Abstract
Human cytomegalovirus (HCMV) exploits host mitochondrial function to promote viral replication. HCMV gene products have been described to directly interact and alter functional or structural aspects of host mitochondria. Current antivirals against HCMV, such as ganciclovir and letermovir, are designed against viral targets. Concerns with the current antivirals include toxicity and viral resistance. Targeting host mitochondrial function is a promising alternative or complimentary antiviral approach as (1) drugs targeting host mitochondrial function interact with host targets, minimizing viral resistance, and (2) host mitochondrial metabolism plays key roles in HCMV replication. This review describes how HCMV alters mitochondrial function and highlights pharmacological targets that can be exploited for novel antiviral development.
1. Introduction
Viruses are obligate parasites and require remodeling of host pathways for efficient viral replication. Hijacking host metabolic pathways permits production of nucleic acids, proteins and lipids required for viral replication. Interfering with host metabolism should directly impact the virus, implicating metabolism as a key factor influencing viral infection and replication. Changes to glycolysis, oxidative phosphorylation (OXPHOS), fatty acid synthesis, glutaminolysis and other metabolic pathways have been characterized during infection and replication by many diverse viruses (reviewed in [1,2,3]). As viruses differ in structure, genetic backbone, and site of replication, diverse metabolic strategies have emerged, dependent on rate of replication and viral size. Reprogramming of metabolic pathways can impact immune response, apoptosis, and establish metabolically favorable conditions for efficient viral replication.
Human cytomegalovirus (HCMV) is a common herpesvirus exhibiting 40–80% seroprevalence. Infection in immunocompetent individuals is typically asymptomatic, but as with all herpesvirus infections, the virus is never cleared and enters a latent phase. Periodic reactivations occur throughout a lifespan and increase in frequency with age. Under immunosuppressed conditions, HCMV infection or reactivation may result in more severe pathology. This includes organ rejection and increased morbidity and mortality under transplantation settings. HCMV is also the leading viral cause of congenital infections.
HCMV is a master manipulator of host cellular metabolism [4]. Upon infection, HCMV upregulates glycolysis and glutaminolysis [5,6,7,8,9,10,11,12]. Metabolites derived from glycolysis and glutaminolysis enter the tricarboxylic acid (TCA) cycle, altering OXPHOS and electron transport chain (ETC) function [5,8,13,14,15,16]. A simultaneous increase in glycolysis and OXPHOS is reported in cancer stem cells and has been observed in diverse viral classes. The elevated carbon flux in the TCA cycle has been shown to support fatty acid synthesis [10,17,18,19]. The inhibition of host mitochondrial pathways negatively impacts HCMV replication [20]. An in-depth description on how metabolism is altered during HCMV replication and how HCMV manipulates host mitochondrial function can be found elsewhere [21,22]. Together, this suggests HCMV dependence on the host mitochondria for successful HCMV infection and replication. This also provides a target for development of novel antivirals.
Mitochondria are also associated with apoptotic pathways [23]. Apoptosis is a type of programmed cell death that is associated with decreased mitochondrial membrane potential (reviewed in [24]). HCMV has been described to prevent apoptosis through numerous mechanisms [25]. Specifically, HCMV can inhibit intrinsic apoptosis by targeting the Bcl-2 family and associated proteins located at the mitochondria [26,27]. This review includes intrinsic apoptosis pathways when related to changes in mitochondrial function. Further reading on the interplay between HCMV and apoptosis, can be found here [28,29].
HCMV antivirals are efficient and have a long history of clinical success. However, current drugs disrupt viral targets making them susceptible to viral resistance. By identifying and optimizing host targets that are essential for HCMV replication, viral resistance can be avoided. Targeting the host mitochondria as an antiviral could be utilized effectively as an adjunct treatment with current HCMV antivirals to reduce treatment duration and increase antiviral efficacy.
In this review, we discuss how HCMV alters host mitochondria function to enhance replication. We examine current and emerging evidence that shows the feasibility of targeting host mitochondria and how pharmacological compounds impact HCMV replication and mitochondrial or cellular health. Throughout this review, we provide examples specific to HCMV but which are likely applicable to many viral classes. As HCMV infection and cancer exhibit similar metabolic changes, we explore novel mitochondria-targeted approaches in the cancer field and apply the lessons learned to develop these treatments as antivirals.
2. HCMV Targets Host Mitochondria
There are five HCMV products currently defined to interact with the host mitochondria (Figure 1). These are HCMV pUL37x1, long non-coding β2.7 (lncβ2.7), pUL13, pUL15A, and US9 [13,14,30,31,32,33]. Strong evidence supporting the essential role of host mitochondria during HCMV replication was provided by infecting Rho cells with HCMV [8]. Human foreskin fibroblasts were used to generate Rho cells that are characterized as having depleted mitochondrial DNA (mtDNA). Rho cells do not have a functional ETC, but through media supplementation, maintain functional mitochondria. The Rho cells displayed 90–95% knock down of mtDNA as determined by ND1 (a mtDNA gene) expression. HCMV-infected Rho cells exhibited a 90% decrease in viral titers, emphasizing the importance of a functional ETC during HCMV infection and replication. Unsurprisingly, the HCMV gene products identified to interact with host mitochondria predominantly interact with the ETC function and/or OHPHOS. HCMV UL37x1 encodes viral mitochondria-localized inhibitor of apoptosis (pUL37x1 or vMIA) that has been described to bind the ETC ATPase inorganic phosphate carrier (PiC). This interaction decreases phosphate transport, resulting in lower ATP production [34]. pUL37x1 has been reported to promote mitochondrial membrane stability [35]. Further, HCMV UL37x1 knockout strains were reported to decrease mitochondrial respiration compared to wild-type strains, suggesting increased mitochondrial respiration is in part due to pUL37x1 [14]. pUL13 targets and remodels cristae, directly interacts with mitochondrial proteins inner membrane mitochondrial protein (IMMT) and coiled-coil-helix-coiled-coil-helix domain containing 3 (CHCHD3) and increases mitochondrial respiration [13]. HCMV US9 targets and disrupts mitochondrial membrane potential and integrity by interfering with translocase of outer membrane 20 (TOM20) and TOM70 [36]. Disruption of the membrane potential releases mitochondrial antiviral signaling protein (MAVS) from the outer mitochondrial membrane [36]. The disruption to MAVS is postulated to be a virus-induced immune evasion strategy. The mitochondrial targets of pUL15A are currently unknown, but localization with host mitochondria was observed late in infection (96 h post infection) [31].
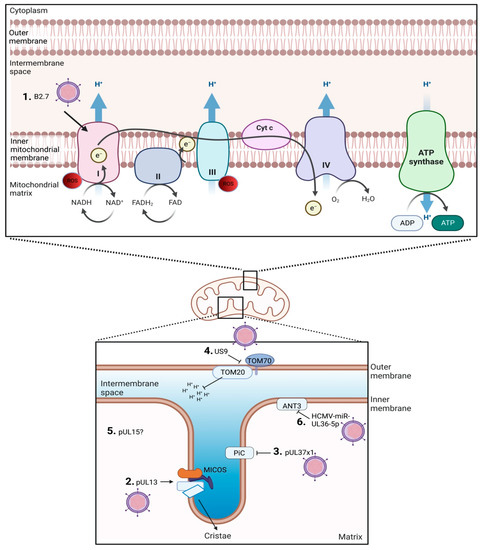
Figure 1.
Overview of HCMV gene products associated with altering host mitochondria function. 1. lncβ2.7 interacts with ETC complex I. 2. pUL13 stabilizes the MICOS complex. 3. pUL37x1 inhibits PiC. 4. US9 disrupts TOM. 5. pUL15 colocalizes with mitochondria but the specific target is unknown. 6. HCMV-miR-UL36-5p inhibits ANT.
Other HCMV products such as HCMV-miR-UL36-5p miRNA may target the mitochondria, but conclusive data are lacking. HCMV-miR-UL36-5p interacts with adenine nucleotide translocator 3 (ANT3), also termed SCL25A6, to inhibit cell death by decreasing ANT3 expression [37]. ANTs have been implicated to have roles in the mitochondrial permeability transition pore (mPTP). Interestingly, lncβ2.7 and pUL13 are expressed early during the HCMV infection and replication cycle, suggesting mitochondrial control and/or stability is a priority during HCMV replication [32,38].
3. ETC and Its Role in HCMV Replication
The ETC consists of transmembrane protein complexes embedded in the cristae, located in the mitochondrial inner membrane. Ubiquinone and cytochrome c (Cyt c) allow electron transfer between complexes. An in-depth review of ETC function is detailed elsewhere [39]. HCMV has been reported to increase ETC activity at transcriptional and protein levels [8,13,15,40]. An overview of the effect of mitochondrial disruptions on HCMV replication is provided in Table 1.

Table 1.
Effect on HCMV replication when altering ETC activity.
3.1. ETC Complex I: NADH-Ubiquinone Oxidoreductase
Complex I transfer electrons from the matrix NADH to ubiquinone. Electrons entering the ETC are transferred by iron–sulfur clusters to ubiquinone (Coenzyme Q10). The transfer of a pair of electrons in complex I (NADH to CoQ) induces translocation of protons from the matrix to the intermembrane space. Complex I may be of critical importance during HCMV replication as it regenerates oxidized NAD+ and is the entry point for electrons into the respiratory chain. Mutations of complex I subunits result in numerous clinical presentations including leukodystrophy, optic neuropathy, and encephalopathy (reviewed in [41,42]). Interestingly, leukodystrophy and congenital CMV are difficult to distinguish [43].
Targeting complex I of the ETC has shown success in mitigating CMV replication [20,32]. The addition of rotenone, a natural isoflavonoid and irreversible inhibitor of complex I, significantly decreased HCMV viral titers three days post infection [32]. Using U737 cells, rotenone addition during HCMV infection increased oxidative stress and decreased ATP production. The HCMV 2.7-kb lncβ2.7 was reported to colocalize with complex I, modulating metabolic intermediates [32]. lncβ2.7 was found to bind GRIM19, protecting infected cells from metabolic-stress-associated apoptosis. Infection with a HCMV lncβ2.7 knockout strain resembled rotenone-treated cells including changes to metabolism, highlighting a possible interaction between complex I and HCMV [32]. Cell cytotoxicity due to rotenone use was not significant. Further, HCMV lncβ2.7 stabilized mitochondrial membrane potential and maintained ATP production in infected cells. Significant decreases in mitochondrial membrane potential were measured when cells were infected with a knockout lncβ2.7 strain. This was similar to treating cells two hours post HCMV infection with a low dose of rotenone (0.1 µM), which reduced HCMV viral titers by 1 log at day 6 post infection [20]. Significant decreases in viral titer were reported beginning at day 4 post infection. Interestingly, expression of the viral protein immediate-early 1 (IE1) was unaffected by rotenone treatment, but expression of early (pp52) and late (pp28) viral proteins were delayed or reduced. Rotenone did decrease cell viability, potentially impacting results.
Metformin has been described to inhibit complex I or mediate metabolic changes through mTOR [44]. Preliminary studies show a single metformin treatment, 2 h post HCMV infection, decreased HCMV titers by 1 log [20]. When glucose levels in the culture medium were restricted (1 g/L versus 4.5 g/L), a 2.5 log reduction in HCMV titers was observed at day 6 post infection. The trends displayed reduced viral titers beginning at day 3 post infection and delayed expression of HCMV early and late proteins. HCMV-infected cells treated with metformin exhibited increased viability although the change was not significant. It is interesting to speculate on the efficacy of metformin if treatment was to be administered daily rather than a single dose shortly after initial infection.
3.2. ETC Complex II: Succinate Dehydrogenase
Complex II bridges metabolism and OXPHOS [45]. Complex II induces the oxidation of succinate to fumarate and is an entry point for electrons. Through iron–sulfur clusters, electrons from succinate are transferred to ubiquinone, permitting reduction to ubiquinol. Malonate is a three-carbon dicarboxylic acid and is considered a competitive inhibitor of complex II succinate dehydrogenase [46]. Diazoxide (DZX) is a mKATP-channel agonist that is known to inhibit complex II [47]. Malonate administration acts as a complex II inhibitor by mimicking DZX and decreasing ROS [48]. Although no direct experiments involving HCMV and ETC complex II have been reported, HCMV has demonstrated the ability to induce ROS production as infection persists [33,49]. Therefore, malonate’s inhibition of complex II and downregulation of ROS suggests that overexpressing malonate could inhibit the progression of the ETC and impact HCMV viral replication. This poses a potential target for future studies but has yet to be explored.
3.3. ETC Complex III: CoQ-Cytochrome c Reductase
Complex III transfers electrons from ubiquinol to Cyt-c. This reaction takes up two protons from the mitochondrial matrix and transfers four protons to the other side of the mitochondrial membrane. Antimycin A, an ETC complex III inhibitor, prevents electrons from transferring from Complex I or FADH2 to Cyt-c [50]. During HCMV infection, a single dose of Antimycin A given 2 h post infection resulted in a 2-log reduction in HCMV titers using HCMV TR, but only a 0.5-log change using HCMV Towne [20]. There were no significant changes in cell death associated with cytotoxicity. A mechanism to describe this difference between strains is unknown but suggests that HCMV laboratory strains may exhibit greater resistance to metabolic stress under in vitro conditions as titers were significantly decreased at day 3 post infection. Complimentary experiments involving daily treatments with Antimycin A would provide insight to these observations. Of note, when released from the mitochondria, Cyt-c plays a role in apoptosis by shuttling electrons from oxidative phosphorylation complex III to IV [51].
3.4. ETC Complex IV: Cytochrome c Oxidase
Complex IV generates H2O by transferring electrons from Cyt-c to a terminal electron acceptor (O2). An experimental design specifically targeting complex IV during HCMV infection is lacking, but studies using rubella virus described a decrease in complex IV activity [52]. Alternately, HIV infection of a CD4+ T cell line increased complex IV activity [53]. The changes were driven by virus-induced apoptosis as inhibition of complex IV using potassium cyanide reduced apoptosis using the same CD4+ T cell model. The role of complex IV during HCMV infection is currently a gap in knowledge that should be addressed.
3.5. Complex V: F1F0 ATPase Synthase
Complex V phosphorylates ADP to ATP. Electron transfer to monooxygen generates H2O and the pumping of protons from the matrix to the inner membrane space (via Complex I, III and IV as described above). Protons pass from the inner membrane space to the matrix transferring stored energy generated by the proton electrochemical gradient from F0 to F1. The induces a conformational change in F1F0 synthase, permitting ATP formation.
Oligomycin differs from other ETC inhibitors because it targets the proton channel of ATP synthase as opposed to directly inhibiting the ETC [50]. Oligomycin binds to a subunit of ATP synthase, preventing protons from passing back into the mitochondria and creating an unfavorable proton gradient for its operation [50,54]. The TCA cycle cannot adequately operate under these conditions, because NADH remains high and NAD+ is too low [54]. By inhibiting ATP synthesis in the mitochondrial matrix, oligomycin limits HCMV replication [20,55]. HCMV-infected cells were more sensitive to the effects of oligomycin than mock-infected cells [14]. This suggests that HCMV-infected cells exhibit greater respiration efficiency and may tolerate greater bioenergetic stress. Oligomycin resistance was more pronounced in cells infected with a HCMV strain lacking UL37x1 compared to the parental strain [14]. The increased susceptibility to the effects of oligomycin in HCMV-infected cells could indicate that pUL37x1 interacts with oligomycin. Further, the increase in respiration activity during HCMV infection was partially dependent on pUL37x1 [14]. Further experiments with HCMV pUL37x1 knockout strains suggest that expression is necessary for HCMV replication and the inhibition of apoptosis [56]. A single treatment of oligomycin after HCMV infection reduced viral titers by 1 log or resulted in no change at day 6 post infection using HCMV TR and Towne, respectively [20]. This result was not influenced by cytotoxicity.
3.6. ETC-Associated Mechanisms
The mitochondrial membrane potential has been reported to be hyperpolarized [8,32,57,58] or depolarized [36,59] following HCMV infection. Chloramphenicol uncouples the ETC and treatment was reported to reduce HCMV titers, but this was not driven by changes in respiration [14]. Addition of the uncoupling compound carbonyl cyanidetrifluoromethoxyphenylhydrazone (FCCP) did delay HCMV replication at day 3 post infection but did not significantly change HCMV viral titers at day 6 post infection [20]. Again, this was single dose, shortly after initial HCMV infection. The arteminisinin-derived compound BG95 has recently been shown to colocalize with the mitochondria and reduce membrane potential [60].
Other artemisinin derivatives, including the semisynthetic analogue artesunate, have been shown to be effective antivirals against HCMV [61,62,63]. Two bioactive excipients, poloxamer 188 and quercetin, were co-formulated to yield Quercetin-P188. When administered with ganciclovir, Quercetin-P188 was shown to synergistically enhance the efficacy of ganciclovir [64]. Interestingly, it was found to colocalize with mitochondria and P188 has been reported to protect mitochondria. Hyperpolarization has been reported to occur during mitochondrial stress and is accompanied with elevated levels of ROS [65,66]. Increased mitochondrial membrane potential and ROS, specifically mitochondrial-derived superoxide, has been reported during CMV infection [8,67]. Increased ROS is associated with inflammasome assembly and the production of the pro-inflammatory cytokine IL-1β. Experimental designs linking OXPHOS, mitochondrial membrane potential, ROS and inflammasome assembly are yet to be explored.
4. Mitochondrial Morphology and Its Role in CMV Replication
Mitochondrial fission and fusion are processes that maintain mitochondrial integrity. This cycle allows the transfer of gene products amongst mitochondria, ensuring optimal functioning. Stress or disruption of this cycle can contribute to various diseases.
Fragmented mitochondria are observed following HCMV infection [8,27,30,34,59]. HCMV pUL37x1 promotes mitochondrial fission by elevating Ca2+ levels in the mitochondria through induction of Ca2+ from the ER to the cytosol [30,35,58,68,69]. Dynamin-related protein 1 (Drp1) regulates fission and is recruited to the mitochondria during Ca2+ flux. Excess mitochondrial fission is often associated with the first steps of apoptosis [69]. Further, pUL37x1 was observed to recruit Bax, an apoptosis-inducing protein, to the mitochondria, disrupting the permeability of the outer mitochondrial membrane [70]. The fragmentation of mitochondria was also observed in DU145 cells and HCT116 Bax−/− cells [71]. These observations suggest pUL37x1 could be a potential drug target [72]. pUL37x1 disruptions to the mitochondria could potentially be inhibited with a Ca2+ chelating agent [69]. Changes in mitochondrial architecture and HCMV mechanisms underlying these changes have been elegantly shown to be dependent on protein posttranslational modifications [73,74]. HCMV pUL13 was shown to localize within the mitochondria by 48 h post infection [13]. Interactions between pUL13 and cristae-shaping proteins altered the mitochondrial architecture, increasing cellular respiration. Specifically, pUL13 interaction with the mitochondrial contact site and cristae organizing system (MICOS) was observed. Remodeling of the inner mitochondrial membrane occurs through interactions with the protein complex MICOS, ATP synthase and inner membrane phospholipids. Poor maintenance or stability of cristae membrane can impair mitochondrial function. pUL13 appears to stabilize MICOS as a mechanism to promote mitochondrial function during a period of extreme mitochondrial demand and stress.
6. Conclusions and Future Perspectives
The new therapeutic strategies described in this review still require thorough analysis on specificity, dosage, toxicity and efficacy prior to moving forward. The list of pathologies associated with HCMV continues to expand, despite the lack of mechanistic understanding. Glioblastoma is an ideal example. The presence of HCMV nucleic acids or proteins in glioblastoma remains controversial, but data suggest that treating patients diagnosed with glioblastoma with the antiviral valganciclovir nearly doubles the median overall survival [98]. Novel strategies to reduce HCMV viremia or viral burden efficiently and efficaciously could benefit patient care outside of individuals normally associated with HCMV susceptibility or disease.
The use of mitochondria-targeted antivirals would ideally be used synergistically with current antivirals. It is expected that coadministration of antiviral and mitochondria- targeted drugs would reduce the duration that patients require antiviral treatment. Theoretically, this would diminish the probability of antiviral resistance occurring and minimize toxicity issues due to prolonged antiviral use. Again, the efficacy and safety studies must be completed to validate this strategy. Collectively, this would improve patient care and ease the financial burden of the patient and to the healthcare system. With continued clinical evidence suggesting HCMV can impact the outcome of patients with cardiovascular disease [99,100], cancer [98,100] or dementia-related disease [97], the use of novel mitochondria-targeted antivirals could provide multiple benefits. A single therapeutic designed to target host mitochondrial pathways could provide benefits by (1) reducing the negative impact of localized or systemic effects of HCMV; and simultaneously (2) treating the primary pathology (i.e., cancer).
Another consideration is the impact of altered metabolism on HCMV latency or reactivation. Herpesviruses reactivation remains broadly defined as a response to stress. Mitochondria constantly communicate with the nucleus, relaying information of metabolic and oxidative stress. Changes in cellular homeostasis are detected by the mitochondria and changes to cellular signaling or function can be initiated to reset homeostasis. Some changes are mediated through epigenetic remodeling, a change that has been shown to be mediated by metabolites. Recent literature suggests that epigenetic status or ROS may impact HCMV reactivation or latency. The effect of mitochondria-targeted antivirals on these pathways is an interesting question that deserves further study. As many of the prospective drugs described in this review decrease mitochondrial membrane potential and drive apoptosis pathways, it is also intriguing to consider the effect on long-term infection. Should these drugs induce apoptosis and prevent HCMV replication, the virus would in theory be unable to replicate and spread. Could this be a strategy to eliminate HCMV or other herpesviruses from our body? The effects of this or reduced reactivation may contribute to healthier aging. To translate the potential outlined in this review into clinical practice, a greater focus on viral mitochondrial dependency and pharmacological interventions must be undertaken.
Author Contributions
Conceptualization, L.O.B. and K.J.Z.; writing original draft preparation, L.O.B. and K.J.Z.; writing review and editing, L.O.B. and K.J.Z.; supervision, K.J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was supported by NIGMS (P20GM103629) and NICHHD (1R01HD107790-01A1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
A data included in this review is publicly available (23 April 2023).
Acknowledgments
Figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Dsouza, L.; Yang, Z. Alteration in Cellular Signaling and Metabolic Reprogramming during Viral Infection. mBio 2021, 12, e0063521. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.M.; Xu, S.; Munger, J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015, 23, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Shenk, T.; Alwine, J.C. Human Cytomegalovirus: Coordinating Cellular Stress, Signaling, and Metabolic Pathways. Annu. Rev. Virol. 2014, 1, 355–374. [Google Scholar] [CrossRef]
- Munger, J.; Bajad, S.U.; Coller, H.A.; Shenk, T.; Rabinowitz, J.D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006, 2, e132. [Google Scholar] [CrossRef]
- Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 2011, 85, 1573–1580. [Google Scholar] [CrossRef]
- Yu, Y.; Maguire, T.G.; Alwine, J.C. ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1951–1956. [Google Scholar] [CrossRef]
- Combs, J.A.; Norton, E.B.; Saifudeen, Z.R.; Bentrup, K.H.Z.; Katakam, P.V.; Morris, C.A.; Myers, L.; Kaur, A.; Sullivan, D.E.; Zwezdaryk, K.J. Human Cytomegalovirus Alters Host Cell Mitochondrial Function during Acute Infection. J. Virol. 2020, 94, e01183-19. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, I.; Schafer, X.L.; Monaghan, M.; Munger, J. The Human Cytomegalovirus UL38 protein drives mTOR-independent metabolic flux reprogramming by inhibiting TSC2. PLoS Pathog. 2019, 15, e1007569. [Google Scholar] [CrossRef]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef]
- McArdle, J.; Schafer, X.L.; Munger, J. Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J. Virol. 2011, 85, 705–714. [Google Scholar] [CrossRef]
- Chambers, J.W.; Maguire, T.G.; Alwine, J.C. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010, 84, 1867–1873. [Google Scholar] [CrossRef]
- Betsinger, C.N.; Jankowski, C.S.R.; Hofstadter, W.A.; Federspiel, J.D.; Otter, C.J.; Jean Beltran, P.M.; Cristea, I.M. The human cytomegalovirus protein pUL13 targets mitochondrial cristae architecture to increase cellular respiration during infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101675118. [Google Scholar] [CrossRef]
- Kaarbo, M.; Ager-Wick, E.; Osenbroch, P.O.; Kilander, A.; Skinnes, R.; Muller, F.; Eide, L. Human cytomegalovirus infection increases mitochondrial biogenesis. Mitochondrion 2011, 11, 935–945. [Google Scholar] [CrossRef]
- Karniely, S.; Weekes, M.P.; Antrobus, R.; Rorbach, J.; van Haute, L.; Umrania, Y.; Smith, D.L.; Stanton, R.J.; Minczuk, M.; Lehner, P.J.; et al. Human Cytomegalovirus Infection Upregulates the Mitochondrial Transcription and Translation Machineries. mBio 2016, 7, e00029. [Google Scholar] [CrossRef]
- Federspiel, J.D.; Cook, K.C.; Kennedy, M.A.; Venkatesh, S.S.; Otter, C.J.; Hofstadter, W.A.; Jean Beltran, P.M.; Cristea, I.M. Mitochondria and Peroxisome Remodeling across Cytomegalovirus Infection Time Viewed through the Lens of Inter-ViSTA. Cell Rep. 2020, 32, 107943. [Google Scholar] [CrossRef]
- Koyuncu, E.; Purdy, J.G.; Rabinowitz, J.D.; Shenk, T. Saturated very long chain fatty acids are required for the production of infectious human cytomegalovirus progeny. PLoS Pathog. 2013, 9, e1003333. [Google Scholar] [CrossRef]
- Purdy, J.G.; Shenk, T.; Rabinowitz, J.D. Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication. Cell Rep. 2015, 10, 1375–1385. [Google Scholar] [CrossRef]
- Spencer, C.M.; Schafer, X.L.; Moorman, N.J.; Munger, J. Human cytomegalovirus induces the activity and expression of acetyl-coenzyme A carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication. J. Virol. 2011, 85, 5814–5824. [Google Scholar] [CrossRef]
- Combs, J.A.; Monk, C.H.; Harrison, M.A.A.; Norton, E.B.; Morris, C.A.; Sullivan, D.E.; Zwezdaryk, K.J. Inhibiting cytomegalovirus replication through targeting the host electron transport chain. Antiviral Res. 2021, 194, 105159. [Google Scholar] [CrossRef]
- Monk, C.H.; Zwezdaryk, K.J. Host Mitochondrial Requirements of Cytomegalovirus Replication. Curr. Clin. Microbiol. Rep. 2020, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tyl, M.D.; Betsinger, C.N.; Cristea, I.M. Virus-host protein interactions as footprints of human cytomegalovirus replication. Curr. Opin. Virol. 2022, 52, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Etherington, A.; Nobre, L.; Nightingale, K.; Antrobus, R.; Nichols, J.; Davison, A.J.; Stanton, R.J.; Weekes, M.P. Human cytomegalovirus protein pUL36: A dual cell death pathway inhibitor. Proc. Natl. Acad. Sci. USA 2020, 117, 18771–18779. [Google Scholar] [CrossRef] [PubMed]
- Collins-McMillen, D.; Kim, J.H.; Nogalski, M.T.; Stevenson, E.V.; Chan, G.C.; Caskey, J.R.; Cieply, S.J.; Yurochko, A.D. Human Cytomegalovirus Promotes Survival of Infected Monocytes via a Distinct Temporal Regulation of Cellular Bcl-2 Family Proteins. J. Virol. 2015, 90, 2356–2371. [Google Scholar] [CrossRef]
- Norris, K.L.; Youle, R.J. Cytomegalovirus proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2 family proteins. J. Virol. 2008, 82, 6232–6243. [Google Scholar] [CrossRef]
- Brune, W.; Andoniou, C.E. Die Another Day: Inhibition of Cell Death Pathways by Cytomegalovirus. Viruses 2017, 9, 249. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Ding, T.; Zhong, Z.; Hu, H.; Xu, Z.; Deng, J. Mitochondrial Dynamics Imbalance: A Strategy for Promoting Viral Infection. Front. Microbiol. 2020, 11, 1992. [Google Scholar] [CrossRef]
- McCormick, A.L.; Smith, V.L.; Chow, D.; Mocarski, E.S. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 2003, 77, 631–641. [Google Scholar] [CrossRef]
- Jean Beltran, P.M.; Mathias, R.A.; Cristea, I.M. A Portrait of the Human Organelle Proteome In Space and Time during Cytomegalovirus Infection. Cell Syst. 2016, 3, 361–373.e6. [Google Scholar] [CrossRef]
- Reeves, M.B.; Davies, A.A.; McSharry, B.P.; Wilkinson, G.W.; Sinclair, J.H. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 2007, 316, 1345–1348. [Google Scholar] [CrossRef]
- Perera, M.R.; Roche, K.L.; Murphy, E.A.; Sinclair, J.H. A Viral Long Non-Coding RNA Protects against Cell Death during Human Cytomegalovirus Infection of CD14+ Monocytes. Viruses 2022, 14, 246. [Google Scholar] [CrossRef]
- Poncet, D.; Pauleau, A.L.; Szabadkai, G.; Vozza, A.; Scholz, S.R.; Le Bras, M.; Briere, J.J.; Jalil, A.; Le Moigne, R.; Brenner, C.; et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Biol. 2006, 174, 985–996. [Google Scholar] [CrossRef]
- Goldmacher, V.S.; Bartle, L.M.; Skaletskaya, A.; Dionne, C.A.; Kedersha, N.L.; Vater, C.A.; Han, J.W.; Lutz, R.J.; Watanabe, S.; Cahir McFarland, E.D.; et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 1999, 96, 12536–12541. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, A.; Kang, S.; Lee, E.; Lee, T.A.; Ra, E.A.; Lee, J.; Lee, S.; Park, B. Human cytomegalovirus-encoded US9 targets MAVS and STING signaling to evade type I interferon immune responses. Nat. Commun. 2018, 9, 125. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Y.; Qi, Y.; Liu, Z.; Ma, Y.; Shao, Y.; Jiang, S.; Sun, Z.; Ruan, Q. Human cytomegalovirus miR-UL36-5p inhibits apoptosis via downregulation of adenine nucleotide translocator 3 in cultured cells. Arch. Virol. 2015, 160, 2483–2490. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Li, M.; Gao, S.; Wang, L.; Qi, Y.; Ji, Y.; Sun, Z.; Ruan, Q. Transcription characteristics of the human cytomegalovirus UL13 gene. Arch. Virol. 2013, 158, 473–477. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Hertel, L.; Mocarski, E.S. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J. Virol. 2004, 78, 11988–12011. [Google Scholar] [CrossRef]
- Rodenburg, R.J. Mitochondrial complex I-linked disease. Biochim. Biophys. Acta 2016, 1857, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Hock, D.H.; Robinson, D.R.L.; Stroud, D.A. Blackout in the powerhouse: Clinical phenotypes associated with defects in the assembly of OXPHOS complexes and the mitoribosome. Biochem. J. 2020, 477, 4085–4132. [Google Scholar] [CrossRef] [PubMed]
- Kameli, R.; Amanat, M.; Rezaei, Z.; Hosseionpour, S.; Nikbakht, S.; Alizadeh, H.; Ashrafi, M.R.; Omrani, A.; Garshasbi, M.; Tavasoli, A.R. RNASET2-deficient leukoencephalopathy mimicking congenital CMV infection and Aicardi-Goutieres syndrome: A case report with a novel pathogenic variant. Orphanet J. Rare Dis. 2019, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef]
- Kim, Y.S. Malonate metabolism: Biochemistry, molecular biology, physiology, and industrial application. J. Biochem. Mol. Biol. 2002, 35, 443–451. [Google Scholar] [CrossRef]
- Akopova, O.; Kolchinskaya, L.; Nosar, V.; Mankovska, I.; Sagach, V. Diazoxide affects mitochondrial bioenergetics by the opening of mKATP channel on submicromolar scale. BMC Mol. Cell Biol. 2020, 21, 31. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Brookes, P.S. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: Implications for ischemic preconditioning. Biochim. Biophys. Acta 2008, 1777, 882–889. [Google Scholar] [CrossRef]
- Speir, E.; Shibutani, T.; Yu, Z.X.; Ferrans, V.; Epstein, S.E. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ. Res. 1996, 79, 1143–1152. [Google Scholar] [CrossRef]
- Frizzell, N.; William, L. Bioenergetics and Oxidative Metabolism. In Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 93–110. [Google Scholar]
- Vempati, U.D.; Diaz, F.; Barrientos, A.; Narisawa, S.; Mian, A.M.; Millán, J.L.; Boise, L.H.; Moraes, C.T. Role of cytochrome C in apoptosis: Increased sensitivity to tumor necrosis factor alpha is associated with respiratory defects but not with lack of cytochrome C release. Mol. Cell. Biol. 2007, 27, 1771–1783. [Google Scholar] [CrossRef]
- Claus, C.; Schonefeld, K.; Hubner, D.; Chey, S.; Reibetanz, U.; Liebert, U.G. Activity increase in respiratory chain complexes by rubella virus with marginal induction of oxidative stress. J. Virol. 2013, 87, 8481–8492. [Google Scholar] [CrossRef]
- Tripathy, M.K.; Mitra, D. Differential modulation of mitochondrial OXPHOS system during HIV-1 induced T-cell apoptosis: Up regulation of Complex-IV subunit COX-II and its possible implications. Apoptosis 2010, 15, 28–40. [Google Scholar] [CrossRef]
- Maruf, A.; Lee, O.; O’Brien, P. Modifications of Mitochondrial Function by Toxicants. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Goldmacher, V.S. vMIA, a viral inhibitor of apoptosis targeting mitochondria. Biochimie 2002, 84, 177–185. [Google Scholar] [CrossRef]
- Landini, M.P.; Rugolo, M. Increased accumulation of a lipophilic cation (tetraphenylphosphonium) in human embryo fibroblasts after infection with cytomegalovirus. J. Gen. Virol. 1984, 65 Pt 12, 2269–2272. [Google Scholar] [CrossRef]
- Hong, C.T.; Chau, K.Y.; Schapira, A.H. The Cytomegalovirus protein pUL37x1 targets mitochondria to mediate neuroprotection. Sci. Rep. 2016, 6, 31373. [Google Scholar] [CrossRef]
- Crowe, W.E.; Maglova, L.M.; Ponka, P.; Russell, J.M. Human cytomegalovirus-induced host cell enlargement is iron dependent. Am. J. Physiol. Cell Physiol. 2004, 287, C1023–C1030. [Google Scholar] [CrossRef]
- Wild, M.; Hahn, F.; Grau, B.; Herrmann, L.; Niesar, A.; Schutz, M.; Lorion, M.M.; Ackermann, L.; Tsogoeva, S.B.; Marschall, M. The Artemisinin-Derived Autofluorescent Compound BG95 Exerts Strong Anticytomegaloviral Activity Based on a Mitochondrial Targeting Mechanism. Int. J. Mol. Sci. 2020, 21, 5578. [Google Scholar] [CrossRef]
- Hutterer, C.; Niemann, I.; Milbradt, J.; Frohlich, T.; Reiter, C.; Kadioglu, O.; Bahsi, H.; Zeittrager, I.; Wagner, S.; Einsiedel, J.; et al. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa B (NF-kappaB) pathway by targeting RelA/p65. Antiviral Res. 2015, 124, 101–109. [Google Scholar] [CrossRef]
- Hahn, F.; Frohlich, T.; Frank, T.; Bertzbach, L.D.; Kohrt, S.; Kaufer, B.B.; Stamminger, T.; Tsogoeva, S.B.; Marschall, M. Artesunate-derived monomeric, dimeric and trimeric experimental drugs—Their unique mechanistic basis and pronounced antiherpesviral activity. Antiviral Res. 2018, 152, 104–110. [Google Scholar] [CrossRef]
- Wild, M.; Bertzbach, L.D.; Tannig, P.; Wangen, C.; Muller, R.; Herrmann, L.; Frohlich, T.; Tsogoeva, S.B.; Kaufer, B.B.; Marschall, M.; et al. The trimeric artesunate derivative TF27 exerts strong anti-cytomegaloviral efficacy: Focus on prophylactic efficacy and oral treatment of immunocompetent mice. Antiviral Res. 2020, 178, 104788. [Google Scholar] [CrossRef]
- Luo, C.L.; Chen, X.P.; Li, L.L.; Li, Q.Q.; Li, B.X.; Xue, A.M.; Xu, H.F.; Dai, D.K.; Shen, Y.W.; Tao, L.Y.; et al. Poloxamer 188 attenuates in vitro traumatic brain injury-induced mitochondrial and lysosomal membrane permeabilization damage in cultured primary neurons. J. Neurotrauma 2013, 30, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Ramzan, R.; Wen, L.; Vogt, S. New extension of the Mitchell Theory for oxidative phosphorylation in mitochondria of living organisms. Biochim. Biophys. Acta 2010, 1800, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Ramzan, R.; Moosdorf, R.; Vogt, S. The role of mitochondrial membrane potential in ischemic heart failure. Mitochondrion 2011, 11, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.A.A.; Hochreiner, E.M.; Benjamin, B.P.; Lawler, S.E.; Zwezdaryk, K.J. Metabolic Reprogramming of Glioblastoma Cells during HCMV Infection Induces Secretome-Mediated Paracrine Effects in the Microenvironment. Viruses 2022, 14, 103. [Google Scholar] [CrossRef]
- Sharon-Friling, R.; Goodhouse, J.; Colberg-Poley, A.M.; Shenk, T. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. USA 2006, 103, 19117–19122. [Google Scholar] [CrossRef]
- Poncet, D.; Larochette, N.; Pauleau, A.L.; Boya, P.; Jalil, A.A.; Cartron, P.F.; Vallette, F.; Schnebelen, C.; Bartle, L.M.; Skaletskaya, A.; et al. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 2004, 279, 22605–22614. [Google Scholar] [CrossRef]
- Karbowski, M.; Norris, K.L.; Cleland, M.M.; Jeong, S.Y.; Youle, R.J. Role of Bax and Bak in mitochondrial morphogenesis. Nature 2006, 443, 658–662. [Google Scholar] [CrossRef]
- Arnoult, D.; Bartle, L.M.; Skaletskaya, A.; Poncet, D.; Zamzami, N.; Park, P.U.; Sharpe, J.; Youle, R.J.; Goldmacher, V.S. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 2004, 101, 7988–7993. [Google Scholar] [CrossRef]
- Murray, L.A.; Sheng, X.; Cristea, I.M. Orchestration of protein acetylation as a toggle for cellular defense and virus replication. Nat. Commun. 2018, 9, 4967. [Google Scholar] [CrossRef]
- Sheng, X.; Cristea, I.M. The antiviral sirtuin 3 bridges protein acetylation to mitochondrial integrity and metabolism during human cytomegalovirus infection. PLoS Pathog. 2021, 17, e1009506. [Google Scholar] [CrossRef]
- Cui, Q.; Wen, S.; Huang, P. Targeting cancer cell mitochondria as a therapeutic approach: Recent updates. Future Med. Chem. 2017, 9, 929–949. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M.; Ouari, O.; Lopez, M.; Joseph, J.; Zielonka, J.; Dwinell, M.B. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biol. 2018, 14, 316–327. [Google Scholar] [CrossRef]
- Rohlenova, K.; Sachaphibulkij, K.; Stursa, J.; Bezawork-Geleta, A.; Blecha, J.; Endaya, B.; Werner, L.; Cerny, J.; Zobalova, R.; Goodwin, J.; et al. Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2(high) Breast Cancer. Antioxid. Redox Signal. 2017, 26, 84–103. [Google Scholar] [CrossRef]
- Lim, S.C.; Carey, K.T.; McKenzie, M. Anti-cancer analogues ME-143 and ME-344 exert toxicity by directly inhibiting mitochondrial NADH: Ubiquinone oxidoreductase (Complex I). Am. J. Cancer Res. 2015, 5, 689–701. [Google Scholar]
- Wang, H.; Luo, J.; Tian, W.; Yan, W.; Ge, S.; Zhang, Y.; Sun, W. gamma-Tocotrienol inhibits oxidative phosphorylation and triggers apoptosis by inhibiting mitochondrial complex I subunit NDUFB8 and complex II subunit SDHB. Toxicology 2019, 417, 42–53. [Google Scholar] [CrossRef]
- Dong, L.F.; Jameson, V.J.; Tilly, D.; Prochazka, L.; Rohlena, J.; Valis, K.; Truksa, J.; Zobalova, R.; Mahdavian, E.; Kluckova, K.; et al. Mitochondrial targeting of alpha-tocopheryl succinate enhances its pro-apoptotic efficacy: A new paradigm for effective cancer therapy. Free Radic. Biol. Med. 2011, 50, 1546–1555. [Google Scholar] [CrossRef]
- Dong, L.F.; Low, P.; Dyason, J.C.; Wang, X.F.; Prochazka, L.; Witting, P.K.; Freeman, R.; Swettenham, E.; Valis, K.; Liu, J.; et al. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene 2008, 27, 4324–4335. [Google Scholar] [CrossRef]
- Cheng, G.; Zielonka, J.; Dranka, B.P.; McAllister, D.; Mackinnon, A.C., Jr.; Joseph, J.; Kalyanaraman, B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012, 72, 2634–2644. [Google Scholar] [CrossRef]
- Alam, M.M.; Sohoni, S.; Kalainayakan, S.P.; Garrossian, M.; Zhang, L. Cyclopamine tartrate, an inhibitor of Hedgehog signaling, strongly interferes with mitochondrial function and suppresses aerobic respiration in lung cancer cells. BMC Cancer 2016, 16, 150. [Google Scholar] [CrossRef]
- Sohoni, S.; Ghosh, P.; Wang, T.; Kalainayakan, S.P.; Vidal, C.; Dey, S.; Konduri, P.C.; Zhang, L. Elevated Heme Synthesis and Uptake Underpin Intensified Oxidative Metabolism and Tumorigenic Functions in Non-Small Cell Lung Cancer Cells. Cancer Res. 2019, 79, 2511–2525. [Google Scholar] [CrossRef]
- Bridges, H.R.; Jones, A.J.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Romero, I.L.; Litchfield, L.M.; Lengyel, E.; Locasale, J.W. Metformin Targets Central Carbon Metabolism and Reveals Mitochondrial Requirements in Human Cancers. Cell Metab. 2016, 24, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Janzer, A.; German, N.J.; Gonzalez-Herrera, K.N.; Asara, J.M.; Haigis, M.C.; Struhl, K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 10574–10579. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Andrzejewski, S.; Gravel, S.P.; Pollak, M.; St-Pierre, J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014, 2, 12. [Google Scholar] [CrossRef]
- Peiris-Pages, M.; Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Mitochondrial fission as a driver of stemness in tumor cells: mDIVI1 inhibits mitochondrial function, cell migration and cancer stem cell (CSC) signalling. Oncotarget 2018, 9, 13254–13275. [Google Scholar] [CrossRef]
- Dong, L.; Neuzil, J. Targeting mitochondria as an anticancer strategy. Cancer Commun. 2019, 39, 63. [Google Scholar] [CrossRef]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Roth, K.G.; Mambetsariev, I.; Kulkarni, P.; Salgia, R. The Mitochondrion as an Emerging Therapeutic Target in Cancer. Trends Mol. Med. 2020, 26, 119–134. [Google Scholar] [CrossRef]
- Kalainayakan, S.P.; Ghosh, P.; Dey, S.; Fitzgerald, K.E.; Sohoni, S.; Konduri, P.C.; Garrossian, M.; Liu, L.; Zhang, L. Cyclopamine tartrate, a modulator of hedgehog signaling and mitochondrial respiration, effectively arrests lung tumor growth and progression. Sci. Rep. 2019, 9, 1405. [Google Scholar] [CrossRef]
- Barnes, L.L.; Capuano, A.W.; Aiello, A.E.; Turner, A.D.; Yolken, R.H.; Torrey, E.F.; Bennett, D.A. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J. Infect. Dis. 2015, 211, 230–237. [Google Scholar] [CrossRef]
- Stragliotto, G.; Rahbar, A.; Solberg, N.W.; Lilja, A.; Taher, C.; Orrego, A.; Bjurman, B.; Tammik, C.; Skarman, P.; Peredo, I.; et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: A randomized, double-blind, hypothesis-generating study. Int. J. Cancer 2013, 133, 1204–1213. [Google Scholar] [CrossRef]
- Jia, Y.J.; Liu, J.; Han, F.F.; Wan, Z.R.; Gong, L.L.; Liu, H.; Zhang, W.; Wardell, T.; Lv, Y.L.; Liu, L.H. Cytomegalovirus infection and atherosclerosis risk: A meta-analysis. J. Med. Virol. 2017, 89, 2196–2206. [Google Scholar] [CrossRef]
- Samson, L.D.; van den Berg, S.P.; Engelfriet, P.; Boots, A.M.; Hendriks, M.; de Rond, L.G.; de Zeeuw-Brouwer, M.L.; Verschuren, W.M.; Borghans, J.A.; Buisman, A.M.; et al. Limited effect of duration of CMV infection on adaptive immunity and frailty: Insights from a 27-year-long longitudinal study. Clin. Transl. Immunol. 2020, 9, e1193. [Google Scholar] [CrossRef]
- Foster, H.; Piper, K.; DePledge, L.; Li, H.F.; Scanlan, J.; Jae-Guen, Y.; Boeckh, M.; Cobbs, C. Human cytomegalovirus seropositivity is associated with decreased survival in glioblastoma patients. Neurooncol. Adv. 2019, 1, vdz020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).