindquist, D.; Olofsson, K. Voice and Quality of Life in Patients with Recurrent Respiratory Papillomatosis in a Northern Sweden Cohort. Acta Otolaryngol. 2014, 134, 401–406. [Google Scholar] [CrossRef]
- Avelino, M.A.G.; Zaiden, T.C.D.T.; Gomes, R.O. Surgical Treatment and Adjuvant Therapies of Recurrent Respiratory Papillomatosis. Braz. J. Otorhinolaryngol. 2013, 79, 636–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benninger, M.S.; Derakhshan, A.; Milstein, C.F. The Use of Cryotherapy for Papilloma and Early Laryngeal Cancers: Long-Term Results. Ann. Otol. Rhinol. Laryngol. 2015, 124, 509–514. [Google Scholar] [CrossRef]
- Benedict, J.J.; Derkay, C.S. Recurrent Respiratory Papillomatosis: A 2020 Perspective. Laryngoscope Investig. Otolaryngol. 2021, 6, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.T. Biologics for the Treatment of Recurrent Respiratory Papillomatosis. Otolaryngol. Clin. N. Am. 2021, 54, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Allen, C. How Enhancing Immunity to Low-Risk HPV Could Cure Recurrent Respiratory Papillomatosis. Laryngoscope 2021, 131, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Dion, G.R.; Teng, S.; Boyd, L.R.; Northam, A.; Mason-Apps, C.; Vieira, D.; Amin, M.R.; Branski, R.C. Adjuvant Human Papillomavirus Vaccination for Secondary Prevention: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 614–622. [Google Scholar] [CrossRef]
- Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Ault, K.A.; Giuliano, A.R.; Wheeler, C.M.; Koutsky, L.A.; Malm, C.; Lehtinen, M.; et al. Prophylactic Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) L1 Virus-like Particle Vaccine in Young Women: A Randomised Double-Blind Placebo-Controlled Multicentre Phase II Efficacy Trial. Lancet Oncol. 2005, 6, 271–278. [Google Scholar] [CrossRef]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final Efficacy, Immunogenicity, and Safety Analyses of a Nine-Valent Human Papillomavirus Vaccine in Women Aged 16-26 Years: A Randomised, Double-Blind Trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- Teutsch, S.M.; Nunez, C.A.; Morris, A.; Booy, R.; McGregor, S.; King, J.; Brotherton, J.M.; Novakovic, D.; Jones, C.A.; Rawlinson, W.; et al. Australian Paediatric Surveillance Unit (APSU) Annual Surveillance Report 2019. Commun. Dis. Intell. 2020, 44. [Google Scholar] [CrossRef]
- Nicol, A.F.; de Andrade, C.V.; Russomano, F.B.; Rodrigues, L.S.L.; Oliveira, N.S.; Provance, D.W., Jr.; Nuovo, G.J. HPV Vaccines: Their Pathology-Based Discovery, Benefits, and Adverse Effects. Ann. Diagn. Pathol. 2015, 19, 418–422. [Google Scholar] [CrossRef]
- Hildesheim, A.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Solomon, D.; Bratti, M.C.; Schiller, J.T.; Gonzalez, P.; Dubin, G.; Porras, C.; et al. Effect of Human Papillomavirus 16/18 L1 Viruslike Particle Vaccine among Young Women with Preexisting Infection: A Randomized Trial. JAMA 2007, 298, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyibizi, J.; Rodier, C.; Wassef, M.; Trottier, H. Risk Factors for the Development and Severity of Juvenile-Onset Recurrent Respiratory Papillomatosis: A Systematic Review. Int. J. Pediatric Otorhinolaryngol. 2014, 78, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Kashima, H.K.; Shah, F.; Lyles, A.; Glackin, R.; Muhammad, N.; Turner, L.; Zandt, S.V.; Whitt, S.; Shah, K. A Comparison of Risk Factors in Juvenile-Onset and Adult-Onset Recurrent Respiratory Papillomatosis. Laryngoscope 1992, 102, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Amiling, R.; Meites, E.; Querec, T.D.; Stone, L.; Singh, V.; Unger, E.R.; Derkay, C.S.; Markowitz, L.E. Juvenile-Onset Recurrent Respiratory Papillomatosis in the United States, Epidemiology and HPV Types—2015–2020. J. Pediatric Infect. Dis. Soc. 2021, 10, 774–781. [Google Scholar] [CrossRef]
- Ruiz, R.; Achlatis, S.; Verma, A.; Born, H.; Kapadia, F.; Fang, Y.; Pitman, M.; Sulica, L.; Branski, R.C.; Amin, M.R. Risk Factors for Adult-Onset Recurrent Respiratory Papillomatosis. Laryngoscope 2014, 124, 2338–2344. [Google Scholar] [CrossRef]
- Szydłowski, J.; Durzyński, Ł.; Myga, M.; Grzegorowski, M.; Goździcka-Józefiak, A. Human Papillomavirus DNA Presence of the Upper Respiratory Tract Mucosa of Healthy Children. Otolaryngol. Pol. 2004, 58, 211–215. [Google Scholar]
- Szydłowski, J.; Jonczyk-Potoczna, K.; Pucher, B.; Buraczyńska-Andrzejewska, B.; Prauzińska, M.; Kolasińska-Lipńska, J.; Krauss, H.; Piątek, J.; Żukiewicz-Sobczak, W. Prevalence of Human Papillomavirus (HPV) in Upper Respiratory Tract Mucosa in a Group of Pre-School Children. Ann. Agric. Environ. Med. 2014, 21, 822–824. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Fitch, J.L.; Holbrook, A. Modal Vocal Fundamental Frequency of Young Adults. Arch. Otolaryngol. 1970, 92, 379–382. [Google Scholar] [CrossRef]
- Glaze, L.E.; Bless, D.M.; Milenkovic, P.; Susser, R.D. Acoustic Characteristics of Children’s Voice. J. Voice 1988, 2, 312–319. [Google Scholar] [CrossRef]
- Levendoski, E.E.; Leydon, C.; Thibeault, S.L. Vocal Fold Epithelial Barrier in Health and Injury: A Research Review. J. Speech Lang. Hear. Res. 2014, 57, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, B.; Suehiro, A.; Echemendia, N.; Sivasankar, M. Raised Intensity Phonation Compromises Vocal Fold Epithelial Barrier Integrity. Laryngoscope 2011, 121, 346–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, C.A.; Sataloff, R.T.; Lyons, K.M.; Hawkshaw, M. Vocal Fold Mucosal Tears: Maintaining a High Clinical Index of Suspicion. J Voice 2001, 15, 451–455. [Google Scholar] [CrossRef]
- Klein, A.M.; Johns, M. Vocal Emergencies. Otolaryngol. Clin. N. Am. 2007, 40, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, S.L.; Rees, L.; Pazmany, L.; Birchall, M.A. At the Crossroads: Mucosal Immunology of the Larynx. Mucosal Immunol. 2009, 2, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Formánek, M.; Jančatová, D.; Komínek, P.; Matoušek, P.; Zeleník, K. Laryngopharyngeal Reflux and Herpes Simplex Virus Type 2 Are Possible Risk Factors for Adult-Onset Recurrent Respiratory Papillomatosis (Prospective Case-Control Study). Clin. Otolaryngol. 2017, 42, 597–601. [Google Scholar] [CrossRef]
- Quiney, R.E.; Hall, D.; Croft, C.B. Laryngeal Papillomatosis: Analysis of 113 Patients. Clin. Otolaryngol. Allied Sci. 1989, 14, 217–225. [Google Scholar] [CrossRef]
- Maran, A.; Amella, C.A.; DiLorenzo, T.P.; Auborn, K.J.; Taichman, L.B.; Steinberg, B.M. Human Papillomavirus Type 11 Transcripts Are Present at Low Abundance in Latently Infected Respiratory Tissues. Virology 1995, 212, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Abramson, A.L.; Nouri, M.; Mullooly, V.; Fisch, G.; Steinberg, B.M. Latent Human Papillomavirus Infection Is Comparable in the Larynx and Trachea. J. Med. Virol. 2004, 72, 473–477. [Google Scholar] [CrossRef]
- Tjon Pian Gi, R.E.A.; San Giorgi, M.R.M.; Slagter–Menkema, L.; van Hemel, B.M.; van der Laan, B.F.A.M.; van den Heuvel, E.R.; Dikkers, F.G.; Schuuring, E.M.D. Clinical Course of Recurrent Respiratory Papillomatosis: Comparison between Aggressiveness of Human Papillomavirus-6 and Human Papillomavirus-11. Head Neck 2015, 37, 1625–1632. [Google Scholar] [CrossRef]
- Doorbar, J. Model Systems of Human Papillomavirus-Associated Disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, M.C.; Brusadelli, M.G.; Carlile, A.; Ruiz-Torres, S.; Lodin, H.; Lee, D.; Kofron, M.; Lambert, P.F.; Lane, A.; Ameziane, N.; et al. Patient-Derived Organotypic Epithelial Rafts Model Phenotypes in Juvenile-Onset Recurrent Respiratory Papillomatosis. Viruses 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Bedard, M.C.; de Alarcon, A.; Kou, Y.-F.; Lee, D.; Sestito, A.; Duggins, A.L.; Brusadelli, M.; Lane, A.; Wikenheiser-Brokamp, K.A.; Wells, S.I.; et al. HPV Strain Predicts Severity of Juvenile-Onset Recurrent Respiratory Papillomatosis with Implications for Disease Screening. Cancers 2021, 13, 2556. [Google Scholar] [CrossRef] [PubMed]
- Uloza, V.; Kuzminienė, A.; Palubinskienė, J.; Balnytė, I.; Ulozienė, I.; Valančiūtė, A. An Experimental Model of Human Recurrent Respiratory Papillomatosis: A Bridge to Clinical Insights. Laryngoscope 2021, 131, E914–E920. [Google Scholar] [CrossRef] [PubMed]
- Ozbun, M.A.; Bondu, V.; Patterson, N.A.; Sterk, R.T.; Waxman, A.G.; Bennett, E.C.; McKee, R.; Sharma, A.; Yarwood, J.; Rogers, M.; et al. Infectious Titres of Human Papillomaviruses (HPVs) in Patient Lesions, Methodological Considerations in Evaluating HPV Infectivity and Implications for the Efficacy of High-Level Disinfectants. EBioMedicine 2021, 63, 103165. [Google Scholar] [CrossRef]
- Jahan-Parwar, B.; Chhetri, D.K.; Bhuta, S.; Hart, S.; Berke, G.S. Development of a Canine Model for Recurrent Respiratory Papillomatosis. Ann. Otol. Rhinol. Laryngol. 2003, 112, 1011–1013. [Google Scholar] [CrossRef]
- Ingle, A.; Ghim, S.; Joh, J.; Chepkoech, I.; Bennett Jenson, A.; Sundberg, J.P. Novel Laboratory Mouse Papillomavirus (MusPV) Infection. Vet. Pathol. 2011, 48, 500–505. [Google Scholar] [CrossRef]
- Hu, J.; Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Christensen, N.D. The Mouse Papillomavirus Infection Model. Viruses 2017, 9, 246. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. MmuPV1: A New Frontier in Animal Models of Papillomavirus Pathogenesis. J. Virol. 2020, 94, e00002–e00020. [Google Scholar] [CrossRef] [Green Version]
- Uberoi, A.; Lambert, P.F. Rodent Papillomaviruses. Viruses 2017, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, M.E.; Uberoi, A.; McGregor, S.M.; Wei, T.; Ward-Shaw, E.; Lambert, P.F. A Novel in Vivo Infection Model to Study Papillomavirus-Mediated Disease of the Female Reproductive Tract. Mbio 2019, 10, e00180-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaine-Sauer, S.; Shin, M.-K.; Matkowskyj, K.A.; Ward-Shaw, E.; Lambert, P.F. A Novel Model for Papillomavirus-Mediated Anal Disease and Cancer Using the Mouse Papillomavirus. Mbio 2021, 12, e01611-21. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Brendle, S.A.; Christensen, N.D.; Schell, T.D.; Hu, J. Mouse Papillomavirus Infection Persists in Mucosal Tissues of an Immunocompetent Mouse Strain and Progresses to Cancer. Sci. Rep. 2017, 7, 16932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Buehler, D.; Ward-Shaw, E.; Lambert, P.F. An Infection-Based Murine Model for Papillomavirus-Associated Head and Neck Cancer. Mbio 2020, 11, e00908-20. [Google Scholar] [CrossRef] [PubMed]
- Bilger, A.; King, R.E.; Schroeder, J.P.; Piette, J.T.; Hinshaw, L.A.; Kurth, A.D.; AlRamahi, R.W.; Barthel, M.V.; Ward-Shaw, E.T.; Buehler, D.; et al. A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia. Viruses 2020, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Yoshida, S.; Lambert, P.F. Development of an in Vivo Infection Model to Study Mouse Papillomavirus-1 (MmuPV1). J. Virol. Methods 2018, 253, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Cooper, T.K.; Balogh, K.K.; Hu, J.; Christensen, N.D. Secondary Infections, Expanded Tissue Tropism, and Evidence for Malignant Potential in Immunocompromised Mice Infected with Mus Musculus Papillomavirus 1 DNA and Virus. J. Virol. 2013, 87, 9391–9395. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Budgeon, L.R.; Cladel, N.M.; Balogh, K.; Myers, R.; Cooper, T.K.; Christensen, N.D. Tracking Vaginal, Anal and Oral Infection in a Mouse Papillomavirus Infection Model. J. Gen. Virol. 2015, 96, 3554–3565. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. Sexual Transmission of Murine Papillomavirus (MmuPV1) in Mus Musculus. Elife 2019, 8, e50056. [Google Scholar] [CrossRef]
- Sangiamo, D.T.; Warren, M.R.; Neunuebel, J.P. Ultrasonic Signals Associated with Different Types of Social Behavior of Mice. Nat. Neurosci. 2020, 23, 411–422. [Google Scholar] [CrossRef]
- Roberts, L.H. The Rodent Ultrasound Production Mechanism. Ultrason 1975, 13, 83–88. [Google Scholar] [CrossRef]
- Yamashita, M.; Bless, D.M.; Welham, N.V. Morphological and Extracellular Matrix Changes Following Vocal Fold Injury in Mice. CTO 2010, 192, 262–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, M.; Bless, D.M.; Welham, N.V. Surgical Method to Create Vocal Fold Injuries in Mice. Ann. Otol. Rhinol. Laryngol. 2009, 118, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/Γcnull Mouse: An Excellent Recipient Mouse Model for Engraftment of Human Cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- National Research Council (US), Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Omland, T.; Akre, H.; Vårdal, M.; Brøndbo, K. Epidemiological Aspects of Recurrent Respiratory Papillomatosis: A Population-Based Study. Laryngoscope 2012, 122, 1595–1599. [Google Scholar] [CrossRef]
- Ullman-Culleré, M.H.; Foltz, C.J. Body Condition Scoring: A Rapid and Accurate Method for Assessing Health Status in Mice. Lab. Anim. Sci. 1999, 49, 319–323. [Google Scholar] [PubMed]
- Foltz, C.J.; Ullman-Culleré, M.H. Guidelines for Assessing the Health and Condition of Mice. Lab Anim. 1999, 28, 28–32. [Google Scholar]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G.; Dintzis, S.M.; Liggitt, D. 6—Nose, Sinus, Pharynx, and Larynx. In Comparative Anatomy and Histology, 2nd ed.; Treuting, P.M., Dintzis, S.M., Montine, K.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 89–114. ISBN 978-0-12-802900-8. [Google Scholar]
- Xue, X.-Y.; Majerciak, V.; Uberoi, A.; Kim, B.-H.; Gotte, D.; Chen, X.; Cam, M.; Lambert, P.F.; Zheng, Z.-M. The Full Transcription Map of Mouse Papillomavirus Type 1 (MmuPV1) in Mouse Wart Tissues. PLoS Pathog. 2017, 13, e1006715. [Google Scholar] [CrossRef]
- Torres, A.D.; Spurgeon, M.E.; Bilger, A.; Blaine-Sauer, S.; Uberoi, A.; Buehler, D.; McGregor, S.M.; Ward-Shaw, E.; Lambert, P.F. The Human Papillomavirus 16 E5 Gene Potentiates MmuPV1-Dependent Pathogenesis. Virology 2020, 541, 1–12. [Google Scholar] [CrossRef]
- Kashima, H.; Leventhal, B.; Mounts, P.; Hruban, R.H. Sites of Predilection in Recurrent Respiratory Papillomatosis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 580–583. [Google Scholar] [CrossRef]
- Handisurya, A.; Day, P.M.; Thompson, C.D.; Buck, C.B.; Pang, Y.-Y.S.; Lowy, D.R.; Schiller, J.T. Characterization of Mus Musculus Papillomavirus 1 Infection in Situ Reveals an Unusual Pattern of Late Gene Expression and Capsid Protein Localization. J. Virol. 2013, 87, 13214–13225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. Mouse Papillomavirus MmuPV1 Infects Oral Mucosa and Preferentially Targets the Base of the Tongue. Virology 2016, 488, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, T.E.; Brooks, R.T.; Thombs, L.A.; Littrell, L.L.; Harris, R.A.; Allen, M.J.; Kadosh, M.D.; Robbins, K.L. Videofluoroscopic Validation of a Translational Murine Model of Presbyphagia. Dysphagia 2015, 30, 328–342. [Google Scholar] [CrossRef]
- Lever, T.E.; Braun, S.M.; Brooks, R.T.; Harris, R.A.; Littrell, L.L.; Neff, R.M.; Hinkel, C.J.; Allen, M.J.; Ulsas, M.A. Adapting Human Videofluoroscopic Swallow Study Methods to Detect and Characterize Dysphagia in Murine Disease Models. J. Vis. Exp. 2015, 52319. [Google Scholar] [CrossRef] [Green Version]
- Osman, K.L.; Kohlberg, S.; Mok, A.; Brooks, R.; Lind, L.A.; McCormack, K.; Ferreira, A.; Kadosh, M.; Fagan, M.K.; Bearce, E.; et al. Optimizing the Translational Value of Mouse Models of ALS for Dysphagia Therapeutic Discovery. Dysphagia 2020, 35, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Welby, L.; Caudill, H.; Yitsege, G.; Hamad, A.; Bunyak, F.; Zohn, I.E.; Maynard, T.; LaMantia, A.-S.; Mendelowitz, D.; Lever, T.E. Persistent Feeding and Swallowing Deficits in a Mouse Model of 22q11.2 Deletion Syndrome. Front. Neurol. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 3483. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Seedat, R. Juvenile-Onset Recurrent Respiratory Papillomatosis Diagnosis and Management—A Developing Country Review. Pediatric Health Med. 2020, 11, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Gélinas, J.-F.; Manoukian, J.; Côté, A. Lung Involvement in Juvenile Onset Recurrent Respiratory Papillomatosis: A Systematic Review of the Literature. Int. J. Pediatric Otorhinolaryngol. 2008, 72, 433–452. [Google Scholar] [CrossRef]
- Karatayli-Ozgursoy, S.; Bishop, J.A.; Hillel, A.; Akst, L.; Best, S.R.A. Risk Factors for Dysplasia in Recurrent Respiratory Papillomatosis in an Adult and Pediatric Population. Ann. Otol. Rhinol. Laryngol. 2016, 125, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Achkar, V.N.R.E.; Duarte, A.; Carlos, R.; León, J.E.; Ribeiro-Silva, A.; Pignatari, S.S.N.; Kaminagakura, E. Histopathological Features of Juvenile-Onset Laryngeal Papillomatosis Related to Severity. Head Neck 2019, 41, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Best, S.R.; Rooper, L.M. HPV RNA In-Situ Hybridization as a Diagnostic Aid in Papillary Laryngeal Lesions. Laryngoscope 2020, 130, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Donne, A.J.; Hampson, L.; Homer, J.J.; Hampson, I.N. The Role of HPV Type in Recurrent Respiratory Papillomatosis. Int. J. Pediatric Otorhinolaryngol. 2010, 74, 7–14. [Google Scholar] [CrossRef]
- Gama, R.R.; Carvalho, A.L.; Filho, A.L.; Scorsato, A.P.; López, R.V.M.; Rautava, J.; Syrjänen, S.; Syrjänen, K. Detection of Human Papillomavirus in Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Laryngoscope 2016, 126, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, J.P.; Stearns, T.M.; Joh, J.; Proctor, M.; Ingle, A.; Silva, K.A.; Dadras, S.S.; Jenson, A.B.; Ghim, S. Immune Status, Strain Background, and Anatomic Site of Inoculation Affect Mouse Papillomavirus (MmuPV1) Induction of Exophytic Papillomas or Endophytic Trichoblastomas. PLoS ONE 2014, 9, e113582. [Google Scholar] [CrossRef]
- Christensen, N.D.; Chen, K.-M.; Hu, J.; Stairs, D.B.; Sun, Y.-W.; Aliaga, C.; Balogh, K.K.; Atkins, H.; Shearer, D.; Li, J.; et al. The Environmental Pollutant and Tobacco Smoke Constituent Dibenzo[Def,p]Chrysene Is a Co-Factor for Malignant Progression of Mouse Oral Papillomavirus Infections. Chem.-Biol. Interact. 2021, 333, 109321. [Google Scholar] [CrossRef]
- Cladel, N.M.; Budgeon, L.R.; Cooper, T.K.; Balogh, K.K.; Christensen, N.D.; Myers, R.; Majerciak, V.; Gotte, D.; Zheng, Z.-M.; Hu, J. Mouse Papillomavirus Infections Spread to Cutaneous Sites with Progression to Malignancy. J. Gen. Virol. 2017, 98, 2520. [Google Scholar] [CrossRef]
- Sievers, C.; Robbins, Y.; Bai, K.; Yang, X.; Clavijo, P.E.; Friedman, J.; Sinkoe, A.; Norberg, S.M.; Hinrichs, C.; Van Waes, C.; et al. Comprehensive Multiomic Characterization of Human Papillomavirus-Driven Recurrent Respiratory Papillomatosis Reveals Distinct Molecular Subtypes. Commun. Biol. 2021, 4, 1416. [Google Scholar] [CrossRef]
- Dmochowski, L.; Grey, C.E.; Sykes, J.A.; Langford, P.; Jesse, R.H.; Maccomb; Ballantyne, A.J.; Dreyer, D.A. A Study of Submicroscopic Structure and of Virus Particles in Cells of Human Laryngeal Papillomas. Tex. Rep. Biol. Med. 1964, 22, 454–491. [Google Scholar]
- Boyle, W.F.; McCoy, E.G.; Fogarty, W.A. Electron Microscopic Identification of Virus-like Particles in Laryngeal Papilloma. Ann. Otol. Rhinol. Laryngol. 1971, 80, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.F.; Riggs, J.L.; Oshiro, L.S.; Lennette, E.H. Electron Microscopic Identification of Papova Virus in Laryngeal Papilloma. Laryngoscope 1973, 83, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, P.-G.; Frithiof, L.; Wersäll, J. Ultrastructural Features of Human Juvenile Laryngeal Papillomas. Acta Oto-Laryngol. 1975, 80, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, D.J.; Kirchner, F.R.; Proud, G.O. Electron Microscopic Study of Human Laryngeal Papillomatosis. Cancer Res. 1963, 23, 1084–1089. [Google Scholar]
- Lack, E.E.; Vawter, G.F.; Smith, H.G.; Healy, G.B.; Lancaster, W.D.; Jenson, A.B. Immunohistochemical Localisation of Human Papillomavirus in Squamous Papillomas of the Larynx. Lancet 1980, 2, 592. [Google Scholar] [CrossRef]
- Lack, E.E.; Jenson, B.; Smith, H.G.; Healy, G.B.; Pass, F.; Vawter, G.F. Immunoperoxidase Localization of Human Papillomavirus in Laryngeal Papillomas. INT 1980, 14, 148–154. [Google Scholar] [CrossRef]
- Costa, J.; Howley, P.M.; Bowling, M.C.; Howard, R.; Bauer, W.C. Presence of Human Papilloma Viral Antigens in Juvenile Multiple Laryngeal Papilloma. Am. J. Clin. Pathol. 1981, 75, 194–197. [Google Scholar] [CrossRef]
- Lancaster, W.D.; Jenson, B. Evidence for Papillomavirus Genus-Specific Antigens and DNA in Laryngeal Papilloma. INT 1981, 15, 204–212. [Google Scholar] [CrossRef]
- Braun, L.; Kashima, H.; Eggleston, J.; Shah, K. Demonstration of Papillomavirus Antigen in Paraffin Sections of Laryngeal Papillomas. Laryngoscope 1982, 92, 640–643. [Google Scholar] [CrossRef]
- Mounts, P.; Shah, K.V.; Kashima, H. Viral Etiology of Juvenile- and Adult-Onset Squamous Papilloma of the Larynx. Proc. Natl. Acad. Sci. USA 1982, 79, 5425–5429. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.M.; Topp, W.C.; Schneider, P.S.; Abramson, A.L. Laryngeal Papillomavirus Infection during Clinical Remission. N. Engl. J. Med. 1983, 308, 1261–1264. [Google Scholar] [CrossRef] [PubMed]
- Abramson, A.L.; Steinberg, B.M.; Winkler, B. Laryngeal Papillomatosis: Clinical, Histopathologic and Molecular Studies. Laryngoscope 1987, 97, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Shikowitz, M.J.; Steinberg, B.M.; Winkler, B.; Abramson, A.L. Squamous Metaplasia in the Trachea: The Tracheotomized Rabbit as an Experimental Model and Implications in Recurrent Papillomatosis. Otolaryngol. Head Neck Surg. 1986, 95, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The Natural History of Human Papillomavirus Infections of the Mucosal Epithelia. APMIS 2010, 118, 422–449. [Google Scholar] [CrossRef] [PubMed]
- Lungova, V.; Verheyden, J.M.; Herriges, J.; Sun, X.; Thibeault, S.L. Ontogeny of the Mouse Vocal Fold Epithelium. Dev. Biol. 2015, 399, 263–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowdall, J.R.; Sadow, P.M.; Hartnick, C.; Vinarsky, V.; Mou, H.; Zhao, R.; Song, P.C.; Franco, R.A.; Rajagopal, J. Identification of Distinct Layers within the Stratified Squamous Epithelium of the Adult Human True Vocal Fold. Laryngoscope 2015, 125, E313–E319. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Vinarsky, V.; Tata, P.R.; Brazauskas, K.; Choi, S.H.; Crooke, A.K.; Zhang, B.; Solomon, G.M.; Turner, B.; Bihler, H.; et al. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 2016, 19, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Nagle, R.B.; Moll, R.; Weidauer, H.; Nemetschek, H.; Franke, W.W. Different Patterns of Cytokeratin Expression in the Normal Epithelia of the Upper Respiratory Tract. Differentiation 1985, 30, 130–140. [Google Scholar] [CrossRef]
- Cohen-Kerem, R.; Lahat, N.; Resnick, M.B.; Elmalah, I.; Doweck, I.; Greenberg, E.; Rahat, M.A. Detection of Cytokeratins in Normal and Malignant Laryngeal Epithelia by Means of Reverse Transcriptase-Polymerase Chain Reaction. Ann. Otol. Rhinol. Laryngol. 2002, 111, 149–154. [Google Scholar] [CrossRef]
- Day, P.M.; Thompson, C.D.; Lowy, D.R.; Schiller, J.T. The HPV16 and MusPV1 Papillomaviruses Initially Interact with Distinct Host Components on the Basement Membrane. Virol. 2015, 481, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Meyers, J.M.; Uberoi, A.; Grace, M.; Lambert, P.F.; Munger, K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-β Tumor Suppressors to Inhibit Differentiation and Sustain Keratinocyte Proliferation. PLoS Pathog. 2017, 13, e1006171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Grace, M.; Uberoi, A.; Romero-Masters, J.C.; Lee, D.; Lambert, P.F.; Munger, K. The Mus Musculus Papillomavirus Type 1 E7 Protein Binds to the Retinoblastoma Tumor Suppressor: Implications for Viral Pathogenesis. Mbio 2021, 12, e02277-21. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Doorbar, J. The Low-Risk Papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Majerciak, V.; Xue, X.-Y.; Uberoi, A.; Lobanov, A.; Chen, X.; Cam, M.; Hughes, S.H.; Lambert, P.F.; Zheng, Z.-M. Mouse Papillomavirus Type 1 (MmuPV1) DNA Is Frequently Integrated in Benign Tumors by Microhomology-Mediated End-Joining. PLoS Pathog. 2021, 17, e1009812. [Google Scholar] [CrossRef]
- Pelleitier, M.; Montplaisir, S. The Nude Mouse: A Model of Deficient T-Cell Function. Methods Achiev. Exp. Pathol. 1975, 7, 149–166. [Google Scholar] [PubMed]
- Bonagura, V.R.; Hatam, L.J.; Rosenthal, D.W.; De Voti, J.A.; Lam, F.; Steinberg, B.M.; Abramson, A.L. Recurrent Respiratory Papillomatosis: A Complex Defect in Immune Responsiveness to Human Papillomavirus-6 and -11. APMIS 2010, 118, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Cannella, F.; Pierangelli, A.; Pilgrim, R.M.; Antonelli, G.; Rowley, D.; Wong, M.; Best, S.; Xing, D.; Roden, R.B.S.; et al. Insights into the Role of Innate Immunity in Cervicovaginal Papillomavirus Infection from Studies Using Gene Deficient Mice. J. Virol. 2020, 94, e00087-21. [Google Scholar] [CrossRef]
- Maglennon, G.A.; McIntosh, P.; Doorbar, J. Persistence of Viral DNA in the Epithelial Basal Layer Suggests a Model for Papillomavirus Latency Following Immune Regression. Virology 2011, 414, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Maglennon, G.A.; McIntosh, P.B.; Doorbar, J. Immunosuppression Facilitates the Reactivation of Latent Papillomavirus Infections. J. Virol. 2014, 88, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Tschirley, A.M.; Stockwell, P.A.; Rodger, E.J.; Eltherington, O.; Morison, I.M.; Christensen, N.; Chatterjee, A.; Hibma, M. The Mouse Papillomavirus Epigenetic Signature Is Characterised by DNA Hypermethylation after Lesion Regression. Viruses 2021, 13, 2045. [Google Scholar] [CrossRef]










| Disease Feature | NSG Mouse Vocal Fold + MmuPV1 | RRP |
|---|---|---|
| Benign disease | Yes | Yes [1,2,3] |
| Moderate or higher dysplasia | Ubiquitous by 3 months | Rare [83,84,85] |
| Progression to cancer | Frequent | Extremely rare [86] |
| Spread to trachea and bronchi | Yes | Yes [81] |
| Spread to lung | No | Extremely rare [82] |
| Viral gene expression | Yes | Yes [91] |
| Capsid production | Squamous metaplasia only | Sparse, absent in some patients [91,92,93,94,95,96,97,98,99,100,101,102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, R.E.; Bilger, A.; Rademacher, J.; Ward-Shaw, E.T.; Hu, R.; Lambert, P.F.; Thibeault, S.L. A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus. Viruses 2022, 14, 1000. https://doi.org/10.3390/v14051000
King RE, Bilger A, Rademacher J, Ward-Shaw ET, Hu R, Lambert PF, Thibeault SL. A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus. Viruses. 2022; 14(5):1000. https://doi.org/10.3390/v14051000
Chicago/Turabian StyleKing, Renee E., Andrea Bilger, Josef Rademacher, Ella T. Ward-Shaw, Rong Hu, Paul F. Lambert, and Susan L. Thibeault. 2022. "A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus" Viruses 14, no. 5: 1000. https://doi.org/10.3390/v14051000
APA StyleKing, R. E., Bilger, A., Rademacher, J., Ward-Shaw, E. T., Hu, R., Lambert, P. F., & Thibeault, S. L. (2022). A Novel In Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus musculus Papillomavirus. Viruses, 14(5), 1000. https://doi.org/10.3390/v14051000






