Antiviral Activity of Micafungin and Its Derivatives against SARS-CoV-2 RNA Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture
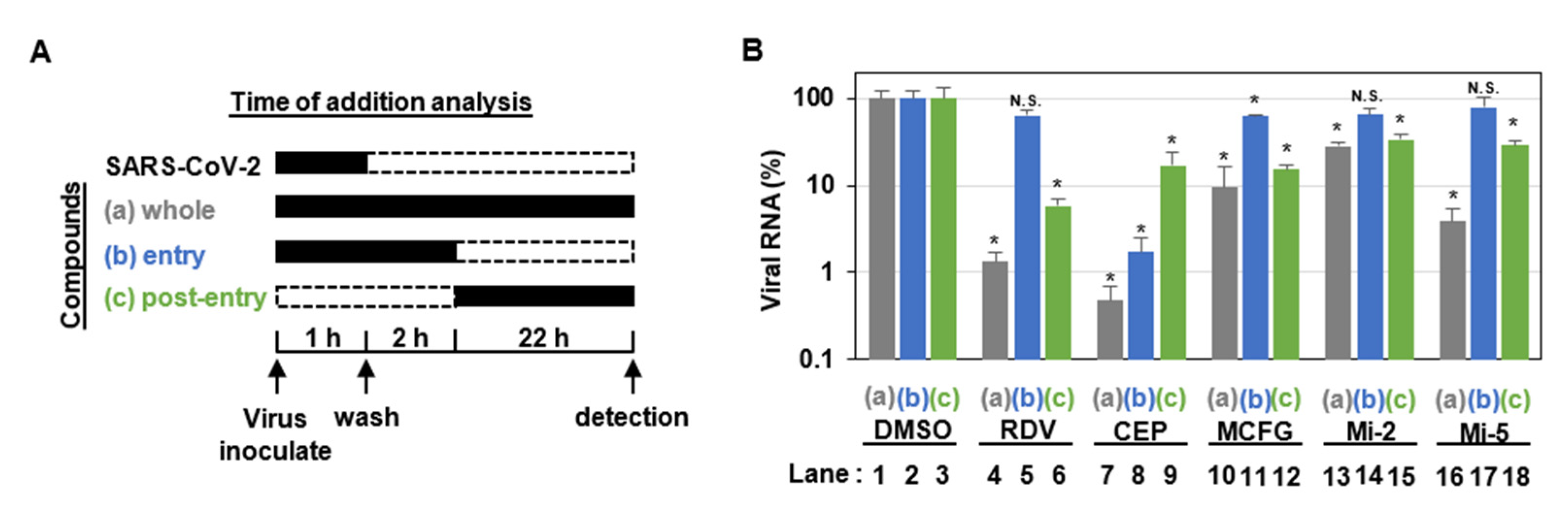
2.3. Infection Assay
2.4. Quantification of Viral RNA
2.5. Cell Viability
2.6. Calculation of IC50, IC90, IC99, and CC50
2.7. Statistics
3. Results
3.1. MCFG and Its Derivatives Show Antiviral Activity against SARS-CoV-2 Infections
3.2. MCFG and Its Derivatives Inhibit SARS-CoV-2 Replication
3.3. Antiviral Activity of MCFG and Its Derivatives against SARS-CoV-2 Variants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, D.; Hui, H.C.; Doerffler, E.; Clarke, M.O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Shukla, A.M.; Chamarthi, G.; Gupte, A. The journey of remdesivir: From Ebola to COVID-19. Drugs Context 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S. Micafungin: A sulfated echinocandin. J. Antibiot. 2009, 62, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Chmielewska, S.; Czyzewska, U.; Malinowska, M.; Tylicki, A. Echinocandins–structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S604–S611. [Google Scholar] [CrossRef]
- Iwamoto, T.; Fujie, A.; Sakamoto, K.; Tsurumi, Y.; Shigematsu, N.; Yamashita, M.; Hashimoto, S.; Okuhara, M.; Kohsaka, M. WF11899A, B and C, novel antifungal lipopeptides. I. Taxonomy, fermentation, isolation and physico-chemical properties. J. Antibiot. 1994, 47, 1084–1091. [Google Scholar] [CrossRef]
- Kim, C.; Kang, H.; Kim, D.E.; Song, J.H.; Choi, M.; Kang, M.; Lee, K.; Kim, H.S.; Shin, J.S.; Jeong, H.; et al. Antiviral activity of micafungin against enterovirus 71. Virol. J. 2016, 13, 99. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.J.; Liu, F.C.; Yeh, C.T.; Yang, C.M.; Lin, C.C.; Lin, T.Y.; Hsieh, P.S.; Hu, M.K.; Gong, Z.; Lu, J.W. Micafungin is a novel anti-viral agent of chikungunya virus through multiple mechanisms. Antivir. Res. 2018, 159, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lu, J.W.; Yeh, C.T.; Lin, T.Y.; Liu, F.C.; Ho, Y.J. Micafungin Inhibits Dengue Virus Infection through the Disruption of Virus Binding, Entry, and Stability. Pharmacy 2021, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.-J.; Park, J.; Shin, H.; Jang, Y.-S.; Woo, J.-S.; Min, D.-H. Identification of a Direct-Acting Antiviral Agent Targeting RNA Helicase via a Graphene Oxide Nanobiosensor. ACS Appl. Mater. Interfaces 2021, 13, 25715–25726. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Chen, Y.C.; Huang, C.K.; Lin, K.C.; Ho, Y.J. Synergistic in-vitro antiviral effects of combination treatment using anidulafungin and T-1105 against Zika virus infection. Antivir. Res. 2021, 195, 105188. [Google Scholar] [CrossRef]
- Ku, K.B.; Shin, H.J.; Kim, H.S.; Kim, B.T.; Kim, S.J.; Kim, C. Repurposing Screens of FDA-Approved Drugs Identify 29 Inhibitors of SARS-CoV-2. J. Microbiol. Biotechnol. 2020, 30, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64, e00819. [Google Scholar] [CrossRef]
- Morikawa, H.; Tomishima, M.; Kayakiri, N.; Araki, T.; Barrett, D.; Akamatsu, S.; Matsumoto, S.; Uchida, S.; Nakai, T.; Takeda, S.; et al. Synthesis and antifungal activity of ASP9726, a novel echinocandin with potent Aspergillus hyphal growth inhibition. Bioorg. Med. Chem. Lett. 2014, 24, 1172–1175. [Google Scholar] [CrossRef]
- Tomishima, M.; Morikawa, H.; Makino, T.; Imanishi, M.; Kayakiri, N.; Asano, T.; Araki, T.; Nakagawa, T. Polypeptide Compound. WO 2009/057568, 7 May 2009. [Google Scholar]
- Toda, A.; Matsuya, T.; Mizuno, H.; Matsuda, H.; Murano, K.; Barrett, D.; Ogino, T.; Matsuda, K. Cyclic Hexapeptide Derivatives. PCT Patent WO 01/60846, 23 August 2001. [Google Scholar]
- Kayakiri, N.; Araki, T.; Makino, T.; Tomishima, M.; Harayama, Y.; Tanabe, D.; Tojo, T.; Nagai, Y. Polypeptide Compound. WO 2012/115159, 30 August 2012. [Google Scholar]
- Ohki, H.; Murano, K.; Tojo, T.; Shiraishi, N.; Matsuya, T.; Matsuda, H.; Mizuno, H.; Barrett, D.; Matsuda, K.; Kawabata, K. New Compound. WO 99/40108, 12 August 1999. [Google Scholar]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef]
- Tsuji, G.; Nakajima, S.; Watashi, K.; Torii, S.; Suzuki, R.; Fukuhara, T.; Ohoka, N.; Inoue, T.; Demizu, Y. Antiviral activity of ciclesonide acetal derivatives blocking SARS-CoV-2 RNA replication. J. Pharmacol. Sci. 2022, 149, 81–84. [Google Scholar] [CrossRef]
- Torii, S.; Kim, K.S.; Koseki, J.; Suzuki, R.; Iwanami, S.; Fujita, Y.; Jeong, Y.D.; Matsuura, Y.; Shimamura, T.; Iwami, S.; et al. Characterization of various remdesivir-resistant mutations of SARS-CoV-2 by mathematical modeling and molecular dynamics simulation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ohashi, H.; Hishiki, T.; Akazawa, D.; Kim, K.S.; Woo, J.; Shionoya, K.; Tsuchimoto, K.; Iwanami, S.; Moriyama, S.; Kinoshita, H.; et al. Different efficacies of neutralizing antibodies and antiviral drugs on SARS-CoV-2 Omicron subvariants, BA.1 and BA.2. Antivir. Res. 2022, 205, 105372. [Google Scholar] [CrossRef]
- Saso, W.; Yamasaki, M.; Nakakita, S.I.; Fukushi, S.; Tsuchimoto, K.; Watanabe, N.; Sriwilaijaroen, N.; Kanie, O.; Muramatsu, M.; Takahashi, Y.; et al. Significant role of host sialylated glycans in the infection and spread of severe acute respiratory syndrome coronavirus 2. PLoS Pathog. 2022, 18, e1010590. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Wang, F.; Stappenbeck, F.; Tsuchimoto, K.; Kobayashi, C.; Saso, W.; Kataoka, M.; Yamasaki, M.; Kuramochi, K.; Muramatsu, M.; et al. Identification of Anti-Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Oxysterol Derivatives In Vitro. Int. J. Mol. Sci. 2021, 22, 3163. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S.; et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience 2021, 24, 102367. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Hobartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2021, 4, 93. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. In silico analysis of echinocandins binding to the main proteases of coronaviruses PEDV (3CL(pro)) and SARS-CoV-2 (M(pro)). Silico Pharm. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, S.; Ali, H.; Secco, I.; Giacca, M.; Gupta, D. Anti-Fungal Drug Anidulafungin Inhibits SARS-CoV-2 Spike-Induced Syncytia Formation by Targeting ACE2-Spike Protein Interaction. Front. Genet. 2022, 13, 866474. [Google Scholar] [CrossRef] [PubMed]

| Compound | VeroE6/TMPRSS2 Cells | Calu-3 Cells | ||
|---|---|---|---|---|
| SARS-CoV-2 RNA | Cell Viability | SARS-CoV-2 RNA | Cell Viability | |
| IC50 (µM) | CC50 (µM) | IC50 (µM) | CC50 (µM) | |
| Micafungin | 26.1 | >64 | 55.3 | >64 |
| Anidulafungin | 7.09 | 24.6 | - | - |
| Caspofungin | >64 | >64 | - | - |
| Mi-1 | 23.7 | >64 | - | - |
| Mi-2 | 5.25 | >64 | 10.1 | >64 |
| Mi-3 | 44.0 | >64 | - | - |
| Mi-4 | 13.0 | >64 | - | - |
| Mi-5 | 6.51 | >64 | 5.71 | 48.5 |
| Mi-6 | >64 | >64 | - | - |
| Remdesivir * | 1.58 | >64 | - | - |
| Cepharanthine * | 0.35 | 25.1 | - | - |
| Compound | SARS-CoV-2 RNA | SARS-CoV-2 RNA | SARS-CoV-2 RNA | SARS-CoV-2 RNA |
|---|---|---|---|---|
| Delta | Omicron | E406W | R10/E796G C799F | |
| IC50 (µM) | IC50 (µM) | IC50 (µM) | IC50 (µM) | |
| Micafungin | 11.8 | 55.9 | 21.7 | 31.3 |
| Mi-2 | <2 | 13.0 | <2 | 9.67 |
| Mi-5 | <2 | 7.56 | 4.48 | 7.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, S.; Ohashi, H.; Akazawa, D.; Torii, S.; Suzuki, R.; Fukuhara, T.; Watashi, K. Antiviral Activity of Micafungin and Its Derivatives against SARS-CoV-2 RNA Replication. Viruses 2023, 15, 452. https://doi.org/10.3390/v15020452
Nakajima S, Ohashi H, Akazawa D, Torii S, Suzuki R, Fukuhara T, Watashi K. Antiviral Activity of Micafungin and Its Derivatives against SARS-CoV-2 RNA Replication. Viruses. 2023; 15(2):452. https://doi.org/10.3390/v15020452
Chicago/Turabian StyleNakajima, Shogo, Hirofumi Ohashi, Daisuke Akazawa, Shiho Torii, Rigel Suzuki, Takasuke Fukuhara, and Koichi Watashi. 2023. "Antiviral Activity of Micafungin and Its Derivatives against SARS-CoV-2 RNA Replication" Viruses 15, no. 2: 452. https://doi.org/10.3390/v15020452






