Expanded Basal Compartment and Disrupted Barrier in Vocal Fold Epithelium Infected with Mouse Papillomavirus MmuPV1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, MmuPV1 Infection, Samples, and Pathology Grading
2.2. Study Design
2.3. Immunofluorescence and Immunohistochemistry Staining
2.4. TUNEL Staining
2.5. MmuPV1 RNA in Situ Hybridization
2.6. Image Acquisition
2.7. Proliferation, Apoptosis, and Basal Cell Quantification
2.8. Statistical Analysis
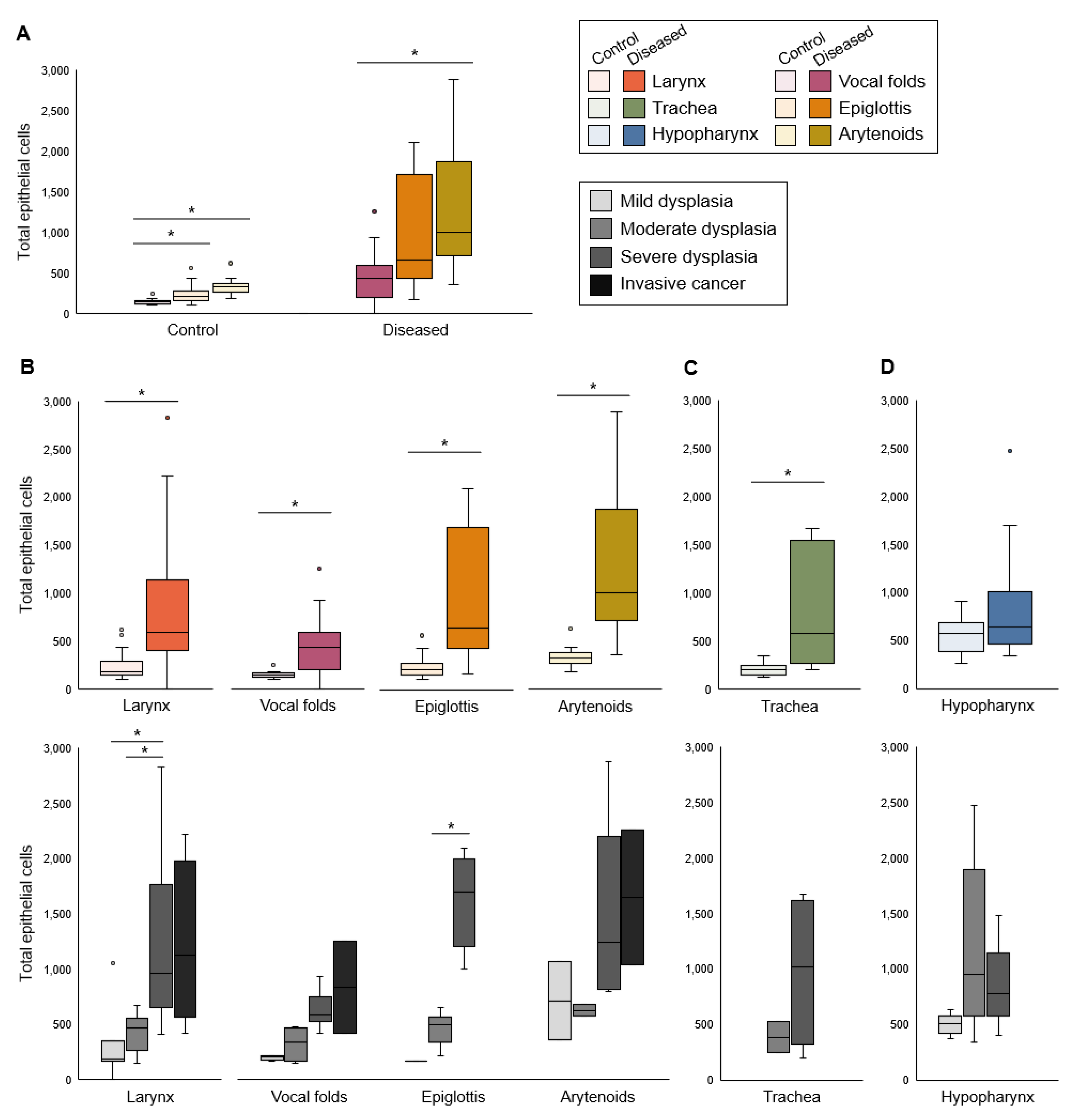
3. Results
3.1. Proliferation
3.2. Apoptosis
3.3. Epithelial Differentiation
3.4. Epithelial Barrier
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedict, J.J.; Derkay, C.S. Recurrent Respiratory Papillomatosis: A 2020 Perspective. Laryngoscope Investig. Otolaryngol. 2021, 6, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Model Systems of Human Papillomavirus-Associated Disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.E.; Bilger, A.; Rademacher, J.; Ward-Shaw, E.T.; Hu, R.; Lambert, P.F.; Thibeault, S.L. A Novel in Vivo Model of Laryngeal Papillomavirus-Associated Disease Using Mus Musculus Papillomavirus. Viruses 2022, 14, 1000. [Google Scholar] [CrossRef]
- Donne, A.J.; Hampson, L.; Homer, J.J.; Hampson, I.N. The Role of HPV Type in Recurrent Respiratory Papillomatosis. Int. J. Pediatric Otorhinolaryngol. 2010, 74, 7–14. [Google Scholar] [CrossRef]
- Garcia, J.A.; Best, S.R.; Rooper, L.M. HPV RNA In-Situ Hybridization as a Diagnostic Aid in Papillary Laryngeal Lesions. Laryngoscope 2020, 130, 955–960. [Google Scholar] [CrossRef]
- Lépine, C.; Voron, T.; Berrebi, D.; Mandavit, M.; Nervo, M.; Outh-Gauer, S.; Péré, H.; Tournier, L.; Teissier, N.; Tartour, E.; et al. Juvenile-Onset Recurrent Respiratory Papillomatosis Aggressiveness: In Situ Study of the Level of Transcription of HPV E6 and E7. Cancers 2020, 12, 2836. [Google Scholar] [CrossRef]
- Sievers, C.; Robbins, Y.; Bai, K.; Yang, X.; Clavijo, P.E.; Friedman, J.; Sinkoe, A.; Norberg, S.M.; Hinrichs, C.; Van Waes, C.; et al. Comprehensive Multiomic Characterization of Human Papillomavirus-Driven Recurrent Respiratory Papillomatosis Reveals Distinct Molecular Subtypes. Commun. Biol. 2021, 4, 1416. [Google Scholar] [CrossRef]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The Natural History of Human Papillomavirus Infections of the Mucosal Epithelia. APMIS 2010, 118, 422–449. [Google Scholar] [CrossRef]
- Tse, J.R.; Zhang, Z.; Long, J.L. Effects of Vocal Fold Epithelium Removal on Vibration in an Excised Human Larynx Model. J. Acoust. Soc. Am. 2015, 138, EL60–EL64. [Google Scholar] [CrossRef] [Green Version]
- Fitch, J.L.; Holbrook, A. Modal Vocal Fundamental Frequency of Young Adults. Arch. Otolaryngol. 1970, 92, 379–382. [Google Scholar] [CrossRef]
- Glaze, L.E.; Bless, D.M.; Milenkovic, P.; Susser, R.D. Acoustic Characteristics of Children’s Voice. J. Voice 1988, 2, 312–319. [Google Scholar] [CrossRef]
- Gunter, H.E. Modeling Mechanical Stresses as a Factor in the Etiology of Benign Vocal Fold Lesions. J. Biomech. 2004, 37, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Titze, I.R. Viscoelastic Shear Properties of Human Vocal Fold Mucosa: Measurement Methodology and Empirical Results. J. Acoust. Soc. Am. 1999, 106, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.D.; Alipour, F.; Titze, I.R.; Hammond, T.H. Biomechanical and Histologic Observations of Vocal Fold Fibrous Proteins. Ann. Otol. Rhinol. Laryngol. 2000, 109, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.D.; Titze, I.R.; Chan, R.; Hammond, T.H. Vocal Fold Proteoglycans and Their Influence on Biomechanics. Laryngoscope 1999, 109, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Hochman, I.; Hillman, R.E.; Sataloff, R.T.; Zeitels, S.M. Ectasias and Varices of the Vocal Fold: Clearing the Striking Zone. Ann. Otol. Rhinol. Laryngol. 1999, 108, 10–16. [Google Scholar] [CrossRef]
- Kashima, H.; Leventhal, B.; Mounts, P.; Hruban, R.H. Sites of Predilection in Recurrent Respiratory Papillomatosis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 580–583. [Google Scholar] [CrossRef]
- Benedict, P.A.; Ruiz, R.; Yoo, M.; Verma, A.; Ahmed, O.H.; Wang, B.; Dion, G.R.; Voigt, A.; Merati, A.; Rosen, C.A.; et al. Laryngeal Distribution of Recurrent Respiratory Papillomatosis in a Previously Untreated Cohort. Laryngoscope 2018, 128, 138–143. [Google Scholar] [CrossRef]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Araujo Neto, C.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent Respiratory Papillomatosis: A State-of-the-Art Review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Levendoski, E.E.; Leydon, C.; Thibeault, S.L. Vocal Fold Epithelial Barrier in Health and Injury: A Research Review. J. Speech Lang. Hear. Res. 2014, 57, 1679. [Google Scholar] [CrossRef] [Green Version]
- Dowdall, J.R.; Sadow, P.M.; Hartnick, C.; Vinarsky, V.; Mou, H.; Zhao, R.; Song, P.C.; Franco, R.A.; Rajagopal, J. Identification of Distinct Layers within the Stratified Squamous Epithelium of the Adult Human True Vocal Fold. Laryngoscope 2015, 125, E313–E319. [Google Scholar] [CrossRef] [PubMed]
- Lungova, V.; Chen, X.; Wang, Z.; Kendziorski, C.; Thibeault, S.L. Human Induced Pluripotent Stem Cell-Derived Vocal Fold Mucosa Mimics Development and Responses to Smoke Exposure. Nat. Commun. 2019, 10, 4161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, M.C.; Brusadelli, M.G.; Carlile, A.; Ruiz-Torres, S.; Lodin, H.; Lee, D.; Kofron, M.; Lambert, P.F.; Lane, A.; Ameziane, N.; et al. Patient-Derived Organotypic Epithelial Rafts Model Phenotypes in Juvenile-Onset Recurrent Respiratory Papillomatosis. Viruses 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.M.; Meade, R.; Kalinowski, S.; Abramson, A.L. Abnormal Differentiation of Human Papillomavirus-Induced Laryngeal Papillomas. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 1167–1171. [Google Scholar] [CrossRef]
- Wu, R.; Sun, S.; Steinberg, B.M. Requirement of STAT3 Activation for Differentiation of Mucosal Stratified Squamous Epithelium. Mol. Med. 2003, 9, 77–84. [Google Scholar] [CrossRef]
- Reppucci, A.D.; Dilorenzo, T.P.; Abramson, A.L.; Steinberg, B.M. In Vitro Modulation of Human Laryngeal Papilloma Cell Differentiation by Retinoic Acid. Otolaryngol. Head Neck Surg. 1991, 105, 528–532. [Google Scholar] [CrossRef]
- Vambutas, A.; Di Lorenzo, T.P.; Steinberg, B.M. Laryngeal Papilloma Cells Have High Levels of Epidermal Growth Factor Receptor and Respond to Epidermal Growth Factor by a Decrease in Epithelial Differentiation. Cancer Res. 1993, 53, 910–914. [Google Scholar]
- Yuan, C.-H.; Filippova, M.; Duerksen-Hughes, P. Modulation of Apoptotic Pathways by Human Papillomaviruses (HPV): Mechanisms and Implications for Therapy. Viruses 2012, 4, 3831–3850. [Google Scholar] [CrossRef] [Green Version]
- Novaleski, C.K.; Carter, B.D.; Sivasankar, M.P.; Ridner, S.H.; Dietrich, M.S.; Rousseau, B. Apoptosis and Vocal Fold Disease: Clinically Relevant Implications of Epithelial Cell Death. J. Speech Lang. Hear. Res. 2017, 60, 1264–1272. [Google Scholar] [CrossRef]
- Poetker, D.M.; Sandler, A.D.; Scott, D.L.; Smith, R.J.H.; Bauman, N.M. Survivin Expression in Juvenile-Onset Recurrent Respiratory Papillomatosis. Ann. Otol. Rhinol. Laryngol. 2002, 111, 957–961. [Google Scholar] [CrossRef]
- Niedzielska, G.; Kocki, J. Evaluation of Bcl-2 Gene Expression in Papilloma of Larynx in Children. Int. J. Pediatric Otorhinolaryngol. 2000, 53, 25–29. [Google Scholar] [CrossRef]
- Manjarrez, M.E.; Ocadiz, R.; Valle, L.; Pacheco, C.; Marroquin, A.; la Torre, C.D.; Selman, M.; Gariglio, P. Detection of Human Papillomavirus and Relevant Tumor Suppressors and Oncoproteins in Laryngeal Tumors. Clin. Cancer Res. 2006, 12, 6946–6951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodman, R.; Mutasa, S.; Dupuis, C.; Spratt, H.; Underbrink, M. Genetic Dysregulation in Recurrent Respiratory Papillomatosis. Laryngoscope 2014, 124, E320–E325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, G.A.; Buda, A.; Moorghen, M.; Dettmar, P.W.; Pignatelli, M. Characterisation of Adherens and Tight Junctional Molecules in Normal Animal Larynx; Determining a Suitable Model for Studying Molecular Abnormalities in Human Laryngopharyngeal Reflux. J. Clin. Pathol. 2005, 58, 1265–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Xiao, Y.; Zhou, S.; Wang, Y.; Wang, J. Transcriptomic Landscape of Gene Expression Profiles and Pathways in JORRP Tumor Tissues and HPV6/11 E6-E7-Overexpressing HNSCC Cell Lines. J. Virol. 2022, 96, e0134221. [Google Scholar] [CrossRef]
- Lundquist, P.-G.; Frithiof, L.; Wersäll, J. Ultrastructural Features of Human Juvenile Laryngeal Papillomas. Acta Oto-Laryngol. 1975, 80, 137–149. [Google Scholar] [CrossRef]
- Horn, T.; Bomholt, A. Ultrastructural Features of the Adult Laryngeal Papilloma. Acta Otolaryngol. 1985, 99, 649–654. [Google Scholar] [CrossRef]
- Incze, J.S.; Lui, P.S.; Strong, M.S.; Vaughan, C.W.; Clemente, M.P. The Morphology of Human Papillomas of the Upper Respiratory Tract. Cancer 1977, 39, 1634–1646. [Google Scholar] [CrossRef]
- Gray, S.D. Cellular Physiology of the Vocal Folds. Otolaryngol. Clin. N. Am. 2000, 33, 679–697. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. Human Papillomavirus and the Stroma: Bidirectional Crosstalk during the Virus Life Cycle and Carcinogenesis. Viruses 2017, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.B.; Stemple, J.C.; Andreatta, R.D.; Andrade, F.H. Establishing a New Animal Model for the Study of Laryngeal Biology and Disease: An Anatomic Study of the Mouse Larynx. J. Speech Lang. Hear. Res. 2009, 52, 802–811. [Google Scholar] [CrossRef]
- Nakano, T.; Muto, H. The “Intermediate Epithelium” Lining the Mouse Larynx. Okajimas Folia Anat. Jpn. 1988, 64, 385–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Muto, H. Distribution and Probable Functional Role of Taste Buds Located in the Intermediate Epithelium on the Mouse Arytenoid Region. Okajimas Folia Anat. Jpn. 1986, 63, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T. Ultrastructural Studies of the Transitional Zone in the Nasopharyngeal Epithelium, with Special Reference to the Keratinizing Process in the Mouse. Cells Tissues Organs 1986, 127, 22–47. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Muto, H. The Transitional Zone in the Epithelium Lining the Mouse Epiglottis. Cells Tissues Organs 1987, 130, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lungova, V.; Thibeault, S.L. Mechanisms of Larynx and Vocal Fold Development and Pathogenesis. Cell. Mol. Life Sci. 2020, 77, 3781–3795. [Google Scholar] [CrossRef]
- Yamashita, M.; Bless, D.M.; Welham, N.V. Morphological and Extracellular Matrix Changes Following Vocal Fold Injury in Mice. Cells Tissues Organs 2010, 192, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.H. Evidence for the Laryngeal Source of Ultrasonic and Audible Cries of Rodents. J. Zool. 1975, 175, 243–257. [Google Scholar] [CrossRef]
- Roberts, L.H. The Rodent Ultrasound Production Mechanism. Ultrasonics 1975, 13, 83–88. [Google Scholar] [CrossRef]
- Pasch, B.; Tokuda, I.T.; Riede, T. Grasshopper Mice Employ Distinct Vocal Production Mechanisms in Different Social Contexts. Proc. R. Soc. B 2017, 284, 20171158. [Google Scholar] [CrossRef] [Green Version]
- Sangiamo, D.T.; Warren, M.R.; Neunuebel, J.P. Ultrasonic Signals Associated with Different Types of Social Behavior of Mice. Nat. Neurosci. 2020, 23, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Riede, T.; Borgard, H.L.; Pasch, B. Laryngeal Airway Reconstruction Indicates That Rodent Ultrasonic Vocalizations Are Produced by an Edge-Tone Mechanism. R. Soc. Open Sci. 2017, 4, 170976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahrt, E.; Agarwal, A.; Perkel, D.; Portfors, C.; Elemans, C.P.H. Mice Produce Ultrasonic Vocalizations by Intra-Laryngeal Planar Impinging Jets. Curr. Biol. 2016, 26, R880–R881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lungova, V.; Verheyden, J.M.; Herriges, J.; Sun, X.; Thibeault, S.L. Ontogeny of the Mouse Vocal Fold Epithelium. Dev. Biol. 2015, 399, 263–282. [Google Scholar] [CrossRef] [Green Version]
- Mohad, V.; Lungova, V.; Verheyden, J.; Thibeault, S.L. Inactivation of Lats1 and Lats2 Highlights the Role of Hippo Pathway Effector YAP in Larynx and Vocal Fold Epithelium Morphogenesis. Dev. Biol. 2021, 473, 33–49. [Google Scholar] [CrossRef]
- Mou, H.; Vinarsky, V.; Tata, P.R.; Brazauskas, K.; Choi, S.H.; Crooke, A.K.; Zhang, B.; Solomon, G.M.; Turner, B.; Bihler, H.; et al. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 2016, 19, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Erickson-DiRenzo, E.; Easwaran, M.; Martinez, J.D.; Dewan, K.; Sung, C.K. Mainstream Cigarette Smoke Impacts the Mouse Vocal Fold Epithelium and Mucus Barrier. Laryngoscope 2021, 131, 2530–2539. [Google Scholar] [CrossRef]
- Kurita, T.; Chitose, S.; Sato, K.; Sakazaki, T.; Fukahori, M.; Sueyoshi, S.; Umeno, H. Pathological Mechanisms of Laryngeal Papillomatosis Based on Laryngeal Epithelial Characteristics. Laryngoscope Investig. Otolaryngol. 2019, 4, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Gartling, G.J.; Sayce, L.; Kimball, E.E.; Sueyoshi, S.; Rousseau, B. A Comparison of the Localization of Integral Membrane Proteins in Human and Rabbit Vocal Folds. Laryngoscope 2021, 131, E1265–E1271. [Google Scholar] [CrossRef]
- Nagle, R.B.; Moll, R.; Weidauer, H.; Nemetschek, H.; Franke, W.W. Different Patterns of Cytokeratin Expression in the Normal Epithelia of the Upper Respiratory Tract. Differentiation 1985, 30, 130–140. [Google Scholar] [CrossRef]
- Lungova, V.; Verheyden, J.M.; Sun, X.; Thibeault, S.L. β-Catenin Signaling Is Essential for Mammalian Larynx Recanalization and the Establishment of Vocal Fold Progenitor Cells. Development 2018, 145, dev157677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/Γcnull Mouse: An Excellent Recipient Mouse Model for Engraftment of Human Cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Wang, W.; Uberoi, A.; Spurgeon, M.; Gronski, E.; Majerciak, V.; Lobanov, A.; Hayes, M.; Loke, A.; Zheng, Z.-M.; Lambert, P.F. Stress Keratin 17 Enhances Papillomavirus Infection-Induced Disease by Downregulating T Cell Recruitment. PLoS Pathog. 2020, 16, e1008206. [Google Scholar] [CrossRef] [PubMed]
- Nyman, P.; Buehler, D.; Lambert, P.F. Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis. Clin. Cancer Res. 2018, 24, 6308–6318. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, M.E.; Chung, S.-H.; Lambert, P.F. Recurrence of Cervical Cancer in Mice after Selective Estrogen Receptor Modulator Therapy. Am. J. Pathol. 2014, 184, 530–540. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, M.E.; Cheng, J.; Bronson, R.T.; Lambert, P.F.; DeCaprio, J.A. Tumorigenic Activity of Merkel Cell Polyomavirus T Antigens Expressed in the Stratified Epithelium of Mice. Cancer Res. 2015, 75, 1068–1079. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- McGraw, K.O.; Wong, S.P. Forming Inferences about Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Abramson, A.L.; Steinberg, B.M.; Winkler, B. Laryngeal Papillomatosis: Clinical, Histopathologic and Molecular Studies. Laryngoscope 1987, 97, 678–685. [Google Scholar] [CrossRef]
- Syrjänen, S.; Syrjänen, K. HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential. Viruses 2021, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.; Pedersen, H.; Stauss, K.; Lungova, V.; Thibeault, S.L. Characterization of Intrauterine Growth, Proliferation and Biomechanical Properties of the Murine Larynx. PLoS ONE 2021, 16, e0245073. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Buehler, D.; Ward-Shaw, E.; Lambert, P.F. An Infection-Based Murine Model for Papillomavirus-Associated Head and Neck Cancer. MBio 2020, 11, e00908-20. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Uberoi, A.; McGregor, S.M.; Wei, T.; Ward-Shaw, E.; Lambert, P.F. A Novel in Vivo Infection Model to Study Papillomavirus-Mediated Disease of the Female Reproductive Tract. MBio 2019, 10, e00180-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaine-Sauer, S.; Shin, M.-K.; Matkowskyj, K.A.; Ward-Shaw, E.; Lambert, P.F. A Novel Model for Papillomavirus-Mediated Anal Disease and Cancer Using the Mouse Papillomavirus. MBio 2021, 12, e01611-21. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.M.; Uberoi, A.; Grace, M.; Lambert, P.F.; Munger, K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-β Tumor Suppressors to Inhibit Differentiation and Sustain Keratinocyte Proliferation. PLOS Pathog. 2017, 13, e1006171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorfer, S.; Strasser, K.; Schröckenfuchs, G.; Bonelli, M.; Bauer, W.; Kittler, H.; Cataisson, C.; Fischer, M.B.; Lichtenberger, B.M.; Handisurya, A. Mus Musculus Papillomavirus 1 Is a Key Driver of Skin Cancer Development upon Immunosuppression. Am. J. Transplant. 2021, 21, 525–539. [Google Scholar] [CrossRef]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G.; Dintzis, S.M.; Liggitt, D. 6-Nose, Sinus, Pharynx, and Larynx. In Comparative Anatomy and Histology, 2nd ed.; Treuting, P.M., Dintzis, S.M., Montine, K.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 89–114. ISBN 978-0-12-802900-8. [Google Scholar]
- Klymenko, T.; Gu, Q.; Herbert, I.; Stevenson, A.; Iliev, V.; Watkins, G.; Pollock, C.; Bhatia, R.; Cuschieri, K.; Herzyk, P.; et al. RNA-Seq Analysis of Differentiated Keratinocytes Reveals a Massive Response to Late Events during Human Papillomavirus 16 Infection, Including Loss of Epithelial Barrier Function. J. Virol. 2017, 91, e01001-17. [Google Scholar] [CrossRef] [Green Version]
- Kranjec, C.; Banks, L. A Systematic Analysis of Human Papillomavirus (HPV) E6 PDZ Substrates Identifies MAGI-1 as a Major Target of HPV Type 16 (HPV-16) and HPV-18 Whose Loss Accompanies Disruption of Tight Junctions. J. Virol. 2011, 85, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Huibregtse, J.M. Human Scribble (Vartul) Is Targeted for Ubiquitin-Mediated Degradation by the High-Risk Papillomavirus E6 Proteins and the E6AP Ubiquitin-Protein Ligase. Mol. Cell. Biol. 2000, 20, 8244–8253. [Google Scholar] [CrossRef]
- Pim, D.; Bergant, M.; Boon, S.S.; Ganti, K.; Kranjec, C.; Massimi, P.; Subbaiah, V.K.; Thomas, M.; Tomaić, V.; Banks, L. Human Papillomaviruses and the Specificity of PDZ Domain Targeting. FEBS J. 2012, 279, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Lambert, P.F. Rodent Papillomaviruses. Viruses 2017, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Zhou, J.; Wang, F.; Shi, H.; Li, Y.; Li, B. HPV-16 E6/E7 Promotes Cell Migration and Invasion in Cervical Cancer via Regulating Cadherin Switch in Vitro and in Vivo. Arch. Gynecol. Obs. 2015, 292, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.-M.; Doorbar, J.; Nindl, I.; Yoon, H.-S.; Hibma, M.H. Deregulation of E-Cadherin by Human Papillomavirus Is Not Confined to High-Risk, Cancer-Causing Types. Br. J. Dermatol. 2010, 163, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-J.; Chang, Y.-W.; Lin, W.-H.; Liang, C.-L.; Lee, J.-L. An Aberrant Nuclear Localization of E-Cadherin Is a Potent Inhibitor of Wnt/β-Catenin-Elicited Promotion of the Cancer Stem Cell Phenotype. Oncogenesis 2015, 4, e157. [Google Scholar] [CrossRef] [Green Version]
- Elston, M.S.; Gill, A.J.; Conaglen, J.V.; Clarkson, A.; Cook, R.J.; Little, N.S.; Robinson, B.G.; Clifton-Bligh, R.J.; McDonald, K.L. Nuclear Accumulation of E-Cadherin Correlates with Loss of Cytoplasmic Membrane Staining and Invasion in Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Liu, X.; Fan, G.; Zhao, X.; Sun, Y.; Wang, T.; Zhao, R.; Wang, G.; Zhao, C.; Zhu, Y.; et al. From Cell Membrane to the Nucleus: An Emerging Role of E-Cadherin in Gene Transcriptional Regulation. J. Cell. Mol. Med. 2014, 18, 1712–1719. [Google Scholar] [CrossRef]
- Chetty, R.; Serra, S. Nuclear E-Cadherin Immunoexpression: From Biology to Potential Applications in Diagnostic Pathology. Adv. Anat. Pathol. 2008, 15, 234–240. [Google Scholar] [CrossRef]
- Salahshor, S.; Naidoo, R.; Serra, S.; Shih, W.; Tsao, M.-S.; Chetty, R.; Woodgett, J.R. Frequent Accumulation of Nuclear E-Cadherin and Alterations in the Wnt Signaling Pathway in Esophageal Squamous Cell Carcinomas. Mod. Pathol. 2008, 21, 271–281. [Google Scholar] [CrossRef]
- Trillsch, F.; Kuerti, S.; Eulenburg, C.; Burandt, E.; Woelber, L.; Prieske, K.; Eylmann, K.; Oliveira-Ferrer, L.; Milde-Langosch, K.; Mahner, S. E-Cadherin Fragments as Potential Mediators for Peritoneal Metastasis in Advanced Epithelial Ovarian Cancer. Br. J. Cancer 2016, 114, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human Pathogens Utilize Host Extracellular Matrix Proteins Laminin and Collagen for Adhesion and Invasion of the Host. FEMS Microbiol. Rev. 2012, 36, 1122–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A Murine Tumor Producing a Matrix of Basement Membrane. J. Exp. Med. 1977, 145, 204–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futaki, S.; Hayashi, Y.; Yamashita, M.; Yagi, K.; Bono, H.; Hayashizaki, Y.; Okazaki, Y.; Sekiguchi, K. Molecular Basis of Constitutive Production of Basement Membrane Components: Gene Expression Profiles of Engelbreth-Holm-Swarm Tumor and F9 Embryonal Carcinoma Cells. J. Biol. Chem. 2003, 278, 50691–50701. [Google Scholar] [CrossRef] [Green Version]
- Tse, J.R.; Long, J.L. Microstructure Characterization of a Decellularized Vocal Fold Scaffold for Laryngeal Tissue Engineering: Decellularized Vocal Fold Microstructure. Laryngoscope 2014, 124, E326–E331. [Google Scholar] [CrossRef] [Green Version]
- Welham, N.V.; Yamashita, M.; Choi, S.H.; Ling, C. Cross-Sample Validation Provides Enhanced Proteome Coverage in Rat Vocal Fold Mucosa. PLoS ONE 2011, 6, e17754, Correction in PLoS ONE 2011, 6, https://doi.org/10.1371/annotation/369b65bc-2f17-461b-ae3f-90eb0778440d. [Google Scholar] [CrossRef]
- Leydon, C.; Imaizumi, M.; Yang, D.; Thibeault, S.L.; Fried, M.P. Structural and Functional Vocal Fold Epithelial Integrity Following Injury. Laryngoscope 2014, 124, 2764–2769. [Google Scholar] [CrossRef] [Green Version]
- Kikkawa, Y.; Hozumi, K.; Katagiri, F.; Nomizu, M.; Kleinman, H.K.; Koblinski, J.E. Laminin-111-Derived Peptides and Cancer. Cell Adh. Migr. 2013, 7, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Tran, M.; Rousselle, P.; Nokelainen, P.; Tallapragada, S.; Nguyen, N.T.; Fincher, E.F.; Marinkovich, M.P. Targeting a Tumor-Specific Laminin Domain Critical for Human Carcinogenesis. Cancer Res. 2008, 68, 2885–2894. [Google Scholar] [CrossRef] [Green Version]
- Meireles Da Costa, N.; Mendes, F.A.; Pontes, B.; Nasciutti, L.E.; Ribeiro Pinto, L.F.; Palumbo Júnior, A. Potential Therapeutic Significance of Laminin in Head and Neck Squamous Carcinomas. Cancers 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, V.R.; Hatam, L.J.; Rosenthal, D.W.; De Voti, J.A.; Lam, F.; Steinberg, B.M.; Abramson, A.L. Recurrent Respiratory Papillomatosis: A Complex Defect in Immune Responsiveness to Human Papillomavirus-6 and -11. APMIS 2010, 118, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Sobti, A.; Sakellariou, C.; Nilsson, M.; Schwartz, S.; Olofsson, K.; Rydell, R.; Lindstedt, M.; Forslund, O. Immune Delineation of Laryngeal Papilloma Reveals Enhanced Neutrophil Associated Gene Profile. Eur. J. Immunol. 2021, 51, 2535–2539. [Google Scholar] [CrossRef] [PubMed]
- DeVoti, J.A.; Rosenthal, D.W.; Wu, R.; Abramson, A.L.; Steinberg, B.M.; Bonagura, V.R. Immune Dysregulation and Tumor-Associated Gene Changes in Recurrent Respiratory Papillomatosis: A Paired Microarray Analysis. Mol. Med. 2008, 14, 608–617. [Google Scholar] [CrossRef]
- Tang, A.; Amagai, M.; Granger, L.G.; Stanley, J.R.; Uddy, M.C. Adhesion of Epidermal Langerhans Cells to Keratinocytes Mediated by E-Cadherin. Nature 1993, 361, 82. [Google Scholar] [CrossRef]
- Mayumi, N.; Watanabe, E.; Norose, Y.; Watari, E.; Kawana, S.; Geijtenbeek, T.B.H.; Takahashi, H. E-Cadherin Interactions Are Required for Langerhans Cell Differentiation. Eur. J. Immunol. 2013, 43, 270–280. [Google Scholar] [CrossRef] [Green Version]
- DeVoti, J.; Hatam, L.; Lucs, A.; Afzal, A.; Abramson, A.; Steinberg, B.; Bonagura, V. Decreased Langerhans Cell Responses to IL-36γ: Altered Innate Immunity in Patients with Recurrent Respiratory Papillomatosis. Mol. Med. 2014, 20, 372–380. [Google Scholar] [CrossRef]
- Israr, M.; DeVoti, J.A.; Lam, F.; Abramson, A.L.; Steinberg, B.M.; Bonagura, V.R. Altered Monocyte and Langerhans Cell Innate Immunity in Patients with Recurrent Respiratory Papillomatosis (RRP). Front. Immunol. 2020, 11, 336. [Google Scholar] [CrossRef]
- Hu, J.; Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Christensen, N.D. The Mouse Papillomavirus Infection Model. Viruses 2017, 9, 246. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. MmuPV1: A New Frontier in Animal Models of Papillomavirus Pathogenesis. J. Virol. 2020, 94, e00002-20. [Google Scholar] [CrossRef] [Green Version]
- Thibeault, S.L.; Rees, L.; Pazmany, L.; Birchall, M.A. At the Crossroads: Mucosal Immunology of the Larynx. Mucosal. Immunol. 2009, 2, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Primary Antibody | Species | Dilution | Supplier | Catalog # | Application |
|---|---|---|---|---|---|
| Ki67 | Rabbit | 1:50 | Abcam | ab16667 | IHC |
| Cytokeratin 8 (K8) | Rabbit | 1:200 | LS Bio, Seattle, WA USA | LS-B7928 | IF |
| Cytokeratin 13 (K13) | Mouse | 1:100 | Fisher Scientific | MA1-35542 | IF |
| p63 | Mouse | 1:200 | Biocare Medical, Pacheco, CA, USA | CM163A | IF |
| Zonula occludens 1 (ZO-1) | Mouse | 1:200 | Fisher Scientific | 33-9100 | IF |
| E-cadherin (E-cad) | Rabbit | 1:200 | Cell Signaling Technology, Danvers, MA. USA | 3195S | IF |
| Laminin (Lam) | Rabbit | 1:200 | Abcam | ab11575 | IF |
| Secondary antibody | Species | Dilution | Supplier | Catalog # | Application |
| Horseradish peroxidase (HRP) | Goat anti-rabbit | 1:500 | Jackson Immuno Research Labs, West Grove, PA, USA | 111-035-144 | IHC |
| Alexa Fluor 488 | Goat anti-rabbit | 1:500 | Fisher Scientific | A-11008 | IF |
| Cy3 | Goat anti-mouse | 1:500 | Jackson Immuno Research Labs | 115-166-003 | IF |
| Disease Feature | Mouse Vocal Fold + MmuPV1 | Human RRP |
|---|---|---|
| Hyperplasia | Yes | Yes [19,71,72] |
| Increased proliferation | Yes | Yes [23] |
| Decreased differentiation | Yes | Yes [24,25] |
| Decreased apoptosis | No | Unclear [30,31,32,33] |
| Altered barrier | Yes | Yes [35,36,37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, R.E.; Ward-Shaw, E.T.; Hu, R.; Lambert, P.F.; Thibeault, S.L. Expanded Basal Compartment and Disrupted Barrier in Vocal Fold Epithelium Infected with Mouse Papillomavirus MmuPV1. Viruses 2022, 14, 1059. https://doi.org/10.3390/v14051059
King RE, Ward-Shaw ET, Hu R, Lambert PF, Thibeault SL. Expanded Basal Compartment and Disrupted Barrier in Vocal Fold Epithelium Infected with Mouse Papillomavirus MmuPV1. Viruses. 2022; 14(5):1059. https://doi.org/10.3390/v14051059
Chicago/Turabian StyleKing, Renee E., Ella T. Ward-Shaw, Rong Hu, Paul F. Lambert, and Susan L. Thibeault. 2022. "Expanded Basal Compartment and Disrupted Barrier in Vocal Fold Epithelium Infected with Mouse Papillomavirus MmuPV1" Viruses 14, no. 5: 1059. https://doi.org/10.3390/v14051059






