Timely Hepatitis C RNA Testing and Treatment in the Era of Direct-Acting Antiviral Therapy among People with Hepatitis C in New South Wales, Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Sources and Record Linkages
2.3. Study Period
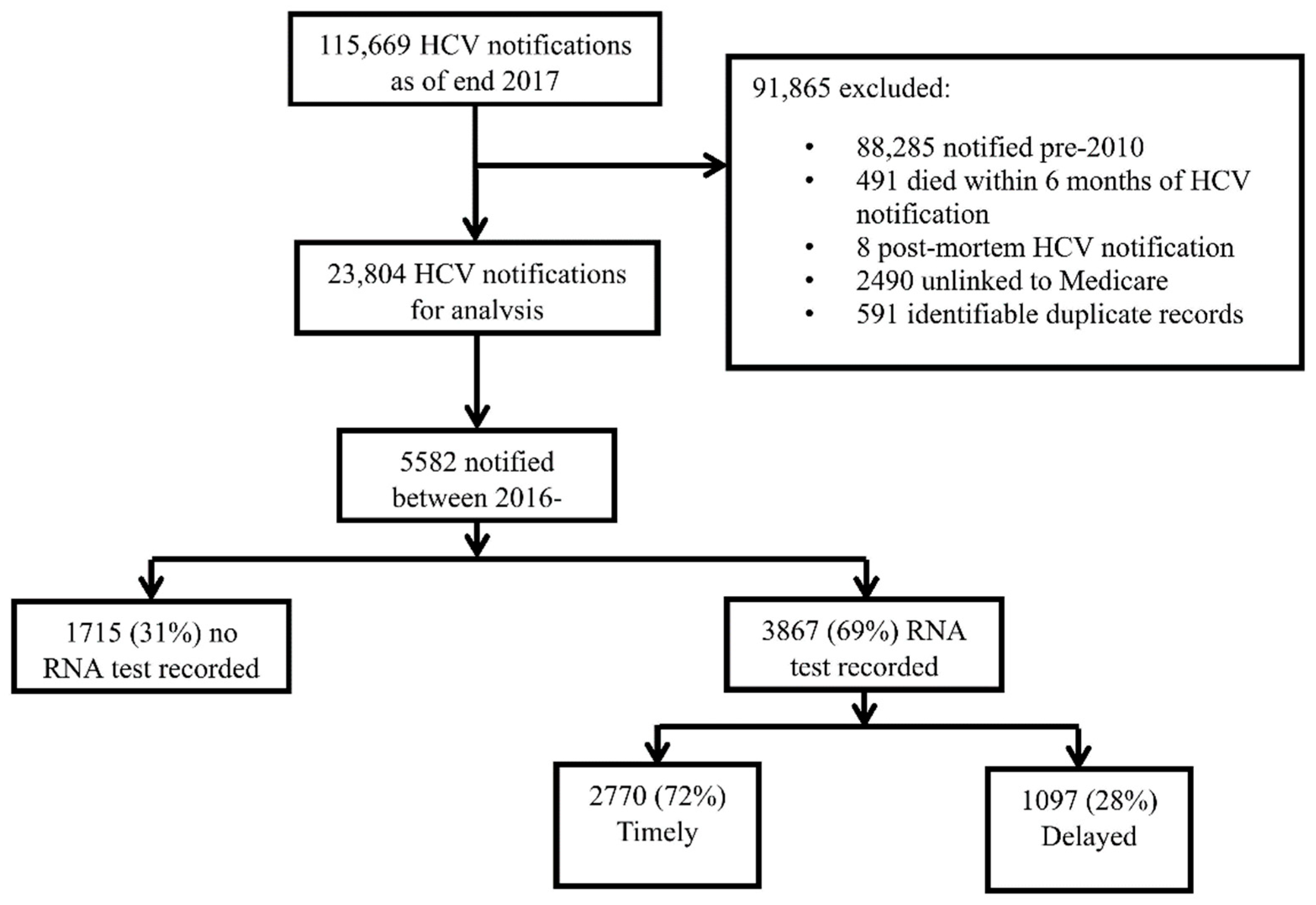
2.4. Study Population
2.5. Exclusion Criteria
2.6. Exposure Variables
2.7. Outcome
2.8. Statistical Analysis
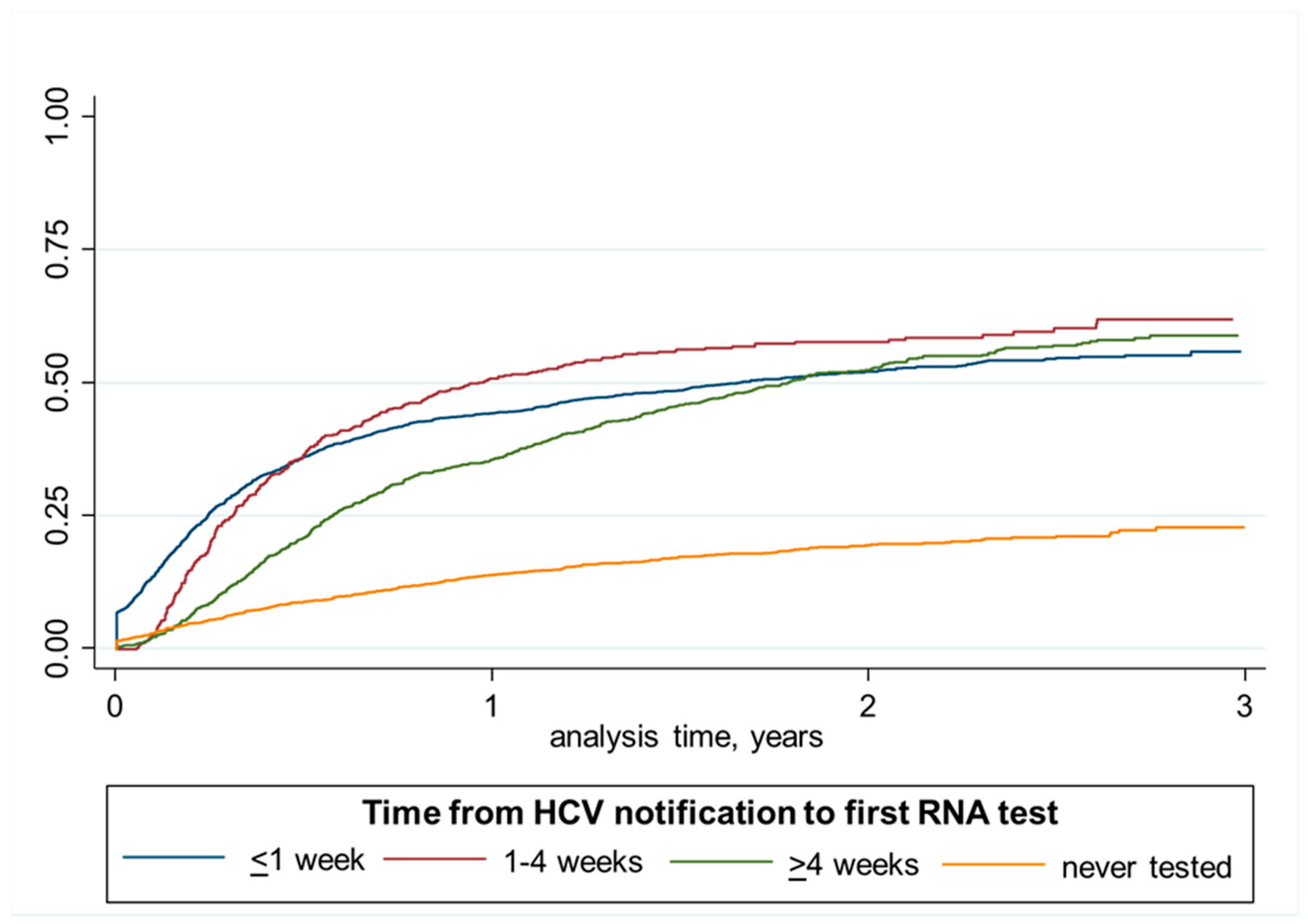
2.8.1. Analysis of Time to Testing and Treatment Initiation Using Kaplan–Meier Failure Curves
2.8.2. Factors Associated with HCV RNA Testing and Treatment Initiation
3. Results
3.1. Descriptive Characteristics
3.1.1. HCV RNA Testing during the DAA Era
3.1.2. DAA Treatment Initiation
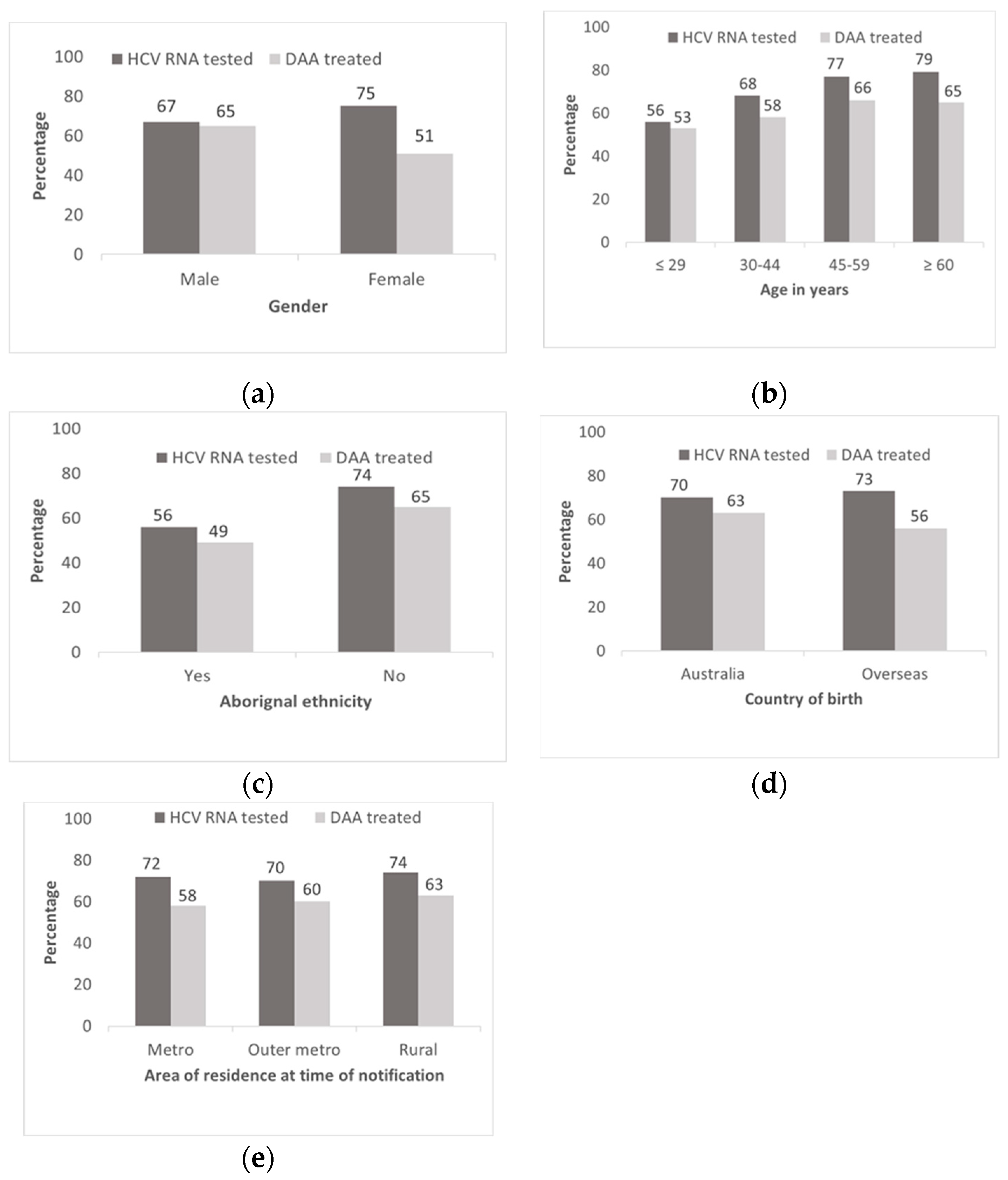
3.1.3. Factors Associated with Timely HCV RNA Testing
3.1.4. Factors Associated with Timely and Ever DAA Treatment Initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wold Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Tramitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lauer, G.M.; Walker, B.D. Hepatitis C virus infection. N. Engl. J. Med. 2001, 345, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021: Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Konerman, M.A.; Lok, A.S.F. Hepatitis C Treatment and Barriers to Eradication. Clin. Transl. Gastroenterol. 2016, 7, e193. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Report on Access to Hepatitis C Treatment. Focus on Overcoming Barriers; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lazarus, J.V.; Roel, E.; Elsharkawy, A.M. Hepatitis C Virus Epidemiology and the Impact of Interferon-Free Hepatitis C Virus Therapy. Cold Spring Harb. Perspect. Med. 2020, 10, a036913. [Google Scholar] [CrossRef]
- Dore, G.J.; Hajarizadeh, B. Elimination of Hepatitis C Virus in Australia: Laying the Foundation. Infect. Dis. Clin. N. Am. 2018, 32, 269–279. [Google Scholar] [CrossRef]
- Hajarizadeh, B.; Grebely, J.; Matthews, G.V.; Martinello, M.; Dore, G.J. Uptake of direct-acting antiviral treatment for chronic hepatitis C in Australia. J. Viral Hepat. 2018, 25, 640–648. [Google Scholar] [CrossRef]
- Stafford, F.; Dore, G.J.; Clackett, S.; Martinello, M.; Matthews, G.V.; Grebely, J.; Balcomb, A.C.; Hajarizadeh, B. Prescribing of direct-acting antiviral therapy by general practitioners for people with hepatitis C in an unrestricted treatment program. Med. J. Aust. 2021, 215, 332–333. [Google Scholar] [CrossRef]
- The Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: Annual Surveillance Report 2018; Kirby Institute: Sydney, Australia; UNSW Sydney: Kensington, Australia, 2018. [Google Scholar]
- Larney, S.; Hickman, M.; Guy, R.; Grebely, J.; Dore, G.J.; Gray, R.T.; Day, C.A.; Kimber, J.; Degenhardt, L. Estimating the number of people who inject drugs in Australia. BMC Public Health 2017, 17, 757. [Google Scholar] [CrossRef]
- Valerio, H.; Alavi, M.; Law, M.; Tillakeratne, S.; Amin, J.; Janjua, N.Z.; Krajden, M.; George, J.; Matthews, G.V.; Hajarizadeh, B.; et al. High hepatitis C treatment uptake among people with recent drug dependence in New South Wales, Australia. J. Hepatol. 2021, 74, 293–302. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Medicare Benefits Schedule (MBS) Data Collection. Available online: https://www.aihw.gov.au/about-our-data/our-data-collections/medicare-benefits-schedule-mbs#:~:text=The%20Medicare%20Benefits%20Schedule%20(MBS,)%2C%20patients%20and%20service%20providers (accessed on 16 February 2022).
- Australian Institute of Health and Welfare. Pharmaceutical Benefits Scheme (PBS) Data Collection. Available online: https://www.aihw.gov.au/about-our-data/our-data-collections/pharmaceutical-benefits-scheme#:~:text=The%20Pharmaceutical%20Benefits%20Scheme%20(PBS,a%20claim%20has%20been%20processed (accessed on 16 February 2022).
- Bartlett, S.R.; Yu, A.; Chapinal, N.; Rossi, C.; Butt, Z.; Wong, S.; Darvishian, M.; Gilbert, M.; Wong, J.; Binka, M.; et al. The population level care cascade for hepatitis C in British Columbia, Canada as of 2018: Impact of direct acting antivirals. Liver Int. 2019, 39, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Valerio, H.; Alavi, M.; Law, M.; McManus, H.; Tillakeratne, S.; Bajis, S.; Martinello, M.; Matthews, G.V.; Amin, J.; Janjua, N.Z.; et al. Opportunities to enhance linkage to hepatitis C care among hospitalised people with recent drug dependence in New South Wales, Australia: A population-based linkage study. Clin. Infect. Dis. 2021, 73, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Janjua, N.Z.; Chong, M.; Grebely, J.; Aspinall, E.J.; Innes, H.; Valerio, H.M.; Hajarizadeh, B.; Hayes, P.C.; Krajden, M.; et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J. Hepatol. 2018, 68, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, M.; Law, M.G.; Valerio, H.; Grebely, J.; Amin, J.; Hajarizadeh, B.; Selvey, C.; George, J.; Dore, G.J. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J. Hepatol. 2019, 71, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Shahid, I.; Alzahrani, A.R.; Al-Ghamdi, S.S.; Alanazi, I.M.; Rehman, S.; Hassan, S. Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises. Diagnostics 2021, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Bui, A.; Prohl, E.; Bhattacharya, D.; Wang, S.; Branch, A.D.; Perumalswami, P.V. Innovations in Hepatitis C Screening and Treatment. Hepatol. Commun. 2021, 5, 371–386. [Google Scholar] [CrossRef]
- Grebely, J.; Applegate, T.L.; Cunningham, P.; Feld, J.J. Hepatitis C point-of-care diagnostics: In search of a single visit diagnosis. Expert Rev. Mol. Diagn. 2017, 17, 1109–1115. [Google Scholar] [CrossRef]
- Alavi, M.; Poustchi, H.; Merat, S.; Kaveh-Ei, S.; Rahimi-Movaghar, A.; Shadloo, B.; Hajarizadeh, B.; Grebely, J.; Dore, G.J.; Malekzadeh, R. An intervention to improve HCV testing, linkage to care, and treatment among people who use drugs in Tehran, Iran: The Enhance study. Int. J. Drug Policy 2019, 72, 99–105. [Google Scholar] [CrossRef]
- Bajis, S.; Applegate, T.L.; Grebely, J.; Matthews, G.V.; Dore, G.J. Novel Hepatitic C Virus (HCV) Diagnosis and Treatment Delivery Systems: Facilitating HCV Elimination by Thinking Outside the Clinic. J. Infect. Dis. 2020, 222, S758–S772. [Google Scholar] [CrossRef]
- Valerio, H.; Alavi, M.; Silk, D.; Treloar, C.; Martinello, M.; Milat, A.; Dunlop, A.; Holden, J.; Henderson, C.; Amin, J.; et al. Progress Towards Elimination of Hepatitis C Infection Among People Who Inject Drugs in Australia: The ETHOS Engage Study. Clin. Infect. Dis. 2020, 73, e69–e78. [Google Scholar] [CrossRef]
- Meyers, S.A.; Earnshaw, V.A.; D’Ambrosio, B.; Courchesne, N.; Werb, D.; Smith, L.R. The intersection of gender and drug use-related stigma: A mixed methods systematic review and synthesis of the literature. Drug Alcohol Depend. 2021, 223, 108706. [Google Scholar] [CrossRef] [PubMed]
- The Kirby Institute. National Update on HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: 2009–2018; The Kirby Institute: Sydney, Australia; UNSW Sydney: Kensington, Australia, 2020. [Google Scholar]
- Bersoff-Matcha, S.J.; Cao, K.; Jason, M.; Ajao, A.; Jones, S.C.; Meyer, T.; Brinker, A. Hepatitis B Virus Reactivation Associated with Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann. Intern. Med. 2017, 166, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Higgs, P.; Carruthers, S. Factors affecting hepatitis C treatment intentions among Aboriginal people in Western Australia: A mixed-methods study. Aust. Health Rev. 2020, 44, 755–762. [Google Scholar] [CrossRef] [PubMed]
| Total | RNA Tested | Never RNA Tested n (%) ** | ||||
|---|---|---|---|---|---|---|
| N (%) § | Ever Tested n (%) ** | Tested < 4 Weeks n (%) ** | Tested > 4 Weeks n (%) ** | |||
| Total | 5582 (100) | 3867 (69) | 2770 (50) | 1097 (20) | 1715 (31) | |
| Age at HCV Diagnosis * | ≤29 years | 1203 (22) | 674 (56) | 411 (34) | 236 (20) | 529 (44) |
| 30–44 | 2057 (37) | 1398 (68) | 981 (48) | 417 (20) | 659 (32) | |
| 45–59 | 1637 (29) | 1252 (77) | 959 (59) | 293 (18) | 385 (24) | |
| ≥60 | 684 (12) | 542 (79) | 418 (61) | 124 (18) | 142 (21) | |
| Sex * | Male | 3862 (69) | 2572 (67) | 1838 (48) | 734 (19) | 1290 (33) |
| Female | 1717 (31) | 1293 (75) | 930 (54) | 363 (21) | 424 (25) | |
| Aboriginal Ethnicity * | No | 3625 (65) | 2680 (74) | 1942 (54) | 738 (20) | 945 (26) |
| Yes | 1180 (21) | 658 (56) | 396 (34) | 262 (22) | 522 (44) | |
| Country of Birth * | Australia | 4042 (72) | 2819 (70) | 1972 (49) | 847 (21) | 1233 (30) |
| Overseas | 853 (15) | 625 (73) | 453 (53) | 172 (20) | 228 (27) | |
| Co-Infection Status | HCV only | 5391 (97) | 3725 (69) | 2658 (49) | 1067 (20) | 1666 (31) |
| HCV/HBV | 108 (2) | 65 (60) | 49 (45) | 16 (15) | 43 (40) | |
| HCV/HIV | 83 (2) | 77 (93) | 63 (76) | 14 (17) | 6 (7) | |
| Area of Residence at The Time of HCV * | Metro | 1100 (20) | 796 (72) | 606 (55) | 190 (17) | 301 (28) |
| Outer Metro | 1533 (28) | 1071 (70) | 762 (50) | 309 (20) | 462 (30) | |
| Rural/regional | 2469 (44) | 1834 (74) | 1324 (54) | 510 (21) | 635 (26) | |
| Incarcerated | No history | 3473 (62) | 2685 (77) | 2060 (59) | 625 (18) | 788 (23) |
| Distant | 836 (15) | 551 (66) | 361 (43) | 190 (23) | 285 (34) | |
| Recent | 1273 (23) | 631 (50) | 349 (27) | 282 (22) | 642 (50) | |
| Drug Dependence | No history | 3077 (55) | 2206 (72) | 1703 (55) | 503 (16) | 871 (28) |
| Distant | 656 (12) | 453 (69) | 329 (50) | 124 (19) | 203 (31) | |
| Recent | 1849 (33) | 1208 (65) | 738 (40) | 470 (25) | 641 (35) | |
| History of AUD | No history | 5137 (92) | 3549 (69) | 2566 (50) | 983 (19) | 1588 (31) |
| History | 445 (8) | 318 (72) | 204 (46) | 114 (26) | 127 (29) | |
| History of ESLD | No history | 5452 (98) | 3763 (69) | 2695 (49) | 1068 (20) | 1689 (31) |
| History | 130 (2) | 104 (80) | 75 (58) | 29 (22) | 26 (20) | |
| Characteristics | Total ⸙ | DAA Treatment Initiation | Never DAA Treated n (%) ** | |||
|---|---|---|---|---|---|---|
| N (%) § | Ever DAA Treated n (%) ** | DAA Initiation ≤ 6 Months n (%) ** | DAA Initiation > 6 Months n (%) ** | |||
| Total | 3925 (100) | 2372 (60) | 1370 (35) | 1002 (26) | 1553 (40) | |
| Age at HCV Diagnosis * | ≤ 29 | 802 (20) | 426 (53) | 191 (24) | 235 (29) | 376 (47) |
| 30–44 | 1424 (36) | 828 (58) | 448 (32) | 380 (27) | 596 (42) | |
| 45–59 | 1199 (31) | 792 (66) | 494 (41) | 298 (25) | 407 (34) | |
| ≥ 60 | 499 (13) | 326 (65) | 237 (48) | 89 (18) | 173 (35) | |
| Sex | Male | 2729 (70) | 1763 (65) | 1029 (38) | 734 (27) | 966 (35) |
| Female | 1196 (31) | 609 (51) | 341 (29) | 268 (22) | 587 (49) | |
| Aboriginal Ethnicity * | No | 2633 (67) | 1702 (65) | 1007 (38) | 695 (26) | 931 (35) |
| Yes | 766 (20) | 376 (49) | 170 (22) | 206 (27) | 390 (51) | |
| Country of Birth * | Australia | 2890 (74) | 1809 (63) | 1016 (35) | 793 (27) | 1081 (37) |
| Overseas | 582 (15) | 328 (56) | 199 (34) | 129 (22) | 254 (44) | |
| Co-Infection Status | HCV only | 3792 (97) | 2292 (60) | 1319 (35) | 973 (26) | 1500 (40) |
| HCV/HBV | 66 (2) | 28 (42) | 13 (20) | 15 (23) | 38 (58) | |
| HCV/HIV | 66 (2) | 52 (79) | 38 (58) | 14 (21) | 14 (21) | |
| Area of Residence at The Time of HCV * | Metro | 759 (19) | 439 (58) | 304 (40) | 135 (18) | 320 (42) |
| Outer Metro | 1079 (28) | 649 (60) | 356 (33) | 293 (27) | 430 (40) | |
| Rural/regional | 1775 (45) | 1121 (63) | 633 (36) | 488 (28) | 654 (37) | |
| Time to RNA Test | < 1 week | 1689 (43) | 1160 (69) | 806 (48) | 354 (21) | 529 (31) |
| 1–4 weeks | 411 (11) | 304 (74) | 187 (46) | 117 (29) | 107 (26) | |
| > 4 weeks | 834 (21) | 583 (70) | 227 (27) | 356 (43) | 251 (30) | |
| No test recorded | 991 (25) | 325 (33) | 150 (15) | 175 (18) | 666 (67) | |
| Incarcerated | No history | 2463 (63) | 1494 (61) | 948 (39) | 546 (22) | 969 (39) |
| Distant | 580 (15) | 348 (60) | 182 (31) | 166 (29) | 232 (40) | |
| Recent | 882 (23) | 530 (60) | 240 (27) | 290 (33) | 352 (40) | |
| Drug Dependence | No history | 2170 (55) | 1316 (61) | 850 (39) | 466 (22) | 854 (39) |
| Distant | 473 (12) | 303 (64) | 178 (38) | 125 (26) | 170 (36) | |
| Recent | 1282 (33) | 753 (59) | 342 (27) | 411 (32) | 529 (41) | |
| History of AUD | No history | 3598 (92) | 2152 (60) | 1266 (35) | 886 (25) | 1446 (40) |
| History | 327 (8) | 220 (67) | 104 (32) | 116 (36) | 107 (33) | |
| History of ESLD | No history | 3834 (98) | 2318 (61) | 1340 (35) | 978 (26) | 1516 (40) |
| History | 91 (2) | 54 (59) | 30 (33) | 24 (26) | 37 (41) | |
| Characteristics, n (%) | RNA Tested within 4 Weeks | Ever RNA Tested | |||
|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | ||
| Age at HCV Diagnosis | ≤29 | reference | reference | reference | reference |
| 30–44 | 1.75 (1.52, 2.04) | 1.52 (1.29, 1.77) | 1.66 (1.44, 1.93) | 1.40 (1.20, 1.64) | |
| 45–59 | 2.72 (2.34, 3.18) | 1.90 (1.60, 2.24) | 2.55 (2.17, 3.00) | 1.8 (1.51, 2.15) | |
| ≥60 | 3.03 (2.49, 3.68) | 1.97 (1.60, 2.43) | 3.0 (2.41, 3.72) | 2.07 (1.63, 2.62) | |
| Sex | Male | reference | reference | reference | reference |
| Female | 1.3 (1.16, 1.45) | 1.26 (1.12, 1.42) | 1.53 (1.35, 1.74) | 1.49 (1.30, 1.71) | |
| Aboriginal Ethnicity | No | reference | reference | reference | reference |
| Yes | 0.44 (0.38, 0.50) | 0.57 (0.49, 0.66) | 0.44 (0.39, 0.51) | 0.56 (0.48, 0.65) | |
| Country of Birth | Australia | reference | reference | reference | reference |
| Overseas | 1.19 (1.03, 1.37) | 0.82 (0.70, 0.97) | 1.19 (1.01, 1.40) | 0.85 (0.70, 1.02) | |
| Co-Infection Status | HCV only | reference | reference | reference | reference |
| HCV/HBV | 0.85 (0.58, 1.25) | 0.84 (0.56, 1.25) | 0.68 (0.46, 1.00) | 0.66 (0.44, 0.99) | |
| HCV/HIV | 3.23 (1.95, 5.37) | 2.83 (1.68, 4.77) | 5.74 (2.50, 13.20) | 5.38 (2.31, 12.51) | |
| Area of Residence at The Time of HCV | Metro | reference | reference | reference | reference |
| Outer Metro | 0.81 (0.69, 0.94) | 0.88 (0.75, 1.04) | 0.89 (0.75, 1.06) | 0.95 (0.80, 1.13) | |
| Rural/regional | 0.94 (0.82, 1.09) | 1.10 (0.94, 1.28) | 1.10 (0.94, 1.29) | 1.24 (1.04, 1.47) | |
| Incarcerated | No history | reference | reference | ||
| Distant | 0.52 (0.45, 0.61) | ** | 0.57 (0.48, 0.67) | ** | |
| Recent | 0.26 (0.23, 0.30) | 0.29 (0.25, 0.33) | |||
| Drug Dependence | No history | reference | reference | reference | reference |
| Distant | 0.81 (0.69, 0.96) | 0.88 (0.73, 1.06) | 0.88 (0.73, 1.06) | 0.89 (0.73, 1.09) | |
| Recent | 0.54 (0.48, 0.60) | 0.63 (0.55, 0.72) | 0.74 (0.66, 0.84) | 0.85 (0.74, 0.98) | |
| History of AUD | No history | reference | reference | reference | reference |
| History | 0.85 (0.70, 1.03) | 0.93 (0.75, 1.14) | 1.12 (0.90, 1.39) | 1.19 (0.94, 1.49) | |
| History of ESLD | No history | reference | reference | reference | reference |
| History | 1.40 (0.98, 1.98) | 1.13 (0.79, 1.63) | 1.80 (1.16, 2.77) | 1.23 (0.79, 1.93) | |
| Characteristics | DAA Initiation within 6 Months | Ever DAA Treated | |||
|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | ||
| Age at HCV Diagnosis * | ≤29 | reference | reference | reference | reference |
| 30–44 | 1.46 (1.21, 1.79) | 1.31 (1.07, 1.61) | 1.23 (1.03, 1.46) | 1.12 (0.93, 1.34) | |
| 45–59 | 2.24 (1.84, 2.74) | 1.71 (1.36, 2.13) | 1.72 (1.43, 2.06) | 1.49 (1.21, 1.83) | |
| ≥60 | 2.90 (2.28, 3.68) | 2.14 (1.64, 2.79) | 1.66 (1.32, 2.10) | 1.54 (1.19, 1.99) | |
| Sex | Male | reference | reference | reference | reference |
| Female | 0.66 (0.57, 0.76) | 0.64 (0.54, 0.75) | 0.57 (0.49, 0.65) | 0.59 (0.51, 0.68) | |
| Aboriginal Ethnicity | No | reference | reference | reference | reference |
| Yes | 0.46 (0.38, 0.55) | 0.59 (0.48, 0.73) | 0.52 (0.45, 0.62) | 0.53 (0.44, 0.64) | |
| Country of Birth | Australia | reference | reference | reference | reference |
| Overseas | 0.96 (0.79, 1.15) | 0.69 (0.56, 0.85) | 0.77 (0.64, 0.92) | 0.66 (0.53, 0.80) | |
| Co-Infection Status | HCV only | reference | reference | reference | reference |
| HCV/HBV | 0.46 (0.25, 0.84) | 0.49 (0.26, 0.91) | 0.48 (0.29, 0.78) | 0.52 (0.31, 0.86) | |
| HCV/HIV | 2.52 (1.54, 4.12) | 1.78 (1.07, 2.99) | 2.39 (1.32, 4.30) | 2.12 (1.15, 3.89) | |
| Area of Residence at The Time of HCV | Metro | reference | reference | reference | reference |
| Outer Metro | 0.74 (0.61, 0.89) | 0.84 (0.69, 1.04) | 1.10 (0.91, 1.32) | 1.21 (1.00, 1.48) | |
| Rural/regional | 0.83 (0.70, 0.99) | 0.89 (0.74, 1.08) | 1.25 (1.05, 1.48) | 1.27 (1.05, 1.52) | |
| Time to RNA Test | <1 week | reference | reference | ||
| 1–4 weeks | 0.91 (0.74,1.14) | 1.30 (1.02, 1.66) | |||
| >4 weeks | 0.41 (0.34, 0.49) | ** | 1.06 (0.89, 1.27) | ** | |
| No test recorded | 0.20 (0.16, 0.24) | 0.22 (0.19, 0.26) | |||
| Incarcerated | No history | reference | reference | reference | reference |
| Distant | 0.73 (0.60, 0.89) | 0.88 (0.71, 1.09) | 0.97 (0.81, 1.18) | 0.96 (0.78, 1.18) | |
| Recent | 0.60 (0.50, 0.71) | 0.91 (0.73, 1.12) | 0.98 (0.83, 1.14) | 1.16 (0.95, 1.42) | |
| Drug Dependence | No history | reference | reference | reference | reference |
| Distant | 0.94 (0.76, 1.15) | 1.00 (0.80, 1.25) | 1.15 (0.94, 1.41) | 1.08 (0.86, 1.35) | |
| Recent | 0.57 (0.49, 0.66) | 0.65 (0.55, 0.77) | 0.92 (0.80, 1.06) | 0.92 (0.78, 1.08) | |
| History of AUD | No history | reference | reference | reference | reference |
| History | 0.86 (0.67, 1.10) | 0.88 (0.68, 1.14) | 1.38 (1.09, 1.76) | 1.26 (0.98, 1.61) | |
| History of ESLD | No history | reference | reference | reference | reference |
| History | 0.92 (0.59, 1.42) | 0.73 (0.46, 1.15) | 0.96 (0.63, 1.46) | 0.72 (0.46, 1.11) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousafzai, M.T.; Alavi, M.; Valerio, H.; Hajarizadeh, B.; Grebely, J.; Dore, G.J. Timely Hepatitis C RNA Testing and Treatment in the Era of Direct-Acting Antiviral Therapy among People with Hepatitis C in New South Wales, Australia. Viruses 2022, 14, 1496. https://doi.org/10.3390/v14071496
Yousafzai MT, Alavi M, Valerio H, Hajarizadeh B, Grebely J, Dore GJ. Timely Hepatitis C RNA Testing and Treatment in the Era of Direct-Acting Antiviral Therapy among People with Hepatitis C in New South Wales, Australia. Viruses. 2022; 14(7):1496. https://doi.org/10.3390/v14071496
Chicago/Turabian StyleYousafzai, Mohammad T., Maryam Alavi, Heather Valerio, Behzad Hajarizadeh, Jason Grebely, and Gregory J. Dore. 2022. "Timely Hepatitis C RNA Testing and Treatment in the Era of Direct-Acting Antiviral Therapy among People with Hepatitis C in New South Wales, Australia" Viruses 14, no. 7: 1496. https://doi.org/10.3390/v14071496
APA StyleYousafzai, M. T., Alavi, M., Valerio, H., Hajarizadeh, B., Grebely, J., & Dore, G. J. (2022). Timely Hepatitis C RNA Testing and Treatment in the Era of Direct-Acting Antiviral Therapy among People with Hepatitis C in New South Wales, Australia. Viruses, 14(7), 1496. https://doi.org/10.3390/v14071496






