Abstract
Enterovirus A71, a non-enveloped single-stranded (+) RNA virus, enters host cells through three stages: attachment, endocytosis and uncoating. In recent years, receptors/co-receptors anchored on the host cell membrane and involved in this process have been continuously identified. Among these, hSCARB-2 was the first receptor revealed to specifically bind to a definite site of the EV-A71 viral capsid and plays an indispensable role during viral entry. It actually acts as the main receptor due to its ability to recognize all EV-A71 strains. In addition, PSGL-1 is the second EV-A71 receptor discovered. Unlike hSCARB-2, PSGL-1 binding is strain-specific; only 20% of EV-A71 strains isolated to date are able to recognize and bind it. Some other receptors, such as sialylated glycan, Anx 2, HS, HSP90, vimentin, nucleolin and fibronectin, were discovered successively and considered as “co-receptors” because, without hSCARB-2 or PSGL-1, they are not able to mediate entry. For cypA, prohibitin and hWARS, whether they belong to the category of receptors or of co-receptors still needs further investigation. In fact, they have shown to exhibit an hSCARB-2-independent entry. All this information has gradually enriched our knowledge of EV-A71’s early stages of infection. In addition to the availability of receptors/co-receptors for EV-A71 on host cells, the complex interaction between the virus and host proteins and various intracellular signaling pathways that are intricately connected to each other is critical for a successful EV-A71 invasion and for escaping the attack of the immune system. However, a lot remains unknown about the EV-A71 entry process. Nevertheless, researchers have been continuously interested in developing EV-A71 entry inhibitors, as this study area offers a large number of targets. To date, important progress has been made toward the development of several inhibitors targeting: receptors/co-receptors, including their soluble forms and chemically designed compounds; virus capsids, such as capsid inhibitors designed on the VP1 capsid; compounds potentially interfering with related signaling pathways, such as MAPK-, IFN- and ATR-inhibitors; and other strategies, such as siRNA and monoclonal antibodies targeting entry. The present review summarizes these latest studies, which are undoubtedly of great significance in developing a novel therapeutic approach against EV-A71.
1. Introduction
The Picornaviridae family is classified into enteroviruses, rhinoviruses, hepatoviruses, cardioviruses and alphathoviruses based on their morphology, physicochemical and biological properties, antigenic structures, genomic sequence and mode of replication [1]. Recently, the family of Picornaviridae has been updated. It is currently composed of 158 species which are grouped into 68 genera (https://www.picornaviridae.com/ (accessed on 11 December 2022)). Enterovirus type A71 (EV-A71), belonging to the genus Enterovirus, is an icosahedral, non-enveloped virus of about 30 nm in diameter. The genome, a 7.2–8.5 kb linear single-stranded (+) RNA, presents, at its 5’ end, a viral protein Vpg and an internal ribosome entry site (IRES) to initiate translation. Having only one open reading frame (ORF), the viral RNA is translated into a polyprotein divided into P1, P2 and P3 (https://viralzone.expasy.org/97 (accessed on 11 December 2022)). P1 is cleaved into VP1, VP2, VP3 and VP4, where portions of VP1, VP2 and VP3 are present at the virion surface, while VP4 is located on the internal part of the capsid. P2 and P3 are cleaved to form proteins associated with replication: 2A, 2B, 2C, 3A, 3B, 3C and 3D [2]. EV-A71 is associated mostly with hand, foot and mouth disease (HFMD), a typical disease occurring in children under the age of 5 years old. It is predominately a mild and self-limiting disease [3]. A series of HFMD outbreaks were reported in the Asia-Pacific region, in countries such as Australia [4], Cambodia [5], China [6], Japan [7], Malaysia [8], Singapore [9], South Korea [10], Taiwan [11], Thailand [12], Vietnam [13], etc., resulting in hospitalizations of children and deaths (the mortality rate ranged from <5% to 19%) [14]. Indeed, in some cases, EV-A71 causes several neurological manifestations, such as brainstem encephalitis, aseptic meningitis, acute flaccid paralysis and pulmonary complications.
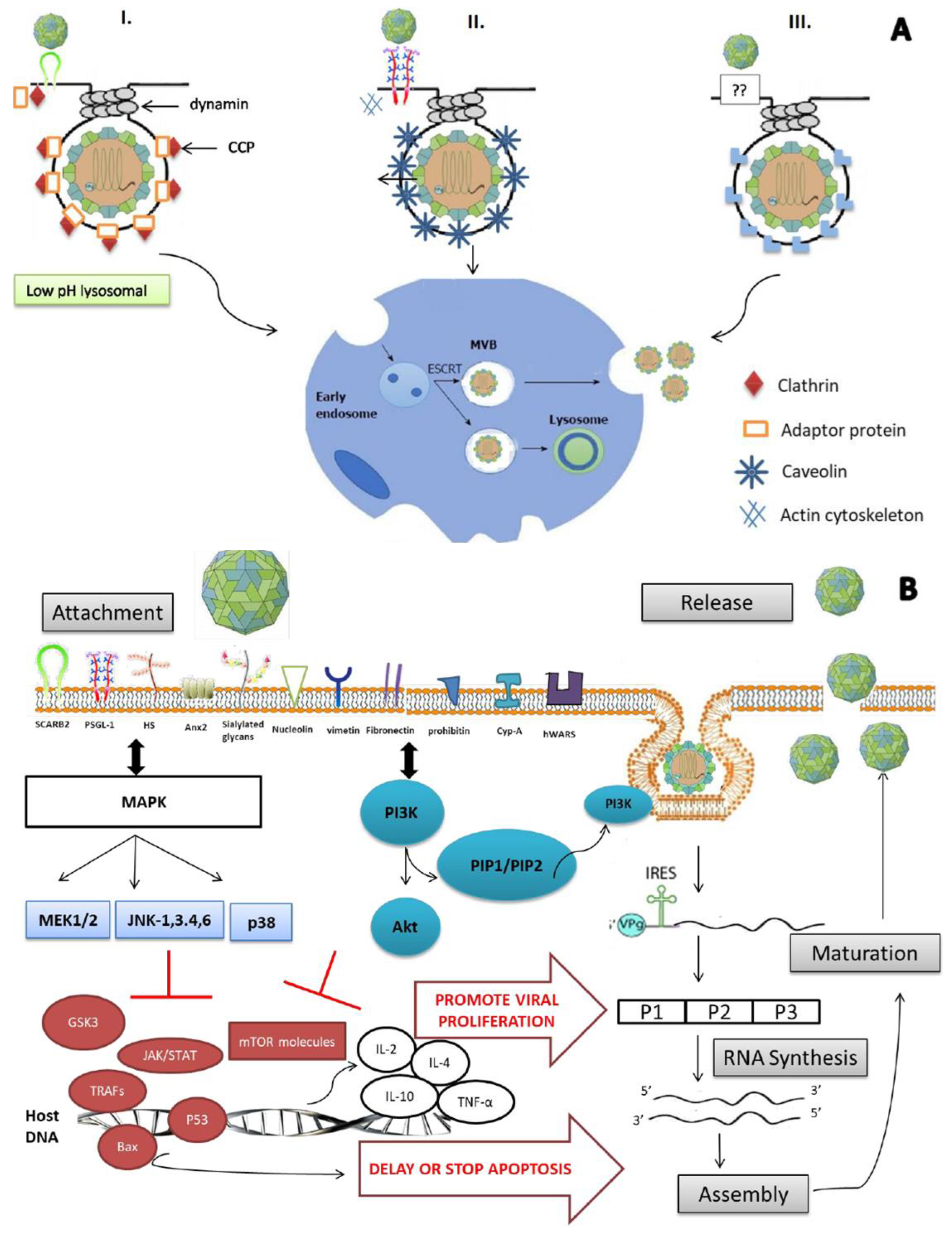
The complex interaction between the virus and host proteins, plus its genome instability, makes it difficult to control and eradicate. Nevertheless, vaccines such as virus-like particles and recombinant VP1 capsids have reached clinical trials; recently, China’s National Medical Products Administration (NMPA) has licensed the commercialization of a whole EV-A71 vaccine [15]. However, to date, treatment is still at the stage of relieving symptoms only, as no FDA-approved antiviral is available. The process of invading the lipid membrane for the non-enveloped virus is not as clear as it is for the enveloped virus. Actually, three different mechanisms (depicted in detail in Figure 1A) have been reported to be involved in viral entry into cells. VP1 and VP2 viral capsids have binding sites for the main receptor, hSCARB-2 (human scavenger receptor class B, member 2), and permit EV-A71 entry into cells through clathrin-mediated endocytosis [16]. In contrast, in the absence of hSCARB-2, the other receptors/co-receptors—PSGL-1 (P-selectin glycoprotein ligand 1), sialylated glycans, Anx 2 (annexin 2), HS (heparan sulfate), HSP90 (heat shock protein 90), cypA (cyclophilin A), vimentin, nucleolin, fibronectin, prohibitin and hWARS (human tryptophanyl-tRNA synthetase)—attach to the virus and initiate caveolae-mediated or endophilin-A2-mediated endocytosis. A general outline of how EV-A71 enters host cells is described in Figure 1.
Figure 1.
Enterovirus A71: mode of entry, virus–host interaction and viral life cycle. (A) Schematic illustration of the 3 different modes of entry identified to date for EV-A71 to invade cells. EV-A71 enters cells through receptor-mediated endocytosis, which includes clathrin-mediated (I), caveolae-mediated (II) and endophilin-A2-mediated (III). (I) In presence of the main receptor, hSCARB2, EV-A71 binds to it with or without the help of one or more co-receptors. The binding induces recruitment of adaptor proteins on the receptor cytoplasmic tail, which afterward bind to clathrin and form “a clathrin-coated pit” (CCP), leading to EV-A71 entry through clathrin-mediated endocytosis. hSCARB2 delivers β-GC from the ER to the lysosomes under physiological conditions. hSCARB2 is abundant in the lysosomal and endosomal compartments, and it also shuttles to the plasma membrane where it encounters EV-A71. After the binding of the virus on the cell surface, the virus–receptor complex is internalized. In the endosome or lysosome, where the pH is low, the virus initiates a conformational change that leads to uncoating. (II) In an alternative process, caveolins are the proteins binding to PSGL-1 cytoplasmic tail in the presence of actin cytoskeleton. Thus, this entrance way is called caveolae-mediated endocytosis. PSGL-1 can bind to EV-A71 and internalize via caveolin-mediated endocytosis, but PSGL-1 cannot initiate uncoating. (III) Recently, endophilin-A2-mediated endocytosis was identified. However, how receptor/co-receptor mediates this kind of entry is not yet elucidated. Once endocytosis initiates, the virus is internalized and delivered to early endosome for translocation, which is assured by the endosomal sorting complex required for transport to multivesicular bodies (ESCRT-MVBs). (B) EV-A71 promotes its production through interaction with intracellular signaling pathways. Once the virus is captured by main or co-receptors, several intracellular signaling pathways related to immune response are activated in order to eliminate the viral infection. However, EV-A71 has to escape immune response and overcome cellular apoptosis/autophagy for its survival by interfering with these pathways by interacting with host proteins. MAPK signaling cascade is activated after release of IL-2, IL-4, IL-10 and TNF-α. Subsequently, together with activated P13K/Akt pathway, MAPK downregulates GSK3, resulting in the delay of apoptosis. Multiple proteins involved in IFN-, apoptosis- and autophagy-related pathways are also regulated, such as JAK/STAT, TRAFs, p53, bax and mTOR. Consequently, many aspects of viral production are promoted, including viral polyprotein processing, RNA synthesis, assembly and maturation and release of new virions.
Virus–host interaction is essential for a successful infection as viruses are host-dependent parasites [17]. Receptors do not only permit entry but also define cell tropism. EV-A71 is known to target cells located in the gastrointestinal tract. However, white blood cells, cells in the respiratory tract and dentritic cells are identified as acting as secondary cell tropism [18,19,20]. This fact, as well as the presence of different symptoms of EV-A71 infection (neurologic and pulmonary complications), implies that multiple alternative entry mechanisms might exist but remain not fully elucidated. Furthermore, the existence of unidentified host proteins that interact with the virus at the stage of entry is highly possible. In fact, host receptor–viral capsid binding also triggers the activation of important signaling pathways, such as MAPK and PI3K/Akt. In addition, IFN is triggered by MDA5 recognition of the double-stranded RNA during EV-A71 replication [21]. Those mechanisms result in the activation of apoptosis and/or autophagy that permit the virus to escape the immune system and to successfully invade cells.
Researchers have always been interested in developing anti-EV-A71 entry inhibitors. Indeed, to date, several virus receptor/co-receptor inhibitors have been developed in order to block EV-A71 at its early stages. These include: soluble forms of hSCARB-2 or PSGL-1, SP-40, lactoferrin and HS mimetics; capsid protein inhibitors that are mostly structurally designed to fit the binding sites of VP1 capsids, such as pleconaril, vapendavir and analogs; related pathway inhibitors, such as picochlorum sp.122 and durvillae Antarctica from plant extracts; and other strategies, such as siRNA and monoclonal antibodies.
In brief, this review highlights the general EV-A71 entry process by emphasizing the functions of corresponding receptors/co-receptors identified to date, the related signaling pathways and the latest progress in the development of entry inhibitors.
2. Enterovirus A71 Receptors/Co-Receptors
EV-A71 enters cells through receptor-mediated endocytosis, which involves three different mechanisms, i.e., (I) clathrin-mediated, (II) caveolae-mediated and (III) endophilin-A2-mediated processes. (I) In the presence of the main receptor, hSCARB2, the viral particle binds to it with or without the help of one or more co-receptors. The binding induces the recruitment of adaptor proteins on the receptor cytoplasmic tail, which afterward bind to clathrin and form “a clathrin-coated pit” (CCP), leading to EV-A71 entry through clathrin-mediated endocytosis. (II) Caveolin is a kind of protein binding to the PSGL-1 cytoplasmic tail in the presence of an actin cytoskeleton. Thus, this entrance way is called caveolae-mediated endocytosis. (III) Recently, an endophilin-A2-mediated endocytosis was identified. However, how receptors/co-receptors mediate this kind of entry has not yet been elucidated. Anyway, once endocytosis initiates, the virus is internalized and delivered to the early endosome for translocation, which is assured by the endosomal sorting complex required for transport to multivesicular bodies (ESCRT-MVBs).
hSCARB-2 was the first receptor identified and was found to act as the main receptor during EV-A71 infection by displaying its recognition activity and uncoating activity in almost all strains. As a lysosomal protein, it is neither abundant nor always present on the surface of cells [22]. Some hSCARB-2-independent entry manners have been reported successively, implying the existence of other alternative mechanisms. Indeed, PSGL-1 was the second receptor discovered. However, its binding is strain-specific, with only 20% of the EV-A71 strains isolated able to recognize it and bind to it. Since recent years, sialylated glycan, Anx 2, HS, HSP90, vimentin, nucleolin and fibronectin have been successively reported to be involved in the process of virus entry. These molecules help hSCARB-2-mediated virus adsorption, recognition and invasion. They are also known as helper receptors or co-receptors. Actually, the presence of a set of receptors/co-receptors that recognizes the capsid protein to permit attachment and entry is the first step to a successful infection. All these receptors/co-receptors are presented according to their chronologic discovery in the following sections.
2.1. hSCARB-2
hSCARB-2 is a receptor protein composed of 478aa, and it presents abundantly in the lysosomal membrane. Thus, it is also called the lysosomal integral membrane protein II or CD38b like-2, and it belongs to the CD36 family [23]. Initially, it was known to function in lysosomal maintenance and transportation, mainly for β-glucocerebrosidase (β-GC). It was later discovered to act as an EV-A71 cell receptor by interacting with the VP1 and (or) VP2 capsid proteins [16,24,25]. The complex structure of EV-A71-hSCARB-2 was determined via cryo-electron microscopy, and the main site of binding was located at α5 (153–163) and α7 (83–193): the G-H loop for VP1 and the E-F loop for VP2 [16]. Among the EV-A71 receptors discovered to date, hSCARB-2 is considered the main receptor as it recognizes all EV-A71 strains and induces a conformational change of the viral capsid that leads to the uncoating of the virion [25,26]. Adult mice are not susceptible to infection by EV-A71, but transgenic mice that express hSCARB-2 become susceptible to EV-A71 infection and develop similar neurological diseases to those found in humans [25,26]. In fact, EV-A71 was able to bind cells expressing hSCARB-2 on their cell surface. Moreover, the soluble form of hSCARB-2 and anti-hSCARB-2 antibodies inhibited EV-A71’s binding to it [27]. Other enteroviruses species, such as CVA7, CVA14 and CVA16, are also known to use hSCARB-2 as a receptor protein [28,29]. Thus, hSCARB-2 acts as the major receptor for EV-A71 infection and assures viral endocytosis and uncoating [30]. In addition, hSCARB-2 was associated with EV-A71 infection susceptibility by comparing the single nucleotide polymorphisms (SNPs) of the hSCARB-2 gene with the clinical HFMD severity [31]. Interestingly, the elucidation of its crystal structure permits the rational design of more and more antivirals as hSCARB-2 inhibitors.
2.2. PSGL-1
PSGL-1 is a glycoprotein receptor expressed on myeloid and T-lymphocyte cells. Initially, it was considered to be a counter-receptor for cell adhesion for P-, E- and L-selectin and to play an important role during inflammation [32,33]. Later, it was identified as one of the EV-A71 receptors [34]. Indeed, EV-A71 was able to bind to PSGL-1 during the infection of Jurkat T cells. The binding site was through 46, 48 and 51Tyr of PSGL-1, with amino acids located at the five-fold axis of EV-A71 [35]. However, the binding was strain-specific, and only 20% of EV-A71 strains isolated to date have been able to recognize and bind to PSGL-1. In fact, EV-A71 strains can be divided into two groups depending on their ability to bind to PSGL-1: PB (PSGL-1 binding strains) and non-PB (non-PSGL-1 binding strains), determined according to the Gly/Glu 145 of VP1, which was acting as a molecule switch [36]. In contrast to hSCARB-2, PSGL-1 may be defined as a non-main receptor and seems to be the one interacting more with the VP2 capsid protein, as mutations in the VP2 gene changed the efficiency of the infection [37]. Yen et al. found that EV-A71 receptor polymorphisms were mostly associated with the susceptibility and clinical severity of EV-A71 infection [31]. In fact, PSGL-1 is only present on the surface of immune cells such as myeloid, dentritic and lymphoid cells. However, both the PSGL-1 P- and L-selectin sites for cellular transduction signaling are recognized by the viral VP1 capsid protein. Consequently, the activation of that signaling induces viral recruitment on the surface of leucocytes and neutrophils to facilitate EV-A71’s invasion of these cells [38]. Thus, PSGL-1 acts as a key receptor for EV-A71 to reach neurologic and pulmonary cells [39]. Nevertheless, in vivo studies and pathogenicity need to be confirmed further.
2.3. Sialylated Glycans
Sulfated or sialylated glycans are sugar residues that are part of the glycolipids of the cell membrane. They were identified as co-receptors for several viruses [40]. Sialic acids (SA), present in terminal monosaccharides (glycan chains of glycoprotein and glycolipid), are synthesized abundantly in the gastrointestinal and respiratory epithelial systems [41]. Yang et al. investigated their potential as an EV-A71 attachment co-receptor using DLD-1 intestinal cells. As a result, the depletion of O-linked glycans as well as pretreatment with sialidase (10 mU, 2 h) and purified SA-α2, 3 Gal and SA-α2, 6Ga significantly decreased the EV-A71 infection. Thus, EV-A71 uses sialic acid-linked glycans as co-receptors [42].
2.4. Anx 2
Anx 2 is a calcium-dependent and phospholipid-binding protein belonging to the annexin family [43]. It is expressed in a wide range of cells but is abundant on the endothelium cell surface. Apart from being a co-receptor protein, Anx2 is involved in multiple functions, such as endocytosis, exocytosis, membrane domain organization, actin remodeling, signaling transduction, protein assembly, transcription, mRNA transport and DNA replication and repair [44,45]. EV-A71 was discovered using Anx2 as an attachment co-receptor. Indeed, EV-A71 binds soluble Anx2, and cells treated with antibodies against Anx2 considerably reduced the viral production. The identified binding site was between VP1 at 40aa and Anx2 at 100aa [46]. More studies should be carried out to fully understand the function of Anx 2 during EV-A71 infection, as it is known to enhance virulence, but, until now, neither viral uncoating nor entry has been reported.
2.5. HS
HS is a polysaccharide that exists in a wide range of mammalian cells. Structurally, it is linear and composed of a repeated arrangement of disaccharide units of N-acetylated or N-sulfated glucosamine and glucuronic acid or iduronic acid [47]. In the presence of core proteins, which are covalently attached to HS chains, it is able to recognize and bind different types of viruses [48,49]. EV-A71 was associated with HS as viral production was reduced in the presence of HS inhibitors and after pre-incubation of EV-A71 with heparin or poly-D lysine and treatment with heparinase I/II/III [50]. A previous study revealed that HS alone was unlikely to assure EV-A71 entry and infection. In fact, a successful viral entry needed the presence of hSCARB-2, as HS alone attached the viral particle through its five-fold symmetry axis in electrostatic interaction and delivered it to the main receptors [51].
2.6. HSP90
Heat shock protein 90 is a chaperone protein of 90 kDa and plays an essential role in the stabilization and/or degradation of cells under temperature stress. It is highly expressed in cells and participates in multiple signaling pathways related to cell regulation [52]. It has emerged as a promising target for cancer, and recently it has been identified to perform an important function during EV-A71 entry [53]. Indeed, HSP90 present on the cell surface was discovered to bind to the viral particle and then attach to surface receptors such as hSCARB-2 or PSGL-1 to facilitate its entry. In addition, the down-regulation of HSP90 by siRNA, antibodies or HSP90 inhibitors considerably reduced viral infection [53]. Alone, HSP90 has never been reported to mediate EV-A71 entry, but it assists by presenting it to the main receptors.
2.7. CypA
Cyclophilins, a family of peptidyl prolyl isomerase, are found in both prokaryotic and eukaryotic cells [54]. Cyclophilin A (cypA) was the first cyclophilin member identified, and it plays several roles: cis-trans isomerase activity, viral production (HIV, HCV), immunosuppressor, mitochondrial function, etc. [55,56,57]. The H-I loop VP1 of EV-A71 was found to interact with cypA [58]. RNAi treatment or cypA inhibitors can impair EV-A71 infection. CypA induces EV-A71 conformational changes and promotes viral entry, implying the existence of other alternative invasion routes independent of hSCARB-2. Thus, cypA represents a valuable target that is worth further study.
2.8. Vimentin
Vimentin is a type III intermediate filament protein that plays multiple functions in human cells: maintaining cell shape and cytoplasm integrity, skeleton stabilization, EMT development, attachment of pathogens, etc. [59,60]. Du et al. discovered that vimentin is also one of the proteins used by EV-A71 as a cell co-receptor [61]. In fact, pull-down experiments revealed a direct interaction between vimentin and EV-A71 VP1 capsid proteins at AA151. Moreover, soluble vimentin and anti-vimentin antibodies reduced EV-A71’s binding and attachment. However, its role in virulence and entry is not yet well understood [61].
2.9. Nucleolin
Nucleolin is a nuclear phosphoprotein of 100-kDa that can also be found on the surface of a wide range of cells. It is involved in multiple functions: ribosome biogenesis, transcriptional regulation, chromatin remodeling, cell proliferation, apoptosis and differentiation [62]. Human immunodeficiency virus (HIV) and respiratory syncytial virus (RSV) have been reported to use nucleolin as a receptor [63,64,65]. However, EV-A71 was later identified to also interact directly with nucleolin through its VP1 capsid protein via glycoproteomics analysis. Furthermore, the knockdown of nucleolin and anti-nucleolin antibodies significantly reduced EV-A71’s binding and infection in vitro, while its expression in vivo (mouse NIH3T3) increased EV-A71’s virulence [66].
2.10. Fibronectin
Fibronectin acts as a multifunctional glycoprotein and is essential for cell adhesion, growth, migration and differentiation [67,68]. It is also able to interact with multiple molecules and mediate attachments on the cell surface. The D2 domain of fibronectin was found to interact with the amino-terminal of the EV-A71 VP1 capsid protein. It is therefore believed that fibronectin represents a cellular co-receptor for EV-A71. Indeed, EV-A71 infection is enhanced by the overexpression of fibronectin. In contrast, the treatment of infected cells with short peptides containing the Arg-Gly-Asp motif or integrin or fibronectin inhibitors considerably reduces EV-A71 infection both in vivo and in vitro [68,69].
2.11. Prohibitin
Prohibitin is a pleiotropic protein found in multiple subcellular compartments, such as the mitochondria, nuclei and plasma membrane [70]. As a multifunctional protein, it plays role in cell differentiation, proliferation, inhibition and morphogenesis [71]. Using a proteomics approach followed by mass spectrometry, Too et al. discovered that this protein is involved in EV-A71 attachment and entry [72]. Indeed, a direct interaction between prohibitin and EV-A71 was revealed by IP. In both siRNA and antibodies specific for prohibitin, Roc-A (a prohibitin inhibitor) inhibited EV-A71 in neuronal cells (NSC-34 cells) [72]. Furthermore, prohibitin might be involved in EV-A71 hSCARB-2-independent entry [73]. Thus, prohibitin is a valuable functional receptor or co-receptor that deserves further investigation.
2.12. hWARS
Human tryptophanyl-tRNA synthetase (hWARS) is known as an enzyme catalyzing the aminoacetylation of tRNA with tryptophan [74]. Recently, it was identified as an IFN-γ-inducible entry factor for EV-A71. In fact, the knockdown of hWARS, treatment with its competitive soluble structure, anti-hWARS antibodies or CRISPR/Cas9-mediated deletion reduced EV-A71 virulence through the attenuation of the cytopathic effect (CPE) and decrease of viral biosynthesis. The decline of EV-A71 infection was observed during both in vitro and in vivo screening (NT2 cells, L929 mouse) with or without the presence of hSCARB-2. Thus, hWARS can successfully induce viral entry in an hSCARB-2-independent manner [75].
3. EV-A71 Entry into Cells
Being recognized/bound by cell surface proteins is not enough for EV-A71 to successfully enter and infect cells. The viral invasion requires several processes by which the related intracellular signaling pathways cooperate, with three different mechanisms responsible for EV-A71 entry. The processes are described in detail in the following sections.
3.1. Adsorption to Cells
Most viruses use endocytosis, a cellular process in which extracellular substances are taken into the cytoplasm through membrane-bound vesicles such as endosomes or lysosomes. EV-A71 has been identified to use this process within multiple routes depending on the cell surface receptors/co-receptors [76]. Indeed, EV-A71 enters cells through receptor-mediated endocytosis: clathrin using a “clathrin-coated pit” (CCP) and caveolae and endophilin A2, both in the presence of dynamin and the actin cytoskeleton. The processes differ according to the type of receptor. However, once the virus is internalized, the translocation into the cytoplasm is the same, occurring via the endosomal sorting complex required for transport to multivesicular bodies (ESCRT-MVBs). Overall, three different mechanisms are involved in the process of viral entry (depicted in detail in Figure 1A).
3.2. hSCARB-2 Uses Clathrin-Mediated Endocytosis (CME)
As a major receptor, the crystal structure of hSCARB-2 was recently elucidated in neutral and acidic conditions [25]. Therefore, hSCARB-2 undergoes a pivotal conformational alteration at α4 and α5 at its “cap position”. Indeed, in the acidic condition, hSCARB-2 presented an opened hydrophobic tunnel that was able to bind to the EV-A71 surface protein and in turn triggered the changes of the EV-A71 structure to the “A-particle”, which uncoated and translocated through the channel [77]. The mechanism of entry was through the clathrin-mediated endocytic (CME) pathway and involved several CME key genes: AP2A1, ARRB1, CLTCL1, ARPC5, PAK1, ROCK1 and WASF1 [30,78]. The binding of the virion to hSCARB-2 triggered the first signaling and induced the binding of the adaptor proteins to the receptor cytoplasmic tail, which afterwards bound to clathrin and formed a “clathrin-coated pit (CCP)”. Furthermore, DNM1/Dynamin-1 or DNM2/Dynamin-2, a member of a class of scission proteins, pinched off the CCP and released the clathrin-coated vesicle (CCV). Auxilin and HSP70 released the clathrin basket from the vesicle and delivered its viral content to early endosomes [79]. In fact, the suppression of clathrin or dynamin via siRNA or CME chemical inhibitors reduced viral entry and infection. However, caveolin inhibitors or siRNA directed to caveolae-mediated endocytosis had no effect, suggesting that CME is the only underlying mechanism of entry through hSCARB-2 [30].
3.3. PSGL-1 Uses Caveolae-Mediated Endocytosis (CaME)
The mechanism of entry of EV-A71 using the PSGL-1 receptor was elucidated by inhibiting CME, which was already known to be used by the major receptor hSCARB-2 under particular pH conditions [30]. Further studies demonstrated that caveolae-mediated endocytosis is the pathway mediated by PSGL-1 [80]. Caveolae, made of caveolins (CAV1/Caveolin-1, CAV-2/Caveolin-2 and CAV3/Caveolin-3), is a specialized lipid raft that forms the flask-shaped invagination of the plasma membrane [81]. When PSGL-1 recognizes and binds to the virion, it is internalized and delivered to early endosomes for translocation. This mechanism is also pH-dependent [79,82].
3.4. Endophilin-A2-Mediated Endocytosis
Caco-2 cells are polarized human intestinal cells within the EV-A71 cell tropism. Studies of EV-A71 infection in caco-2 cells revealed that neither clathrin- nor caveolae-mediated endocytosis, as previously described, was adopted [83]. In fact, the inhibition of CME, CaME and macropynocytosis by their corresponding inhibitors or siRNA targeted genes (ENTH, EPIV 2, AP2B1 for clathrin, and CLTA, CLTB, CLTC, APFA1 for caveolin) rather enhanced than reduced EV-A71’s infection in caco-2 cells. Further studies showed that endophilin-A2-mediated endocytosis is the mechanism of entry of EV-A71 in such cells, with a dependence on dynamin-2 and the actin cytoskeleton. Indeed, while EV-A71 recognized and bound the receptors or attachments, the SH3 domain of endophilin also bound to the cytoplasmic motifs of EV-A71, activated receptors and mediated endocytosis with actin polymerization and dynamin. The translocation is assured by ESCRT-MVBs [83].
3.5. Relevant Signaling Pathways
Mapping host factors and signaling pathways is a very important task for the understanding of EV-A71 infection and for the development of antivirals. Currently, mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase/Akt (PI3K/Akt), interferon (IFN), apoptosis and autophagy pathways have been reported to be associated with EV-A71 at the early stage of its life cycle (Figure 1B) [84,85,86].
3.5.1. MAPK Signaling Pathway
MAPKs are a series of regulator protein kinases essential for cell proliferation, cell differentiation, innate immunity, cell movement and cell death. The MAPK pathway is one of the most studied pathways associated with EV-A71 entry and replication, as several RNA viruses induced Raf/MEK/ERK signaling for their multiplication [87]. In fact, EV-A71 activated the MEK1-ERK signaling pathway, and its suppression by siRNA or inhibitors efficiently blocked viral production. MAPK activation is initiated after the binding of viral particles to host receptors. As a result, the MAPK group, ERK, JNK and p38, is phosphorylated. Subsequently, the MEK1/ERK1 signaling cascade is activated, during which the activation of ERK1/2 by MEK1/2 is controlled by Raf. This pathway is essential for a successful EV-A71 infection, mostly in immature dendritic cells [88,89,90]. Moreover, during the study of differential gene expressions (PCR array) of MAPK signaling in EV-A71 infected RD, EV-A71 was proved to induce the activation of ERK and JNK through the release of IL-2, IL-4, IL-10 and TNF-α [91]. Furthermore, together with PI3K/Akt, the MAPK signaling pathway delayed apoptosis by downregulating GSK3 [92].
3.5.2. PI3K/Akt Signaling Pathway
PI3K/Akt is an essential pathway that regulates signaling and biological processes, such as RNA processing, translation, autophagy and apoptosis. During EV-A71 infection, the PI3K pathway is favorable for virus entry. Indeed, the virus has used this pathway to escape early apoptosis in order to promote progeny propagation [93]. The underlying mechanism was similar to that for MAPK, where GSK3 is downregulated while phosphorylated PI3K/Akt activated PIP2/PIP3 and targeted the apoptosis gene in order to delay it. Moreover, the PI3K/Akt signaling pathway is directly associated with viral entry. In fact, the binding of VP1 to the major receptor or one of the co-receptors activated PI3K; in a pH-dependent manner, the virus was able to enter cells through PI3K/Akt-supported endocytosis [92]. This entry mechanism was also reported to be used by other types of viruses, such as the influenza A virus, the vaccinia virus, HCV, and the avian leucosis virus (ALV) [94,95,96,97].
3.5.3. IFN Signaling Pathway
IFN is one of the primary responders of the immune system during viral infections. The first step, in which the virus is recognized by receptors/co-receptors on the cell surface, triggers the production of a large number of cytokines and interferons [98]. However, the virus has the ability to escape the response of the host immune system. In fact, EV-A71 proteins are able to interact with multiple host proteins responsible for the activation of the IFN signaling pathway and consequently promote viral production and escape immune responses. Interestingly, 2Apro and 3Cpro are essential proteins of EV-A71, able to counteract and suppress type I IFN [99,100]. Moreover, EV-A71 suppresses interferon responses by blocking the JAK/signaling transducer and activator of STAT signaling by inducing karyopherin-α1 degradation [101,102]. Meanwhile, cellular endogenous microRNA-628-5p facilitates viral infection by suppressing TRAF3 signaling [98]. Moreover, type III interferon signaling restricts EV-A71 infection in goblet cells [103].
3.5.4. Apoptosis Signaling Pathway
Apoptosis is a form of programmed cell death and a pathway used by the organism to kill infected cells. During infection, viral proteins trigger the activation of the apoptosis pathway through the activation of numerous related proteins [104]. However, EV-A71 is able to corrupt the apoptosis mechanism for its own benefit. In fact, essential molecules involved in apoptosis, such as p53, STAT1 and bax, are involved during infection. Thus, they represent an interesting target for EV-A71 drug screening [105,106].
3.5.5. Autophagy Signaling Pathway
Together with apoptosis, autophagy plays an important function under stress situations or during an external attack on the cells such as a viral infection. It maintains homeostasis in cells and is responsible for the delivery of infected cells to lysosomes in order to eliminate them [107]. However, some viruses escape autophagy by the interaction of viral proteins with host proteins. Liu et al. compared two recombinant viruses with one attenuated VP1 and discovered that the VP1 played a vital role in disrupting autophagy through the mTOR signaling pathway [108].
4. Drug Development Targeting EV-A71 Entry
Strategies blocking viral entry include developing inhibitors targeting host receptors/co-receptors, viral capsid proteins, some key intracellular pathways and other techniques. In this field, significant progress has been made in recent years. Presented in Table 1 are several drug candidates discovered lately. These will be discussed in detail in the following section.
4.1. Virus Receptor Inhibitors
Soluble form of EV-A71 receptors: Several studies have demonstrated that a soluble form of the EV-A71 receptor, such as soluble hSCARB2 or PSGL-1, is able to inhibit viral attachment and entry [25,34]. It acts as a competitive inhibitor that interferes with the active site of the viral capsid so that the cell receptor can no longer bind it [109,110,111]. Moreover, glycosylation or sialylation considerably affects virus binding and subsequent entry [112]. Neuraminidase has already been used to remove cell surface sialic acids and reduce EV-A71 binding and infection rates [113].
SP40 and L-SP40: are peptides designed after the VP1 capsid protein of EV-A71. They were able to interfere with their receptors/co-receptors and inhibit viral attachment and entry. Their affinity towards multiple EV-A71 receptors was assessed by molecular docking, and nucleolin was discovered to have the highest binding affinity, followed by anx-2, hSCARB-2 and hWARS [114,115,116].
Lactoferrin: is a multifunctional iron-binding glycoprotein with a broad range of bioactivities against bacteria, fungi and viruses [117,118]. It was able to interact directly with heparin sulfate glycosaminoglycane, which served as an EV-A71 binding co-receptor. Both bovine and human lactoferrin inhibited EV-A71 uncoating and entry in vitro (IC50 = 10.5–24.5 µg/ml and 103.3–185 µg/ml, respectively) [119]. The underlying mechanism was revealed through the binding of the VP1 capsid protein [120].
Heparan sulfate mimetics: Carbohydrates such as HS and sialylated glycans (SG) are used by EV-A71 for attachment and uncoating. Several attempts to inhibit EV-A71 entry by disrupting these kinds of co-receptors have been made [112]. Heparin, heparan sulfates and pentosan polysulfate mimetics were evaluated for EV-A71 inhibition [111]. All of the three compounds exhibited antiviral activity, with heparin being the most potent (SI = 236.24). The underlying mechanism inhibiting EV-A71 infection is the hindrance of viral attachment/entry to the cell surface.
4.2. Virus Capsid Protein Inhibitors
Pyridyl Imidazolinone: is a capsid inhibitor known to prevent viral attachment, uncoating and the entry of several viruses such as poliovirus, the best-known picornaviridae [121,122]. Later, a series of designed pyridyl imidazolinones were tested against enterovirus 71 entry [123,124]. The mechanism was through the binding of the viral capsid protein VP1. For instance, the complex binding of WIN51711 to VP1 was reported [125]. These inhibitors represent a promising candidate for EV-A71 entry inhibition despite some resistance due to VP1 mutations [126,127]. Recently, a viral capsid inhibitor G197 and a host-targeting phosphatidylinositol 4-kinase III beta inhibitor N373, especially when used in combination, were able to significantly improve the survival of infected mice [128].
Pleconaril, vapendavir and their analogs: are among the best-known capsid inhibitors for picornaviruses. They were designed to be capsid-binding compounds that recognize, bind and neutralize viral protein VP1 and inhibit EV-A71 entry. They represent an excellent candidate due to their potency against almost all EV-A71 strains. Further analogs have been designed at the level of the amino group in the central chain coupled to a methylisoxazol moiety of those compounds in order to enhance their activity [129,130,131].
NF449 (4, 49, 40,409- [carbonylbis [imino-5,1,3-benzenetriyl bis(carbonyl-imino)]] tetrakis (benzene-1,3-disulfonic acid) octasodium salt): is known as a Gs-α inhibitor and has been identified to block EV-A71 activity (SI > 150). During the screening of anti-EV-A71 activity, NF449 was discovered to inhibit EV-A71 at the entry step. Furthermore, the progeny virus isolated from cells treated with NF449 presented mutations at nt 2734 and 3173 in the VPI capsid protein. Thus, NF449 is known as a capsid protein inhibitor [132].
Kappa carrageenan: is a sulfate polysaccharide from seaweed that exhibits broad bioactivities [133,134,135]. It has shown anti-EV-A71 activity in more than one stage of its life cycle. Kappa carrageenan inhibits EV-A71 binding, viral replication and apoptosis. It was able to directly bind to EV-A71 in a dose-dependent manner [136]. In fact, it has a similar structure as HS and is considered as its analog [137].
Sertraline: Tseng et al. screened the anti-EV-A71 activity of 774 FDA-approved drugs using a cell-based biosensor, “Hela-G2AwtR”. Among them, Sertraline, known to increase serotonin concentration by inhibiting the serotonin transporter and used traditionally as an anti-depressant, had the strongest antiviral activity against not only EV-A71 but CVA16, CVB1, CVB2, Echo9 and Echo30 as well. The selectivity index (SI) for EV-A71 was 19.92 and 19.95 for RD and Hela, respectively. Further studies revealed that its mode of action was independent of its empiric application, and the EV-A71 inhibition was at an early stage. Actually, sertraline inhibited viral entry by neutralizing endolysosomal acidification through an increase in the pH value to circa 7.0 [138].
Ni (2+)-chitosan beads: Chitosan is a polysaccharide known for having various biological activities and being nontoxic toward cells. It is easy to modify due to its unique structural features [139,140]. Lin et al. developed metal–ion composite chitosan beads to characterize and evaluate EV-A71 adsorption/removal. Interestingly, Ni (2+)-chitosan beads were found to be the most effective. Therefore, they represent an interesting strategy to inhibit EV-A71 infection despite the fact that the adsorption by the compound did not affect EV-A71 antigenicity [141].
CPZ and DNS: Since EV-A71 entry was discovered to be mainly through clathrin- and dynamin-mediated endocytosis, endocytosis inhibitors such as chlorpromazine (CPZ) and dynasore (DNS) were used to suppress EV-A71 entry and infection. However, this was shown to be not always the case, as EV-A71 used multiple entry routes and pathways [76].
PTC-209HBr: is a Bmi-1 inhibitor known as anticancer. In later studies, this small molecule was discovered to inhibit EV-A71 infection by blocking the binding between VP1 and hSCARB2 [142].
4.3. Related Signaling Pathway Inhibitors
Saururus chimensis: is an endemic plant in Asia (China, India, Japan, Korea, the Philippines and Vietnam) used to treat inflammation [143]. It has been found in later studies to exhibit different bioactivities, being anti-oxidative [144], anticancer [145,146] and antiviral [147]. Recently, this compound was detected to have anti-EV-A71 activity, targeting the MEK1/ERK signaling pathway, which is essential for EV-A71 entry and replication. In fact, treatment of Vero cells infected by EV-A71 with saururus chimensis considerably reduced the viral yield as well as the number of host proteins associated with the MEK pathway [147]. Thus, it acts as a potent EV-A71 drug candidate that also targets several essential pathways related to the virus such as AP-1 or PI3K.
U0126: is a chemical compound that specifically inhibits MAP kinase activity by blocking MEK1/2 [147]. A study has shown that U0126 was able to block EV-A71 infection by targeting MEK/ERK signaling, a pathway related to viral entry and replication [148].
Formononetin: is a flavonoid present in several plants and herbs such as “the red clover”. It has multiple biological activities such as anti-inflammatory, anti-oxidant, wound healing and anti-tumor. Reportedly, it inhibited EV-A71-induced CPE by suppressing ERK, p38 and JNK through regulating cox-2/PGE2 expression, which was associated with the MAPK signaling pathway [149].
GS-9620: is a heterocyclic compound known as a TLR7 antagonist that exhibits antiviral activity against HBV and HIV. An in vivo study of this compound against EV-A71 showed that it inhibited viral infection by targeting the IFN and PI3K/Akt pathways [150].
Berberine: from berberis vulgaris L., is an isoquinoline alkaloid that has antiviral activity against EV-A71. The mechanism of action is through the inhibition of phosphorylated MEK/ERK-related proteins during the MAPK signaling pathway [151].
Se@PEI@siRNA: is a siRNA targeting VP1 and loaded with selenium nanoparticles of polyethylenimine (PEI) on the surface. It has been able to inhibit EV-A71 infection by targeting the VP1 capsid and activating bax-mediated apoptosis simultaneously. It also maintained cells in the sub-G1 phase [106].
Picochlorum sp.122: is a polysaccharide exhibiting antiviral activity. It reduced VP1 RNA and viral protein levels in EV-A71-infected Vero cells. The mechanism revealed that picochlorum sp.122 protected cells from apoptosis via the AKT and ATM/ATR signaling pathways [152].
Durvillae Antarctica: is a green alga containing polysaccharides with strong antiviral activity against multiple viruses, such as HIV, herpes simplex virus (HSV), HCV, etc. In later studies, it has been proven to reduce viral RNA replication of EV-A71 in a dose-dependent manner via inhibition of p53 and the STAT1 signaling transducer [105].
4.4. Other Strategies Blocking Entry
Lipid rafts: Cholesterol plays an essential role in virus entry by endocytosis [153]. In fact, the disruption of those lipids on the cell membrane can be a target to inhibit viral entry. Zhu et al. investigated the effects of depletion or addition of cholesterol mainly by MβCD, a pharmacological agent, during EV-A71 infection. The results showed that lipid raft depletion before infection considerably inhibited EV-A71 entry and infection [154].
Monoclonal antibodies: several attempts have been made using monoclonal antibodies as candidates for EV-A71 treatment [155,156,157]. D5, H7 and C4 are monoclonal antibodies (mAbs) targeting the VP1 GH loop. Thus, studies revealed that all of them inhibited EV-A71 infection both pre- and post-infection. Further studies confirmed that those mAbs not only inhibited viral entry but also attachment, internalization, uncoating and RNA release. These mAbs were able to bind to the viral receptor binding sites of HS, hSCARB-2 and PSGL-1, thus interfering with the viral binding to these receptors [156]. Furthermore, mAb 51 and 53 belong to isotopes IgM and IgG1, respectively, and are monoclonal antibodies targeting the VP1 capsid protein. MAb 51 was able to protect 2-week-old AG129 mice from developing certain symptoms of EV-A71 infection [158]. In addition, 10D3, a neutralizing monoclonal antibody targeting a highly conserved region of the VP3 capsid, has also been identified as inhibiting EV-A71 [159].
siRNA: is a recent strategy that is being used to target EV-A71 receptors, such as hSCARB-2 or the capsid protein or related pathways, including MEK1/2, PI3K/Akt, IFN, apoptosis and autophagy [160,161]. In addition, the CME/CaME-related genes can also be targeted by siRNA in order to inhibit viral entry.

Table 1.
A list of antivirals targeting the EV-A71 entry stage.
Table 1.
A list of antivirals targeting the EV-A71 entry stage.
| Drug Name | Target | EC50 | Cell Line/Animal Model | Ref. |
|---|---|---|---|---|
| Host receptor inhibitors | ||||
| Soluble form of receptors | hSCARB-2/PSGL-1 | — | RD/L-PSGL-1.1 | [24,34] |
| SP40 and L-SP40 | Nucleolin/Anx-2/hSCARB-2/hWARS/HS | 6–9.3 µM | RD/Hela/HT-29/Vero/neonatal mice | [114,115,116] |
| Lactoferrin bovine/human | HS | 10.5–24.5 µg/mL 103.3–185 µg/mL | RD | [119] |
| HS mimetics | HS | 205 µg/mL | Vero | [111] |
| Heparin/heparan | 290 µg/mL | |||
| sulfates/pentosan | 238 µg/mL | |||
| Capsid protein inhibitors | ||||
| Pyridyl Imidazolinone | ||||
| VP1 | 0.31–1.26 µM | Vero/MRC-5 | [123,124] |
| 0.51–1.37 µM | |||
| 0.04 µM | |||
| 0.339 µM | RD | [126] | |
| 0.04 µM | |||
| 0.011 µM | |||
| 0.04 µM | |||
| 0.13 µM | |||
| 0.0012 µM | |||
| 0.008 µM | |||
| 0.0088 µM | |||
| — | [125] | ||
| — | [128] | ||
| Pleconaril | VP1 | 0.13–0.54 µg/mL | RD/1-day-old ICR | [129] |
| Pirodavir | 0.36–0.727 µg/mL | RD | [130] | |
| Vapendavir | 0.498–0.957 µg/mL | [131] | ||
| NF449 | Capsid protein | 6.5 µM | [132] | |
| Kappa carrageenan | VP1/HS | — | Vero | [136] |
| Sertraline | EV-A71 entry/uncoating | 1.92/1.67 µM | RD/Hela | [138] |
| Ni(2+) chitosan beads | Capsid protein | 5.5 µM | Vero | [141] |
| CPZ and DNS | — | RD | [76] | |
| PTC-209HBr | hSCARB2/VP1 | 0.79–2.92 µM | RD/Hela/HEK293T/Vero | [142] |
| Related signaling pathway inhibitors | ||||
| Saururus Chinensis | MEK/ERK, AP-1, PI3K | — | Vero/SK--SH | [147] |
| U0126 | 3.45/3.98 µM | Mouse model | [148] | |
| Formononetin | PI3K/Akt | — | Vero | [149] |
| GS-9620 | MEK/ERK | 73.10 µM | SK-N-SH | [150] |
| Berberine | bax apoptosis | — | [151] | |
| Selenium | Akt | — | [106] | |
| Picochlorumsp.122 | P53, STAT1 | 13.7 µg/ml | RD | [152] |
| Durvillae A. | — | 2-day-old Balb/c | [105] | |
| Other strategies blocking entry | ||||
| Lipid rafts | Capsid protein | 31.5 µg/mL | Vero | [154] |
| Monoclonal antibodies | ||||
| EV-A71 endocytosis | — | RD/Vero/Jurkat T cells/2-day-old BALB/c mice | [155] |
| VP1 | — | Hela | [156] |
| VP1 | — | 2-week-old AG129 mice | [158] |
| VP3 | — | [159] | |
| siRNA | ||||
| VP1 GH Loop | — | Vero/Hela/HepG2/293T/L929 | [160] |
| hSCARB-2 | — | 293T/L929 | [161] |
| MEK/ERK | — | HEK293T | [88] |
“—” means not determined in the original reference. “EC50”: the concentration applied on which 50% of total cell numbers survived representing a value of drug ability; the lower the value, the better the antiviral activity is.
5. Discussion and Perspectives
Since EV-A71 has provoked epidemics and severe diseases throughout the world, more and more receptors/co-receptors have been identified. However, not all of them have the ability to induce binding, uncoating and entry. In fact, EV-A71 enters cells through receptor-dependent endocytosis, and only five receptors, which are hSCARB-2, PSGL-1, cypA, prohibitin and hWARS, out of all those discovered to date, are able to mediate viral entry independently. Moreover, the presence of hSCARB-2, which is able to recognize all EV-A71 strains and even some other types of enteroviruses, such as CVA7, CVA14 and CVA16, is the only productive receptor for both attachment and uncoating, but other receptors aid in the attachment or uncoating processes [25,29]. This fact makes hSCARB-2 the main receptor and the others co-receptors. Correspondingly, the attachment mediates a conformational alteration of EV-A71 from 160S to 135S particles and permits uncoating and entry [162]. Thus, exploring the underlying mechanisms and clarifying the role of EV-A71 receptors/co-receptors are helpful to understand the scenario of viral entry and to develop well-directed inhibitors.
Although EV-A71 cell tropism is defined, its mode of entry also depends on the receptors/co-receptors available on the cell surface. Indeed, hSCARB-2 participates in clathrin-mediated endocytosis to permit EV-A71 entry, while PSGL-1 involves caveolae-mediated endocytosis. Alternatively, endophylin-A2-mediated endocytosis was observed in EV-A71-infected caco-2 cells. Previous studies confirmed the existence of other modes of entry, particularly the unknown dynamin- and hSCARB2-independent entry manner that was adopted during viral entry in A549 cells, as well as the prohibitin-, cyp-A- and hWARS-mediated endocytosis [18,75].
Several intracellular signaling pathways are involved in the viral life cycle. The MAPK signaling pathway is among the first that was found to be associated with EV-A71 entry. However, it is believed that more pathways are being controlled by EV-A71 at the early stages of the infection. Thus, mapping the entire signaling pathway will definitely provide not only new targets but more understanding of how EV-A71 infects cells and leads to serious disorders. For instance, the PI3K/Akt pathway is taken over by EV-A71 to help the virus invade. In addition, EV-A71 delays apoptosis to enhance its replication and corrupts IFN-, apoptosis- and autophagy-related signaling pathways by targeting numerous host proteins, such as STAT/KPNA1, TRAF3, type III IFN, p53 and bax. Indeed, 2Apro and 3Cpro are essential proteins of EV-A71 that promote viral spread and, importantly, are able to interfere with IFN pathways during immune responses.
Overall, the EV-A71 mechanism of entry is not yet fully elucidated, including the clear role of all receptors/co-receptors identified to date, the existence of unknown ones and the interaction between the host and the viral proteins that change virulence and pathogenicity. Indeed, studies demonstrated that other molecules, such as SLC35B2 and B3GAT3, are related to the successful entry of EV-A71, as their knockout but not hSCARB-2 decreased EV-A71 infection. Furthermore, the infection efficiency of EV-A71 was positively correlated with the level of host cell sulfation, not only with the amount of HS, suggesting that an unidentified sulfated protein(s) must contribute to EV-A71 infection [163]. Moreover, as this virus possesses an unstable genome, the viral capsid is prone to mutation, which leads to drug resistance and makes the development of inhibitors challenging. In addition, an appropriate animal model with similar symptoms to humans is lacking, making it difficult for an antiviral drug to reach clinical trials and FDA approval. Effectively, drug approval requires safety and efficiency tests in a large population of young children, which is hard in many cases due to ethics and culture. All these limitations need to be overcome in the near future. Last but not least, it is possible to combine all of the entry inhibitors mentioned above with other types of inhibitors that target other EV-A71 replication steps or related host proteins. For instance, 3Cpro, an essential viral protein ensuring viral spread and infection [164,165], has been inhibited by quercetin, a flavonoid bioactive compound of plants [166]. Its combination treatment with one of the entry inhibitors will ultimately increase the drug efficiency against EV-A71 and therefore eradicate its infection and related diseases.
Author Contributions
K.H., R.O.D. and Y.W.: conceptualization, literature review, critical and comparative analysis of available information, manuscript writing; R.O.D.: tables and figures preparation (original draft); R.O.D., C.Y. and H.L.: review and editing; K.H. and Y.W.: supervision and editing manuscript and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key R&D Project of Hubei Province (Social Development), China (2022BCA018) to K.H.; the Open Project Funding of the Key Laboratory of Fermentation Engineering (Ministry of Education) (202209FE06) to Y.W.; the Initial Project for High Level Talents of HBUT (337193) to K.H.; cooperative Innovation Center of Industrial Fermentation (Ministry of Education & Hubei Province), China (2022KF16) to K.H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALV | avian leucosis virus |
| Anx-2 | annexin 2 |
| AP-1 | activating protein-1 |
| ATM/ATR | ataxia-telangiectasia-mutated/ataxia telangiectasia |
| CCP | clathrin-coated pit |
| CCV | clathrin-coated vesicle |
| CVA 7/14/16 | coxsackievirus A 7/14/16 |
| CME | clathrin-mediated endocytosis |
| CaME | caveolae-mediated endocytosis |
| CPE | cytopathic effect |
| CPZ | chlorpromazine |
| CypA | cyclophilin A |
| DNS | dynasore |
| EMT | epithelial–mesenchymal transition |
| ESCRT-MVBs | endosomal sorting complex required for transport to multivesicular bodies |
| EV-A71 | enterovirus 71 |
| FDA | food and drug administration |
| GSK3 | glycogen synthase kinase-3 |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| HS | heparan sulfate |
| hSCARB-2 | human scavenger receptor class B, member 2 |
| HSP90 | heat shock protein 90 |
| HSV | herpex simplex virus |
| hWARS | human tryptophanyl-tRNA synthetase |
| IFN | interferon |
| IP | immunoprecipitation |
| IRES | internal ribosome sites |
| JAK | Janus kinase |
| mAbs | monoclonal antibodies |
| MAPKs | itogen-activated protein kinases |
| non-PB | non-PSGL-1 binding |
| ORF | open reading frame |
| PCR | polymerase chain reaction |
| PB | PSGL-1 binding |
| PI3K/Akt | phosphatidylinositol-3-kinase/protein kinase B |
| PKC ζ | protein kinase C zeta |
| PSGL-1 | P-selectin glycoprotein ligand 1 |
| RdRp | RNA dependent RNA-polymerase |
| RNA | ribonucleic acid |
| RSV | respiratory syncytial virus |
| SA | sialic acid |
| SG | sialic glycans |
| SI | selectivity index |
| SNPs | single nucleotide polymorphisms |
| siRNA | small interfering RNA |
| STAT | signal transducer and activator of transcription |
| TLR7 | toll-like receptor 7 |
| TRAF3 | TNF receptor-associated factor 3 |
References
- Zell, R. Picornaviridae-the ever-growing virus family. Arch. Virol. 2018, 163, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Perera, R.; Cardosa, J.; Kuhn, R.J.; Rossmann, M.G. Structure determination of enterovirus 71. Acta Crystallogr. D. Biol. Crystallogr. 2012, 68, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Liu, Y.; Ma, H.C.; Paul, A.V.; Wimmer, E. Picornavirus morphogenesis. Microbiol. Mol. Biol. Rev. 2014, 78, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Horsley, E.; Just, E.; Torres, C.; Huhtinen, E.; Forssman, B.; Slade, R. Enterovirus 71 outbreak in Northern Sydney, 2013: Case series and initial response. J. Paediatr. Child Health 2014, 50, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.; Mey, C.; Eloit, M.; Zhu, H.; Danet, L.; Huang, Z.; Zou, G.; Tarantola, A.; Cheval, J.; Perot, P.; et al. Molecular epidemiology of human enterovirus 71 at the origin of an epidemic of fatal hand, foot and mouth disease cases in Cambodia. Emerg. Microbes Infect. 2016, 5, e104. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008–2012: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef]
- Tagaya, I.; Takayama, R.; Hagiwara, A. A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn. J. Med. Sci. Biol. 1981, 34, 191–196. [Google Scholar] [CrossRef]
- Chua, K.B.; Kasri, A.R. Hand foot and mouth disease due to enterovirus 71 in Malaysia. Virol. Sin. 2011, 26, 221–228. [Google Scholar] [CrossRef]
- Lim, C.T.; Jiang, L.; Ma, S.; James, L.; Ang, L.W. Basic reproduction number of coxsackievirus type A6 and A16 and enterovirus 71: Estimates from outbreaks of hand, foot and mouth disease in Singapore, a tropical city-state. Epidemiol. Infect. 2016, 144, 1028–1034. [Google Scholar] [CrossRef]
- Baek, K.; Yeo, S.; Lee, B.; Park, K.; Song, J.; Yu, J.; Rheem, I.; Kim, J.; Hwang, S.; Choi, Y.; et al. Epidemics of enterovirus infection in Chungnam Korea, 2008 and 2009. Virol. J. 2011, 8, 297. [Google Scholar] [CrossRef]
- Chia, M.Y.; Chiang, P.S.; Chung, W.Y.; Luo, S.T.; Lee, M.S. Epidemiology of enterovirus 71 infections in Taiwan. Pediatr. Neonatol. 2014, 55, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Linsuwanon, P.; Puenpa, J.; Huang, S.W.; Wang, Y.F.; Mauleekoonphairoj, J.; Wang, J.R.; Poovorawan, Y. Epidemiology and seroepidemiology of human enterovirus 71 among Thai populations. J. Biomed. Sci. 2014, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Khanh, T.H.; Sabanathan, S.; Thanh, T.T.; Thoa, L.P.K.; Thuong, T.C.; Hang, V.T.; Farrar, J.F.; Hien, T.T.; Chau, N.V.; van Doorn, H.R. Enterovirus 71-associated hand, foot, and mouth disease, Southern Vietnam, 2011. Emerg. Infect. Dis. 2012, 18, 2002–2005. [Google Scholar] [CrossRef]
- Puenpa, J.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. The history of enterovirus A71 outbreaks and molecular epidemiology in the asia-pacific region. J. Biomed. Sci. 2019, 26, 75. [Google Scholar] [CrossRef]
- Li, M.L.; Shih, S.R.; Tolbert, B.S.; Brewer, G. Enterovirus A71 vaccines. Vaccines 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef]
- Sangar, D.V. The replication of picornaviruses. J. Gen Virol. 1979, 45, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Shih, S.R. Cell and tissue tropism of enterovirus 71 and other enteroviruses infections. J. Biomed. Sci. 2014, 21, 18. [Google Scholar] [CrossRef]
- Chang, C.S.; Liao, C.C.; Liou, A.T.; Chang, Y.S.; Chang, Y.T.; Tzeng, B.H.; Chen, C.C.; Shih, C. Enterovirus 71 targets the cardiopulmonary system in a robust oral infection mouse model. Sci. Rep. 2019, 9, 11108. [Google Scholar] [CrossRef]
- Lin, Y.W.; Wang, S.W.; Tung, Y.Y.; Chen, S.H. Enterovirus 71 infection of human dendritic cells. Exp. Biol. Med. 2009, 234, 1166–1173. [Google Scholar] [CrossRef]
- Huang, H.I.; Lin, J.Y.; Chen, S.H. EV71 infection induces IFNβ expression in neural cells. Viruses 2019, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Koike, S. Cellular receptors for enterovirus A71. J. Biomed. Sci. 2020, 27, 23. [Google Scholar] [CrossRef]
- Calvo, D.; Dopazo, J.; Vega, M.A. The CD36, CLA-1 (CD36L1), and LIMPII (CD36L2) gene family: Cellular distribution, chromosomal location, and genetic evolution. Genomics 1995, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A.; Khoury, J.E. Scavenger receptors. Curr. Biol. 2020, 30, R790–R795. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Fujii, K.; Koike, S. Receptors for enterovirus 71. Emerg. Microbes Infect. 2014, 3, e53. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, N.; Zhou, S.; Zheng, K.; Sun, L.; Zhang, Y.; Cao, L.; Zhang, X.; Xiang, Q.; Chen, Z.; et al. Severity of enterovirus A71 infection in a human SCARB2 knock-in mouse model is dependent on infectious strain and route. Emerg. Microbes Infect. 2018, 7, 205. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Wang, N.; Zhang, J.; Li, J.; Guo, H.; Yin, X.; Rao, Z.; Wang, X.; Zhang, L. The binding of a monoclonal antibody to the apical region of SCARB2 blocks EV71 infection. Protein Cell 2017, 8, 590–600. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shimizu, H. Cellular receptors for human enterovirus species a. Front. Microbiol. 2012, 3, 105. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Iizuka, S.; Yamashita, T.; Minagawa, H.; Mizuta, K.; Okamoto, M.; Nishimura, H.; Sanjoh, K.; Katsushima, N.; Itagaki, T.; et al. Human SCARB2-dependent infection by coxsackievirus A7, A14, and A16 and enterovirus 71. J. Virol. 2012, 86, 5686–5696. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Lin, H.Y.; Tsou, Y.L.; Chitra, E.; Hsiao, K.N.; Shao, H.Y.; Liu, C.C.; Sia, C.; Chong, P.; Chow, Y.H. Human SCARB2-mediated entry and endocytosis of EV71. PLoS ONE 2012, 7, e30507. [Google Scholar] [CrossRef]
- Yen, T.Y.; Shih, W.L.; Huang, Y.C.; Lee, J.T.; Huang, L.M.; Chang, L.Y. Polymorphisms in enterovirus 71 receptors associated with susceptibility and clinical severity. PLoS ONE 2018, 13, e0206769. [Google Scholar] [CrossRef] [PubMed]
- Laszik, Z.; Jansen, P.J.; Cummings, R.D.; Tedder, T.F.; McEver, R.P.; Moore, K.L. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood 1996, 88, 3010–3021. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.S.; Tang, J.; Shaw, G.D.; Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell 2000, 103, 467–479. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009, 15, 794–797. [Google Scholar] [CrossRef]
- Nishimura, Y.; Wakita, T.; Shimizu, H. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS Pathog. 2010, 6, e1001174. [Google Scholar] [CrossRef]
- Nishimura, Y.; Lee, H.; Hafenstein, S.; Kataoka, C.; Wakita, T.; Bergelson, J.M.; Shimizu, H. Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS Pathog. 2013, 9, e1003511. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tijsma, A.; Mirabelli, C.; Baggen, J.; Wahedi, M.; Franco, D.; De Palma, A.; Leyssen, P.; Verbeken, E.; van Kuppeveld, F.J.M.; et al. Intra-host emergence of an enterovirus A71 variant with enhanced PSGL1 usage and neurovirulence. Emerg. Microbes Infect. 2019, 8, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Müller, H.; Kuwano, Y.; Ley, K. PSGL-1-dependent myeloid leukocyte activation. J. Leukoc. Biol. 2009, 86, 1119–1124. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Shi, K.; Yao, S.; Yang, X.; Liu, Y.H.; Tang, L.; Dang, Y.W.; Chen, G.; Feng, Z.B.; Pan, H.B. Morphological characteristics of fatal pediatric hand, foot and mouth disease: A clinicopathological study with related receptors of EV71. Pathol. Res. Pract. 2017, 213, 1144–1151. [Google Scholar] [CrossRef]
- Maginnis, M.S. Virus-Receptor Interactions: The Key to Cellular Invasion. J. Mol. Biol. 2018, 430, 2590–2611. [Google Scholar] [CrossRef]
- Varki, N.M.; Varki, A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. 2007, 87, 851–857. [Google Scholar] [CrossRef]
- Yang, B.; Chuang, H.; Yang, K.D. Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Virol. J. 2009, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Rintala-Dempsey, A.C.; Rezvanpour, A.; Shaw, G.S. S100-annexin complexes--structural insights. FEBS J. 2008, 275, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Skeate, J.G.; Kast, W.M. Annexin A2 in Virus Infection. Front. Microbiol. 2018, 9, 2954. [Google Scholar] [CrossRef]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J. Virol. 2011, 85, 11809–11820. [Google Scholar] [CrossRef] [PubMed]
- Kjellén, L.; Lindahl, U. Proteoglycans: Structures and interactions. Annu. Rev. Biochem. 1991, 60, 443–475. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef]
- Tan, C.W.; Poh, C.L.; Sam, I.C.; Chan, Y.F. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J. Virol. 2013, 87, 611–620. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sudaka, Y.; Takashino, A.; Imura, A.; Fujii, K.; Koike, S. Amino acid variation at VP1-145 of enterovirus 71 determines attachment receptor usage and neurovirulence in human scavenger receptor B2 transgenic mice. J. Virol. 2018, 92, e00681-18. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Ivy, S.P. Heat shock protein 90. Curr. Opin. Oncol. 2003, 15, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.L.; Lin, Y.W.; Chang, H.W.; Lin, H.Y.; Shao, H.Y.; Yu, S.L.; Liu, C.C.; Chitra, E.; Sia, C.; Chow, Y.H. Heat shock protein 90: Role in enterovirus 71 entry and assembly and potential target for therapy. PLoS ONE 2013, 8, e77133. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heitman, J. The cyclophilins. Genome Biol. 2005, 6, 226. [Google Scholar] [CrossRef]
- Olejnik, P.; Nuc, K. Cyclophilins—proteins with many functions. (Article in Polish). Postepy. Biochem. 2018, 64, 46–54. [Google Scholar] [CrossRef]
- Zhou, D.; Mei, Q.; Li, J.; He, H. Cyclophilin A and viral infections. Biochem. Biophys. Res. Commun. 2012, 424, 647–650. [Google Scholar] [CrossRef]
- Liu, J.; Farmer, J.D., Jr.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Qing, J.; Wang, Y.; Sun, Y.; Huang, J.; Yan, W.; Wang, J.; Su, D.; Ni, C.; Li, J.; Rao, Z.; et al. Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential role in viral infection as an uncoating regulator. PLoS Pathog. 2014, 10, e1004422. [Google Scholar] [CrossRef]
- Goldman, R.D.; Khuon, S.; Chou, Y.H.; Opal, P.; Steinert, P.M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J. Cell Biol. 1996, 134, 971–983. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Shahar, Y.B.; Pollak, Y.; Dorfman, T.; Shefer, H.K.; Assi, Z.E.; Mor-Vaknin, N.; Coran, A.G. The role of intermediate filaments in maintaining integrity and function of intestinal epithelial cells after massive bowel resection in a rat. Pediatr. Surg. Int. 2018, 3, 217–225. [Google Scholar] [CrossRef]
- Du, N.; Cong, H.; Tian, H.; Zhang, H.; Zhang, W.; Song, L.; Tien, P. Cell surface vimentin is an attachment receptor for enterovirus 71. J. Virol. 2014, 88, 5816–5833. [Google Scholar] [CrossRef] [PubMed]
- Ginisty, H.; Sicard, H.; Roger, B.; Bouvet, P. Structure and functions of nucleolin. J. Cell Sci. 1999, 112, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Mastrangelo, P.; Griffin, J.K.; Moraes, T.J.; Hegele, R.G. Respiratory syncytial virus receptor expression in the mouse and viral tropism. Histol. Histopathol. 2015, 30, 401–411. [Google Scholar] [PubMed]
- Mastrangelo, P.; Chin, A.A.; Tan, S.; Jeon, A.H.; Ackerley, C.A.; Siu, K.K.; Lee, J.E.; Hegele, R.G. Identification of RSV Fusion Protein Interaction Domains on the Virus Receptor, Nucleolin. Viruses 2021, 13, 261. [Google Scholar] [CrossRef]
- Hovanessian, A.G. Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin. Cell Res. 2006, 16, 174–181. [Google Scholar] [CrossRef]
- Su, P.Y.; Wang, Y.F.; Huang, S.W.; Lo, Y.C.; Wang, Y.H.; Wu, S.R.; Shieh, D.B.; Chen, S.H.; Wang, J.R.; Lai, M.D.; et al. Cell surface nucleolin facilitates enterovirus 71 binding and infection. J. Virol. 2015, 89, 4527–4538. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Adams, J.C.; Chiquet-Ehrismann, R.; Tucker, R.P. The evolution of tenascins and fibronectin. Cell Adh. Migr. 2015, 9, 22–33. [Google Scholar] [CrossRef]
- He, Q.Q.; Ren, S.; Xia, Z.C.; Cheng, Z.K.; Peng, N.F.; Zhu, Y. Fibronectin Facilitates Enterovirus 71 Infection by Mediating Viral Entry. J. Virol. 2018, 92, e02251-17. [Google Scholar] [CrossRef]
- Wei, Y.; Chiang, W.C.; Sumpter, R., Jr.; Mishra, P.; Levine, B. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 2017, 168, 224–238. [Google Scholar] [CrossRef]
- Chowdhury, I.; Thompson, W.E.; Thomas, K. Prohibitins role in cellular survival through Ras-Raf-MEK-ERK pathway. J. Cell Physiol. 2014, 229, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Too, I.H.K.; Bonne, I.; Tan, E.L.; Chu, J.J.H.; Alonso, S. Prohibitin plays a critical role in Enterovirus 71 neuropathogenesis. PLoS Pathog. 2018, 14, e1006778. [Google Scholar] [CrossRef] [PubMed]
- Too, I.H.; Yeo, H.; Sessions, O.M.; Yan, B.; Libau, E.A.; Howe, J.L.; Lim, Z.Q.; Suku-Maran, S.; Ong, W.Y.; Chua, K.B.; et al. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci. Rep. 2016, 6, 36983. [Google Scholar] [CrossRef] [PubMed]
- Kisselev, L.L. Mammalian tryptophanyl-tRNA synthetases. Biochimie 1993, 75, 1027–1039. [Google Scholar] [CrossRef]
- Yeung, M.L.; Jia, L.; Yip, C.C.Y.; Chan, J.F.W.; Teng, J.L.L.; Chan, K.H.; Cai, J.P.; Zhang, C.; Zhang, A.J.; Wong, W.M.; et al. Human tryptophanyl-tRNA synthetase is an IFN-γ-inducible entry factor for Enterovirus. J. Clin. Investig. 2018, 128, 5163–5177. [Google Scholar] [CrossRef]
- Yuan, M.; Yan, J.; Xun, J.; Chen, C.; Zhang, Y.; Wang, M.; Chu, W.; Song, Z.; Hu, Y.; Zhang, S.; et al. Enhanced human enterovirus 71 infection by endocytosis inhibitors reveals multiple entry pathways by enterovirus causing hand-foot-and-mouth diseases. Virol. J. 2018, 15, 1. [Google Scholar] [CrossRef]
- Dang, M.; Wang, X.; Wang, Q.; Wang, Y.; Lin, J.; Sun, Y.; Li, X.; Zhang, L.; Lou, Z.; Wang, J.; et al. Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71. Protein Cell 2014, 5, 692–703. [Google Scholar] [CrossRef]
- Hussain, K.M.; Leong, K.L.; Ng, M.M.; Chu, J.J. The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71. J. Biol. Chem. 2011, 286, 309–321. [Google Scholar] [CrossRef]
- Mudhakir, D.; Harashima, H. Learning from the viral journey: How to enter cells and how to overcome intracellular barriers to reach the nucleus. AAPS J. 2009, 11, 65–77. [Google Scholar] [CrossRef]
- Lin, H.Y.; Yang, Y.T.; Yu, S.L.; Hsiao, K.N.; Liu, C.C.; Sia, C.; Chow, Y.H. Caveolar endocytosis is required for human PSGL-1-mediated enterovirus 71 infection. J. Virol. 2013, 87, 9064–9076. [Google Scholar] [CrossRef]
- Parton, R.G.; Howes, M.T. Revisiting caveolin trafficking: The end of the caveosome. J. Cell Biol. 2010, 191, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Hayer, A.; Stoeber, M.; Ritz, D.; Engel, S.; Meyer, H.H.; Helenius, A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010, 191, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Liu, Y.G.; Zhou, Y.T.; Zhao, P.; Ren, H.; Xiao, M.; Zhu, Y.Z.; Qi, Z.T. Endophilin-A2-mediated endocytic pathway is critical for enterovirus 71 entry into caco-2 cells. Emerg. Microbes Infect. 2019, 8, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Tung, W.H.; Lee, I.T.; Hsieh, H.L.; Yang, C.M. EV71 induces COX-2 expression via c-Src/PDGFR/PI3K/Akt/p42/p44 MAPK/AP-1 and NF-kappaB in rat brain astrocytes. J. Cell Physiol. 2010, 224, 376–386. [Google Scholar] [CrossRef]
- Bian, L.; Wang, Y.; Liu, Q.; Xia, J.; Long, J.E. Prediction of signaling pathways involved in enterovirus 71 infection by algorithm analysis based on miRNA profiles and their target genes. Arch. Virol. 2015, 160, 173–182. [Google Scholar] [CrossRef]
- Fu, F.; Zhao, J.; Xi, X. Identification of genes involved in enterovirus 71 infected SK-N-SH cells. Int. J. Clin. Exp. Pathol. 2017, 1, 11588–11595. [Google Scholar]
- Pleschka, S. RNA viruses and the mitogenic Raf/MEK/ERK signal transduction cascade. Biol. Chem. 2008, 389, 1273–1282. [Google Scholar] [CrossRef]
- Shi, W.; Hou, X.; Peng, H.; Zhang, L.; Li, Y.; Gu, Z.; Jiang, Q.; Shi, M.; Ji, Y.; Jiang, J. MEK/ERK signaling pathway is required for enterovirus 71 replication in immature dendritic cells. Virol. J. 2014, 11, 227. [Google Scholar] [CrossRef]
- Wang, C.; Wang, P.; Chen, X.; Wang, W.; Jin, Y. Saururus chinensis (Lour.) Baill blocks enterovirus 71 infection by hijacking MEK1-ERK signaling pathway. Antivir. Res. 2015, 119, 47–56. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef]
- Shi, W.; Hou, X.; Li, X.; Peng, H.; Shi, M.; Jiang, Q.; Liu, X.; Ji, Y.; Yao, Y.; He, C.; et al. Differential gene expressions of the MAPK signaling pathway in enterovirus 71-infected rhabdomyosarcoma cells. Braz. J. Infect. Dis. 2013, 17, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.R.; Chen, Y.Y.; Yang, S.M.; Chen, Y.L.; Horng, J.T. Phosphorylation of PI3K/Akt and MAPK/ERK in an early entry step of enterovirus 71. Life Sci. 2005, 78, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Diehl, N.; Schaal, H. Make yourself at home: Viral hijacking of the PI3K/Akt signaling pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.T.; Liu, H.J. PI3K-Akt signaling and viral infection. Recent Pat. Biotechnol. 2008, 2, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Eaton, H.E.; Saffran, H.A.; Wu, F.W.; Quach, K.; Smiley, J.R. Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J. Virol. 2014, 88, 7379–7388. [Google Scholar] [CrossRef]
- Izmailyan, R.; Hsao, J.C.; Chung, C.S.; Chen, C.H.; Su, P.W.; Liao, C.L.; Chang, W. Integrin β1 mediates vaccinia virus entry through activation of PI3K/Akt signaling. J. Virol. 2012, 86, 6677–6687. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, T.; Mo, G.; Zhang, Z.; Lin, L.; Zhang, X. ACSL1 Inhibits ALV-J Replication by IFN-I Signaling and PI3K/Akt Pathway. Front. Immunol. 2021, 12, 774323. [Google Scholar] [CrossRef]
- Gongora, C.; Mechti, N. Interferon signaling pathways. (Article in French). Bull. Cancer 1999, 86, 911–919. [Google Scholar]
- Wen, W.; Qi, Z.; Wang, J. The function and mechanism of enterovirus 71 (EV71) 3C protease. Curr. Microbiol. 2020, 77, 968–1975. [Google Scholar] [CrossRef]
- Visser, L.J.; Langereis, M.A.; Rabouw, H.H.; Wahedi, M.; Muntjewerff, E.M.; de Groot, R.J.; van Kuppeveld, F.J.M. Essential role of enterovirus 2A protease in counteracting stress granule formation and the induction of type I interferon. J. Virol. 2019, 93, e00222-19. [Google Scholar] [CrossRef]
- Wang, C.; Sun, M.; Yuan, X.; Ji, L.; Jin, Y.; Cardona, C.J.; Xing, Z. Enterovirus 71 suppresses interferon responses by blocking Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling through inducing karyopherin-α1 degradation. J. Biol Chem. 2017, 292, 10262–10274. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Zhang, W.; Zhang, C.; Sun, T.; Du, Y.; Ding, R.; Gao, Y.; Jin, Y.; Duan, G. MicroRNA-628-5p facilitates enterovirus 71 infection by suppressing TRAF3 signaling. Cell Mol. Immunol. 2021, 18, 1320–1322. [Google Scholar] [CrossRef]
- Good, C.; Wells, A.I.; Coyne, C.B. Type III interferon signaling restricts enterovirus 71 infection of goblet cells. Sci. Adv. 2019, 5, eaau4255. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M. Viral infection and apoptosis. Viruses 2017, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, Y.; Wu, H.L.; Chen, H.; Wu, H.; Guo, M.; Zhao, M.; Wang, C.; Lin, T.; Lin, Z.; et al. The inhibition of enterovirus 71 induced apoptosis by Durvillaea antarctica through P53 and STAT1 signaling pathway. J. Med. Virol. 2021, 93, 3532–3538. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Xu, T.; Guo, M.; Wang, C.; Zhao, M.; Chen, H.; Kuang, J.; Li, W.; Zhang, Y.; et al. Inhibition of enterovirus 71 by selenium nanoparticles loaded with siRNA through bax signaling pathways. ACS. Omega. 2020, 13, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lin, E.; He, L.; Yu, J.; Tan, P.; Zhou, Y. Autophagy and viral infection. Adv. Exp. Med. Biol. 2019, 1209, 55–78. [Google Scholar]
- Liu, Z.W.; Zhuang, Z.C.; Chen, R.; Wang, X.R.; Zhang, H.L.; Li, S.H.; Wang, Z.Y.; Wen, H.L. Enterovirus 71 VP1 protein regulates viral replication in SH-SY5Y cells via the mTOR autophagy signaling pathway. Viruses 2019, 12, 11. [Google Scholar] [CrossRef]
- Lin, Y.W.; Yu, S.L.; Shao, H.Y.; Lin, H.Y.; Liu, C.C.; Hsiao, K.N.; Chitra, E.; Tsou, Y.L.; Chang, H.W.; Sia, C.; et al. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS ONE 2013, 8, e57591. [Google Scholar] [CrossRef]
- Fujii, K.; Nagata, N.; Sato, Y.; Ong, K.C.; Wong, K.T.; Yamayoshi, S.; Shimanuki, M.; Shitara, H.; Taya, C.; Koike, S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14753–14758. [Google Scholar] [CrossRef]
- Pourianfar, H.R.; Poh, C.L.; Fecondo, J.; Grollo, L. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against Enterovirus 71. Virus Res. 2012, 169, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Mizuta, K.; Koike, S. Heparan sulfate attachment receptor is a major selection factor for attenuated enterovirus 71 mutants during cell culture adaptation. PLoS Pathog. 2020, 16, e1008428. [Google Scholar] [CrossRef] [PubMed]
- Su, P.Y.; Liu, Y.T.; Chang, H.Y.; Huang, S.W.; Wang, Y.F.; Yu, C.K.; Wang, J.R.; Chang, C.F. Cell surface sialylation affects binding of enterovirus 71 to rhabdomyosarcoma and neuroblastoma cells. BMC Microbiol. 2012, 12, 162. [Google Scholar] [CrossRef]
- Masomian, M.; Lalani, S.; Poh, C.L. Molecular Docking of SP40 Peptide towards Cellular Receptors for Enterovirus 71 (EV-A71). Molecules 2021, 26, 6576. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Tan, S.H.; Tan, K.O.; Lim, H.X.; Ong, K.C.; Wong, K.T.; Poh, C.L. Molecular mechanism of L-SP40 peptide and in vivo efficacy against EV-A71 in neonatal mice. Life Sci. 2021, 287, 120097. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chan, Y.F.; Sim, K.M.; Tan, E.L.; Poh, C.L. Inhibition of enterovirus 71 (EV-71) infections by a novel antiviral peptide derived from EV-71 capsid protein VP1. PLoS ONE 2012, 7, e34589. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin--a natural immunity molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [PubMed]
- Antoshin, A.A.; Shpichka, A.I.; Huang, G.; Chen, K.; Lu, P.; Svistunov, A.A.; Lychagin, A.V.; Lipina, M.M.; Sinelnikov, M.Y.; Reshetov, I.V.; et al. Lactoferrin as a regenerative agent: The old-new panacea. Pharmacol. Res. 2021, 167, 105564. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chu, C.; Chiu, C.H. Lactoferrin inhibits enterovirus 71 infection of human embryonal rhabdomyosarcoma cells in vitro. J. Infect. Dis. 2002, 186, 1161–1164. [Google Scholar] [CrossRef]
- Weng, T.Y.; Chen, L.C.; Shyu, H.W.; Chen, S.H.; Wang, J.R.; Yu, C.K.; Lei, H.Y.; Yeh, T.M. Lactoferrin inhibits enterovirus 71 infection by binding to VP1 protein and host cells. Antivir. Res. 2005, 67, 31–34. [Google Scholar] [CrossRef]
- Swain, S.P.; Mohanty, S. Imidazolidinones and Imidazolidine-2,4-diones as Antiviral Agents. ChemMedChem 2019, 14, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.W.; Racaniello, V.R. An antiviral compound that blocks structural transitions of poliovirus prevents receptor binding at low temperatures. J. Virol. 2000, 74, 3929–3931. [Google Scholar] [CrossRef]
- Shia, K.-S.; Li, W.-T.; Chang, C.-M.; Hsu, M.-C.; Chern, J.-H.; Leong, M.K.; Tseng, S.-N.; Lee, C.-C.; Lee, Y.-C.; Chen, S.-J.; et al. Design, synthesis, and structure-activity relationship of pyridyl imidazolidinones: A novel class of potent and selective human enterovirus 71 inhibitors. J. Med. Chem. 2002, 45, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.-H.; Shia, K.-S.; Hsu, T.-A.; Tai, C.-L.; Lee, C.-C.; Lee, Y.-C.; Chang, C.-S.; Tseng, S.-N.; Shih, S.-R. Design, synthesis, and structure-activity relationships of pyrazolo[3,4-d] pyrimidines: A novel class of potent enterovirus inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Perera, R.; Yap, M.L.; Cardosa, J.; Kuhn, R.J.; Rossmann, M.G. Structure of human enterovirus 71 in complex with a capsid-binding inhibitor. Proc. Natl. Acad. Sci. USA 2013, 110, 5463–5467. [Google Scholar] [CrossRef]
- Shih, S.-R.; Tsai, M.-C.; Tseng, S.-N.; Won, K.-F.; Shia, K.-S.; Li, W.-T.; Chern, J.-H.; Chen, G.-W.; Lee, C.-C.; Lee, Y.-C.; et al. Mutation in enterovirus 71 capsid protein VP1 confers resistance to the inhibitory effects of pyridyl imidazolidinone. Antimicrob. Agents Chemother. 2004, 48, 3523–3529. [Google Scholar] [CrossRef]
- Chen, T.-C.; Liu, S.-C.; Huang, P.-N.; Chang, H.-Y.; Chern, J.-H.; Shih, S.-R.J. Antiviral activity of pyridyl imidazolidinones against enterovirus 71 variants. Biomed. Sci. 2008, 15, 291–300. [Google Scholar] [CrossRef]
- Tan, Y.W.; Yam, W.K.; Kooi, R.J.W.; Westman, J.; Arbrandt, G.; Chu, J.J.H. Novel capsid binder and PI4KIIIbeta inhibitors for EV-A71 replication inhibition. Sci. Rep. 2021, 11, 9719. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, F.; Gu, B.; Ding, C.; Feng, D.; Xie, F.; Wang, J.; Zhang, C.; Cao, Q.; Deng, Y.; et al. In vitro and in vivo evaluation of ribavirin and pleconaril antiviral activity against enterovirus 71 infection. Arch. Virol. 2012, 157, 669–679. [Google Scholar] [CrossRef]
- Barnard, D.L.; Hubbard, V.D.; Smee, D.F.; Sidwell, R.W.; Watson, K.G.; Tucker, S.P.; Reece, P.A. In vitro activity of expanded-spectrum pyridazinyl oxime ethers related to pirodavir: Novel capsid-binding inhibitors with potent antipicornavirus activity. Antimicrob. Agents Chemother. 2004, 48, 1766–1772. [Google Scholar] [CrossRef]
- Tijsma, A.; Franco, D.; Tucker, S.; Hilgenfeld, R.; Froeyen, M.; Leyssen, P.; Neyts, J. The capsid binder Vapendavir and the novel protease inhibitor SG85 inhibit enterovirus 71 replication. Antimicrob. Agents Chemother. 2014, 58, 6990–6992. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Wakita, T.; Shimizu, H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008, 89, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Chiu, H.; Chan, Y.L.; Tsai, L.W.; Li, T.L.; Wu, C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012, 95, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.M.; Maugard, T.; Fruitier-Arnaudin, I. λ-Carrageenan Oligosaccharides of Distinct Anti-Heparanase and Anticoagulant Activities Inhibit MDA-MB-231 Breast Cancer Cell Migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef]
- Tseng, K.C.; Hsu, B.Y.; Ling, P.; Lu, W.W.; Lin, C.W.; Kung, S.H. Antidepressant Sertraline Is a Broad-Spectrum Inhibitor of Enteroviruses Targeting Viral Entry through Neutralization of Endolysosomal Acidification. Viruses 2022, 14, 109. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Thangavelu, A.; Stelin, K.S.; Vannala, V.; Mahabob, N.; Hayyan, F.M.B.; Sundaram, R. An Overview of Chitosan and Its Role in Periodontics. J. Pharm. Bioallied Sci. 2021, 13, S15–S18. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, S.T.; Chen, C.Y.; Wu, S.C. Enterovirus 71 adsorption on metal ion-composite chitosan beads. Biotechnol. Prog. 2012, 28, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xu, Z.C.; Zhang, F.; Cai, Y.; Chen, Y.N.; Lu, B.J.; Zhou, H.B.; Lan, K.; Wu, S.W. Identification of a novel binding inhibitor that blocks the interaction between hSCARB2 and VP1 of enterovirus 71. Cell Insight 2022, 1, 100016. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kang, H.J.; Jung, H.J.; Kim, K.; Lim, C.J.; Park, E.H. Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of Saururus chinensis extract. J. Ethnopharmacol. 2008, 120, 282–286. [Google Scholar] [CrossRef]
- Cho, H.Y.; Cho, C.W.; Song, Y.S. Antioxidative and anti-inflammatory effects of Saururus chinensis methanol extract in RAW 264.7 macrophages. J. Med. Food 2005, 8, 190–197. [Google Scholar] [PubMed]
- Gong, Q.Z.; Xiao, D.; Feng, F.; Wen, X.D.; Wei, Q. Ent-Sauchinone as Potential Anticancer Agent Inhibiting Migration and Invasion of Human Liver Cancer Cells via Suppressing the STAT3 Signaling Pathway. Chem. Biodivers. 2018, 15, e1800024. [Google Scholar] [CrossRef]
- Park, J.S.; Bang, O.S.; Kim, J. Screening of Stat3 inhibitory effects of Korean herbal medicines in the A549 human lung cancer cell line. Integr. Med. Res. 2014, 3, 67–73. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.Y.; Xu, Y.X.; Li, E. Effect and mechanism of Saururus chinensis against herpes dimplex virus. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2012, 37, 1642–1645. (In Chinese) [Google Scholar]
- Wang, B.; Zhang, H.; Zhu, M.; Luo, Z.; Peng, Y. MEK1-ERKs signal cascade is required for the replication of Enterovirus 71 (EV71). Antivir. Res. 2012, 93, 110–117. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhang, D.J.; Ge, M.; Li, Z.R.; Jiang, J.D.; Li, Y.H. Formononetin inhibits enterovirus 71 replication by regulating COX-2/PGE₂ expression. Virol. J. 2015, 12, 35. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Chen, X.; Song, N.; Wu, J.; Li, G.; Yu, P.; Han, Y.; Liu, J.; Qin, C. GS-9620 inhibits enterovirus 71 replication mainly through the NF-κB and PI3K-AKT signaling pathways. Antivir. Res. 2018, 153, 39–48. [Google Scholar] [CrossRef]
- Wang, H.; Li, K.; Ma, L.; Wu, S.; Hu, J.; Yan, H.; Jiang, J.; Li, Y. Berberine inhibits enterovirus 71 replication by downregulating the MEK/ERK signaling pathway and autophagy. Virol. J. 2017, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zheng, R.; Wu, H.L.; Chen, D.; Su, J.; Xu, T.; Wu, H.; Xiang, W.; Li, Y.; Zhu, B. Inhibition of enterovirus 71 infection by polysaccharides extracted from Picochlorum sp. 122 via the AKT and ATM/ATR signaling pathways. Arch. Virol. 2021, 166, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Ripa, I.; Andreu, S.; López-Guerrero, J.A.; Bello-Morales, R. Membrane R: Portals for viral entry. Front. Microbiol. 2021, 12, e631274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Wu, D.G.; Ren, H.; Xu, Q.Q.; Zheng, K.C.; Chen, W.; Chen, S.L.; Qian, X.J.; Tao, Q.Y.; Wang, Y.; et al. The role of lipid rafts in the early stage of Enterovirus 71 infection. Cell Physiol. Biochem. 2015, 35, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Luo, Y.J.; Wu, X.Y.; Si, B.Y.; Lin, L.; Zhu, Q.Y. Monoclonal antibody induced with inactived EV71-Hn2 virus protects mice against lethal EV71-Hn2 virus infection. Virol. J. 2010, 7, 106. [Google Scholar] [CrossRef]
- Ku, Z.; Ye, X.; Shi, J.; Wang, X.; Liu, Q.; Huang, Z. Single neutralizing monoclonal antibodies targeting the VP1 GH loop of enterovirus 71 inhibit both virus attachment and internalization during viral entry. J. Virol. 2015, 89, 12084–12095. [Google Scholar] [CrossRef]
- Lee, H.; Cifuente, J.O.; Ashley, R.E.; Conway, J.F.; Makhov, A.M.; Tano, Y.; Shimizu, H.; Nishimura, Y.; Hafenstein, S. A strain-specific epitope of enterovirus 71 identified by cryo-electron microscopy of the complex with fab from neutralizing antibody. J. Virol. 2013, 87, 11363–11370. [Google Scholar] [CrossRef]
- Lim, X.F.; Jia, Q.; Khong, W.X.; Yan, B.; Premanand, B.; Alonso, S.; Chow, V.T.; Kwang, J. Characterization of an isotype-dependent monoclonal antibody against linear neutralizing epitope effective for prophylaxis of enterovirus 71 infection. PLoS ONE 2012, 7, e29751. [Google Scholar] [CrossRef]
- Kiener, T.K.; Jia, Q.; Meng, T.; Chow, V.T.; Kwang, J. A novel universal neutralizing monoclonal antibody against enterovirus 71 that targets the highly conserved “knob” region of VP3 protein. PLoS Negl. Trop. Dis. 2014, 8, e2895. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Sun, M.; Ji, H.; Dou, H.; Hu, J.; Yan, Y.; Wang, X.; Chen, L. Honeysuckle-encoded microRNA2911 inhibits Enterovirus 71 replication via targeting VP1 gene. Antivir. Res. 2018, 152, 117–123. [Google Scholar] [CrossRef]
- Feng, C.; Fu, Y.; Chen, D.; Wang, H.; Su, A.; Zhang, L.; Chang, L.; Zheng, N.; Wu, Z. miR-127-5p negatively regulates enterovirus 71 replication by directly targeting SCARB2. FEBS Open Bio. 2017, 7, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Song, Z.; Qi, Y.; Feng, X.; Xu, N.; Sun, Y.; Wu, X.; Yao, X.; Mao, Q.; Li, X.; et al. Molecular determinants of enterovirus 71 viral entry: Cleft around GLN-172 on VP1 protein interacts with variable region on scavenge receptor B 2. J. Biol. Chem. 2012, 287, 6406–6420. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yu, X.; Wang, D.; Li, Z.; Zhou, Y.; Xu, G.; Yuan, B.; Qin, Y.; Chen, M. SLC35B2 acts in a dual role in the host sulfation required for EV71 infection. J. Virol. 2022, 96, e0204221. [Google Scholar] [CrossRef] [PubMed]
- Falah, N.; Montserret, R.; Lelogeais, V.; Schuffenecker, I.; Lina, B.; Cortay, J.C.; Violot, S. Blocking human enterovirus 71 replication by targeting viral 2A protease. J. Antimicrob. Chemother. 2012, 67, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Diarimalala, R.O.; Hu, M.; Wei, Y.; Hu, K. Recent advances of enterovirus 71 3Cpro targeting Inhibitors. Virol. J. 2020, 17, 173. [Google Scholar] [CrossRef]
- Yao, C.; Xi, C.; Hu, K.; Gao, W.; Cai, X.; Qin, J.; Lv, S.; Du, C.; Wei, Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018, 15, 116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).