Rotavirus A in Domestic Pigs and Wild Boars: High Genetic Diversity and Interspecies Transmission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. RNA Extraction and Real-Time RT-PCR
2.3. VP7 and VP4 Genotyping
2.4. Genotype Assignment and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. RVA Prevalence in Domestic Pigs and Wild Boars
3.2. VP7 and VP4 Genotype Diversity in RVA Strains Circulating in Domestic Pigs and Wild Boars
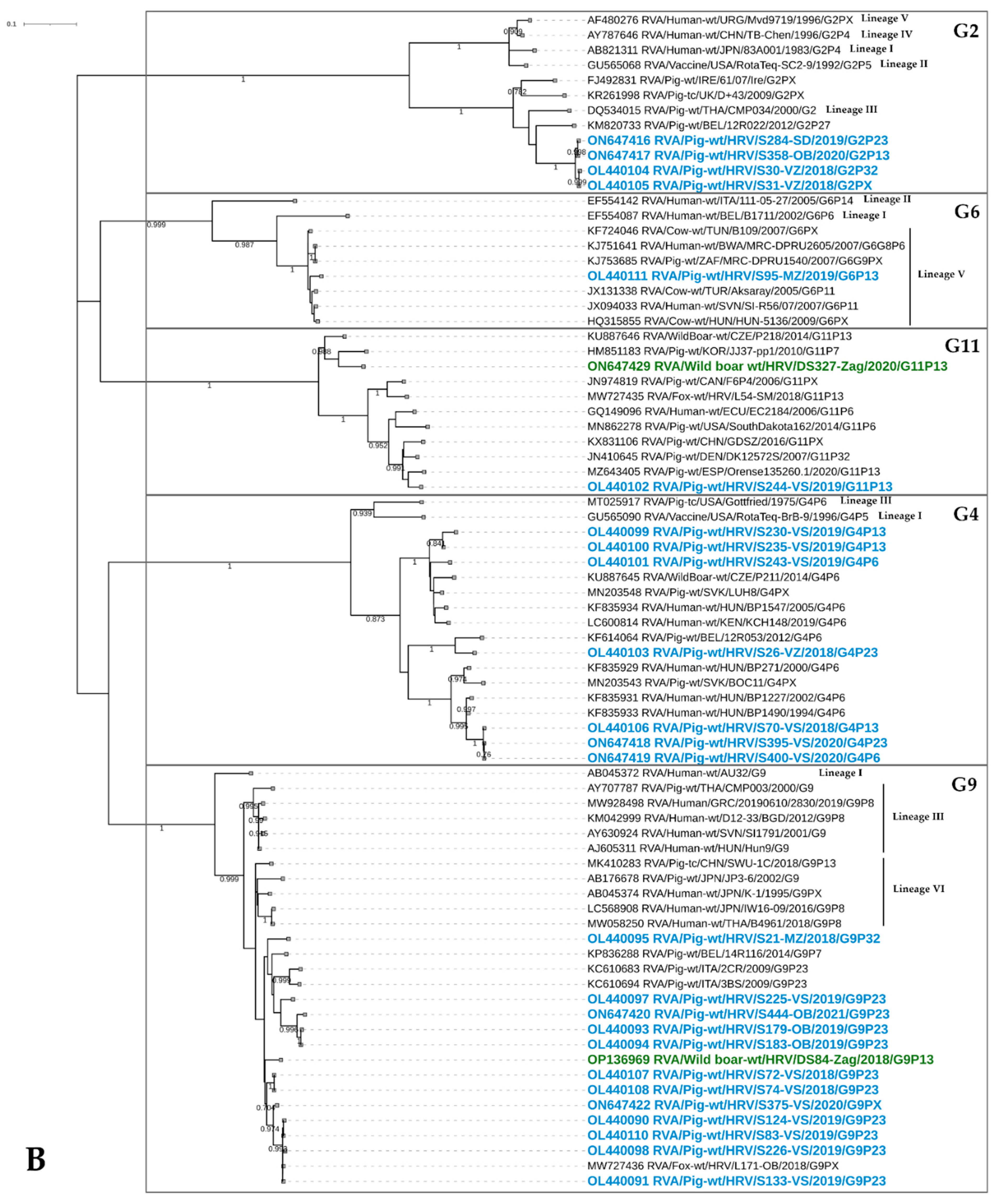
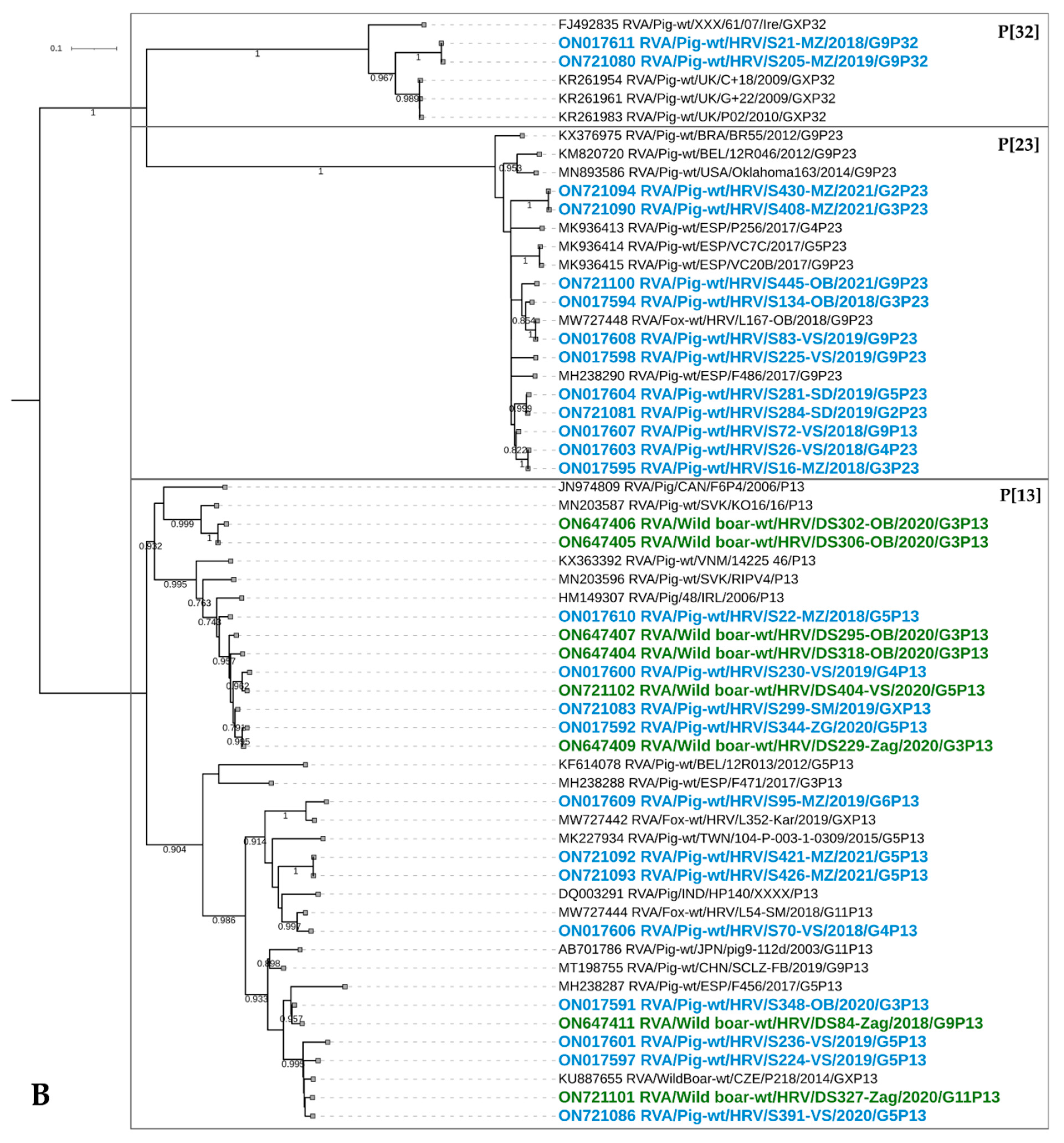
3.3. The Results of Phylogenetic Analysis of RVA Strains in Domestic Pigs and Wild Boars
3.3.1. VP7 Genotyping
G1
G2
G3
G4
G5
G6
G9
G11
3.3.2. VP4 Genotyping
P[6]
P[7]
P[8]
P[11]
P[13]
P[23]
P[32]
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M. Reoviridae. In Fenner’s Veterinary Virology, 5th ed.; MacLachlan, N.J., Dubovi, E.J., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 299–317. [Google Scholar]
- Papp, H.; Laszlo, B.; Jakab, F.; Ganesh, B.; De Grazia, S.; Matthijnssens, J.; Ciarlet, M.; Martella, V.; Banyai, K. Review of group A rotavirus strains reported in swine and cattle. Vet. Microbiol. 2013, 165, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kobayashi, N. Exotic rotaviruses in animals and rotaviruses in exotic animals. VirusDisease 2014, 25, 158–172. [Google Scholar] [CrossRef] [PubMed]
- ICTV. Rotavirus Taxonomy. 2021. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 25 May 2022).
- Doro, R.; Farkas, S.L.; Martella, V.; Banyai, K. Zoonotic transmission of rotavirus: Surveillance and control. Expert Rev. Anti Infect. Therap. 2015, 13, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Banyai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gomara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef]
- RCWG. List of Accepted Genotypes by Rotavirus Classification Working Group. 2021. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 28 May 2022).
- Midgley, S.E.; Banyai, K.; Buesa, J.; Halaihel, N.; Hjulsager, C.K.; Jakab, F.; Kaplon, J.; Larsen, L.E.; Monini, M.; Poljsak-Prijatelj, M.; et al. Diversity and zoonotic potential of rotaviruses in swine and cattle across Europe. Vet. Microbiol. 2012, 156, 238–245. [Google Scholar] [CrossRef]
- Martella, V.; Banyai, K.; Matthijnssens, J.; Buonavoglia, C.; Ciarlet, M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010, 140, 246–255. [Google Scholar] [CrossRef]
- Chang, K.-O.; Saif, J.L.; Kim, Y. Reoviruses (Rotaviruses and Reoviruses). In Diseases of Swine, 10th ed.; Zimmerman, J., Karriker, L., Ramirez, A., Schwartz, K., Stevenson, G., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 621–634. [Google Scholar]
- Vlasova, N.A.; Amimo, O.J.; Saif, J.L. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gomara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo, L.V.; Benito, A.A.; Lázaro-Gaspar, S.; Arnal, J.L.; Martin-Jurado, D.; Menjon, R.; Quílez, J. Occurrence of Rotavirus A Genotypes and Other Enteric Pathogens in Diarrheic Suckling Piglets from Spanish Swine Farms. Animals 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Okadera, K.; Abe, M.; Ito, N.; Morikawa, S.; Yamasaki, A.; Masatani, T.; Nakagawa, K.; Yamaoka, S.; Sugiyama, M. Evidence of natural transmission of group A rotavirus between domestic pigs and wild boars (Sus scrofa) in Japan. Infect. Genet. Evol. 2013, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Moutelikova, R.; Dufkova, L.; Kamler, J.; Drimaj, J.; Plhal, R.; Prodelalova, J. Epidemiological survey of enteric viruses in wild boars in the Czech Republic: First evidence of close relationship between wild boar and human rotavirus A strains. Vet. Microbiol. 2016, 193, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Aguirre, I.; Steyer, A.; Boben, J.; Gruden, K.; Poljšak-Prijatelj, M.; Ravnikar, M. Sensitive Detection of Multiple Rotavirus Genotypes with a Single Reverse Transcription-Real-Time Quantitative PCR Assay. J. Clin. Microbiol. 2008, 46, 2547–2554. [Google Scholar] [CrossRef]
- Jamnikar-Ciglenecki, U.; Kuhar, U.; Sturm, S.; Kirbis, A.; Racki, N.; Steyer, A. The first detection and whole genome characterization of the G6P[15] group A rotavirus strain from roe deer. Vet. Microbiol. 2016, 191, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Čolić, D.; Krešić, N.; Mihaljević, Ž.; Andreanszky, T.; Balić, D.; Lolić, M.; Brnić, D. A Remarkable Genetic Diversity of Rotavirus A Circulating in Red Fox Population in Croatia. Pathogens 2021, 10, 485. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Abe, M.; Ito, N.; Morikawa, S.; Takasu, M.; Murase, T.; Kawashima, T.; Kawai, Y.; Kohara, J.; Sugiyama, M. Molecular epidemiology of rotaviruses among healthy calves in Japan: Isolation of a novel bovine rotavirus bearing new P and G genotypes. Virus Res. 2009, 144, 250–257. [Google Scholar] [CrossRef]
- Eurorotanet. Rotavirus Detection and Typing 2009; p. 25. Available online: https://www.eurorotanet.com/project-information/documents-and-methods/ (accessed on 2 April 2018).
- Mijatovic-Rustempasic, S.; Esona, M.D.; Williams, A.L.; Bowen, M.D. Sensitive and specific nested PCR assay for detection of rotavirus A in samples with a low viral load. J. Virol. Methods 2016, 236, 41–46. [Google Scholar] [CrossRef]
- Theuns, S.; Desmarets, L.M.B.; Heylen, E.; Zeller, M.; Dedeurwaerder, A.; Roukaerts, I.D.M.; Van Ranst, M.; Matthijnssens, J.; Nauwynck, H.J. Porcine group a rotaviruses with heterogeneous VP7 and VP4 genotype combinations can be found together with enteric bacteria on Belgian swine farms. Vet. Microbiol. 2014, 172, 23–34. [Google Scholar] [CrossRef]
- Pickett, B.E.; Sadat, E.L.; Zhang, Y.; Noronha, J.M.; Squires, R.B.; Hunt, V.; Liu, M.; Kumar, S.; Zaremba, S.; Gu, Z.; et al. ViPR: An open bioinformatics database and analysis resource for virology research. Nucleic Acids Res. 2011, 40, D593–D598. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.J.; Rodrigues, A.P.; ElSawy, K.M.; McCammon, J.A.; Caves, L.S. Bio3d: An R package for the comparative analysis of protein structures. Bioinformatics 2006, 22, 2695–2696. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 8 June 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Yu, G. Scatterpie: Scatter Pie Plot, R Package Version 0.1.7, 2021. Available online: https://CRAN.R-project.org/package=scatterpie (accessed on 15 July 2022).
- Steyer, A.; Poljsak-Prijatelj, M.; Barlic-Maganja, D.; Marin, J. Human, porcine and bovine rotaviruses in Slovenia: Evidence of interspecies transmission and genome reassortment. J. Gen. Virol. 2008, 89, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.M.; Esona, M.D.; Betrapally, N.S.; De La Cruz De Leon, L.A.; Neira, Y.R.; Rey, G.J.; Bowen, M.D. Whole-gene analysis of inter-genogroup reassortant rotaviruses from the Dominican Republic: Emergence of equine-like G3 strains and evidence of their reassortment with locally-circulating strains. Virology 2019, 534, 114–131. [Google Scholar] [CrossRef]
- Bonura, F.; Bányai, K.; Mangiaracina, L.; Bonura, C.; Martella, V.; Giammanco, G.M.; De Grazia, S. Emergence in 2017–2019 of novel reassortant equine-like G3 rotavirus strains in Palermo, Sicily. Transbound. Emerg. Dis. 2022, 69, 813–835. [Google Scholar] [CrossRef]
- Afrad, M.H.; Matthijnssens, J.; Afroz, S.F.; Rudra, P.; Nahar, L.; Rahman, R.; Hossain, M.E.; Rahman, S.R.; Azim, T.; Rahman, M. Differences in lineage replacement dynamics of G1 and G2 rotavirus strains versus G9 strain over a period of 22 years in Bangladesh. Infect. Genet. Evol. 2014, 28, 214–222. [Google Scholar] [CrossRef]
- Phan, T.G.; Khamrin, P.; Quang, T.D.; Dey, S.K.; Takanashi, S.; Okitsu, S.; Maneekarn, N.; Ushijima, H. Detection and Genetic Characterization of Group A Rotavirus Strains Circulating among Children with Acute Gastroenteritis in Japan. J. Virol. 2007, 81, 4645–4653. [Google Scholar] [CrossRef]
- Phan, T.G.; Okitsu, S.; Maneekarn, N.; Ushijima, H. Genetic heterogeneity, evolution and recombination in emerging G9 rotaviruses. Infect. Genet. Evol. 2007, 7, 656–663. [Google Scholar] [CrossRef]
- Wandera, E.A.; Hatazawa, R.; Tsutsui, N.; Kurokawa, N.; Kathiiko, C.; Mumo, M.; Waithira, E.; Wachira, M.; Mwaura, B.; Nyangao, J.; et al. Genomic characterization of an African G4P[6] human rotavirus strain identified in a diarrheic child in Kenya: Evidence for porcine-to-human interspecies transmission and reassortment. Infect. Genet. Evol. 2021, 96, 105133. [Google Scholar] [CrossRef] [PubMed]
- Papp, H.; Borzák, R.; Farkas, S.; Kisfali, P.; Lengyel, G.; Molnár, P.; Melegh, B.; Matthijnssens, J.; Jakab, F.; Martella, V.; et al. Zoonotic transmission of reassortant porcine G4P[6] rotaviruses in Hungarian pediatric patients identified sporadically over a 15 year period. Infect. Genet. Evol. 2013, 19, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Monini, M.; Zaccaria, G.; Ianiro, G.; Lavazza, A.; Vaccari, G.; Ruggeri, F.M. Full-length genomic analysis of porcine rotavirus strains isolated from pigs with diarrhea in Northern Italy. Infect. Genet. Evol. 2014, 25, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, D.; Vivancos, R.; Read, J.M.; Pitzer, V.E.; Cunliffe, N.; French, N.; Iturriza-Gomara, M. In-season and out-of-season variation of rotavirus genotype distribution and age of infection across 12 European countries before the introduction of routine vaccination, 2007/08 to 2012/13. Euro Surveill. 2016, 21, 30106. [Google Scholar] [CrossRef]
- Ferrari, E.; Salogni, C.; Martella, V.; Alborali, G.L.; Scaburri, A.; Boniotti, M.B. Assessing the Epidemiology of Rotavirus A, B, C and H in Diarrheic Pigs of Different Ages in Northern Italy. Pathogens 2022, 11, 467. [Google Scholar] [CrossRef]
- Marthaler, D.; Homwong, N.; Rossow, K.; Culhane, M.; Goyal, S.; Collins, J.; Matthijnssens, J.; Ciarlet, M. Rapid detection and high occurrence of porcine rotavirus A, B, and C by RT-qPCR in diagnostic samples. J. Virol. Method. 2014, 209, 30–34. [Google Scholar] [CrossRef]
- Wu, F.-T.; Liu, L.T.-C.; Jiang, B.; Kuo, T.-Y.; Wu, C.-Y.; Liao, M.-H. Prevalence and diversity of rotavirus A in pigs: Evidence for a possible reservoir in human infection. Infect. Genet. Evol. 2022, 98, 105198. [Google Scholar] [CrossRef]
- Wenske, O.; Rückner, A.; Piehler, D.; Schwarz, B.-A.; Vahlenkamp, T.W. Epidemiological analysis of porcine rotavirus A genotypes in Germany. Vet. Microbiol. 2018, 214, 93–98. [Google Scholar] [CrossRef]
- Kozyra, I.; Kozyra, J.; Dors, A.; Rzeżutka, A. Molecular chracterisation of porcine group A rotaviruses: Studies on the age-related occurrence and spatial distribution of circulating virus genotypes in Poland. Vet. Microbiol. 2019, 232, 105–113. [Google Scholar] [CrossRef]
- Brnić, D.; Šimić, I.; Lojkić, I.; Krešić, N.; Jungić, A.; Balić, D.; Lolić, M.; Knežević, D.; Hengl, B. The emergence of porcine epidemic diarrhoea in Croatia: Molecular characterization and serology. BMC Vet. Res. 2019, 15, 249. [Google Scholar] [CrossRef] [Green Version]
- Banyai, K.; Laszlo, B.; Duque, J.; Steele, A.D.; Nelson, E.A.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012, 30 (Suppl. 1), A122–A130. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Bostock, R.; Hancox, L.R.; Nawaz, S.; Watts, O.; Iturriza-Gomara, M.; Mellits, K.H. Genetic diversity of porcine group A rotavirus strains in the UK. Vet. Microbiol. 2014, 173, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.S.; Costa, F.B.; Amorim, A.R.; Mendes, G.S.; Rojas, M.; Santos, N. Rotavirus A, C, and H in Brazilian pigs: Potential for zoonotic transmission of RVA. J. Vet. Diagn. Investig. 2021, 33, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Junga, J.O.; Ogara, W.O.; Vlasova, A.N.; Njahira, M.N.; Maina, S.; Okoth, E.A.; Bishop, R.P.; Saif, L.J.; Djikeng, A. Detection and genetic characterization of porcine group A rotaviruses in asymptomatic pigs in smallholder farms in East Africa: Predominance of P[8] genotype resembling human strains. Vet. Microbiol. 2015, 175, 195–210. [Google Scholar] [CrossRef]
- Hoxie, I.; Dennehy, J.J. Rotavirus A Genome Segments Show Distinct Segregation and Codon Usage Patterns. Viruses 2021, 13, 1460. [Google Scholar] [CrossRef]
- Collins, P.J.; Martella, V.; Buonavoglia, C.; O’Shea, H. Identification of a G2-like porcine rotavirus bearing a novel VP4 type, P[32]. Vet. Res. 2010, 41, 73. [Google Scholar] [CrossRef]
- Baumann, S.; Sydler, T.; Rosato, G.; Hilbe, M.; Kümmerlen, D.; Sidler, X.; Bachofen, C. Frequent Occurrence of Simultaneous Infection with Multiple Rotaviruses in Swiss Pigs. Viruses 2022, 14, 1117. [Google Scholar] [CrossRef]
- Arana, A.; Montes, M.; Jere, K.C.; Alkorta, M.; Iturriza-Gómara, M.; Cilla, G. Emergence and spread of G3P[8] rotaviruses possessing an equine-like VP7 and a DS-1-like genetic backbone in the Basque Country (North of Spain), 2015. Infect. Genet. Evol. 2016, 44, 137–144. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brnić, D.; Čolić, D.; Kunić, V.; Maltar-Strmečki, N.; Krešić, N.; Konjević, D.; Bujanić, M.; Bačani, I.; Hižman, D.; Jemeršić, L. Rotavirus A in Domestic Pigs and Wild Boars: High Genetic Diversity and Interspecies Transmission. Viruses 2022, 14, 2028. https://doi.org/10.3390/v14092028
Brnić D, Čolić D, Kunić V, Maltar-Strmečki N, Krešić N, Konjević D, Bujanić M, Bačani I, Hižman D, Jemeršić L. Rotavirus A in Domestic Pigs and Wild Boars: High Genetic Diversity and Interspecies Transmission. Viruses. 2022; 14(9):2028. https://doi.org/10.3390/v14092028
Chicago/Turabian StyleBrnić, Dragan, Daniel Čolić, Valentina Kunić, Nadica Maltar-Strmečki, Nina Krešić, Dean Konjević, Miljenko Bujanić, Ivica Bačani, Dražen Hižman, and Lorena Jemeršić. 2022. "Rotavirus A in Domestic Pigs and Wild Boars: High Genetic Diversity and Interspecies Transmission" Viruses 14, no. 9: 2028. https://doi.org/10.3390/v14092028
APA StyleBrnić, D., Čolić, D., Kunić, V., Maltar-Strmečki, N., Krešić, N., Konjević, D., Bujanić, M., Bačani, I., Hižman, D., & Jemeršić, L. (2022). Rotavirus A in Domestic Pigs and Wild Boars: High Genetic Diversity and Interspecies Transmission. Viruses, 14(9), 2028. https://doi.org/10.3390/v14092028









