Unraveling the Molecular and Cellular Pathogenesis of COVID-19-Associated Liver Injury
Abstract
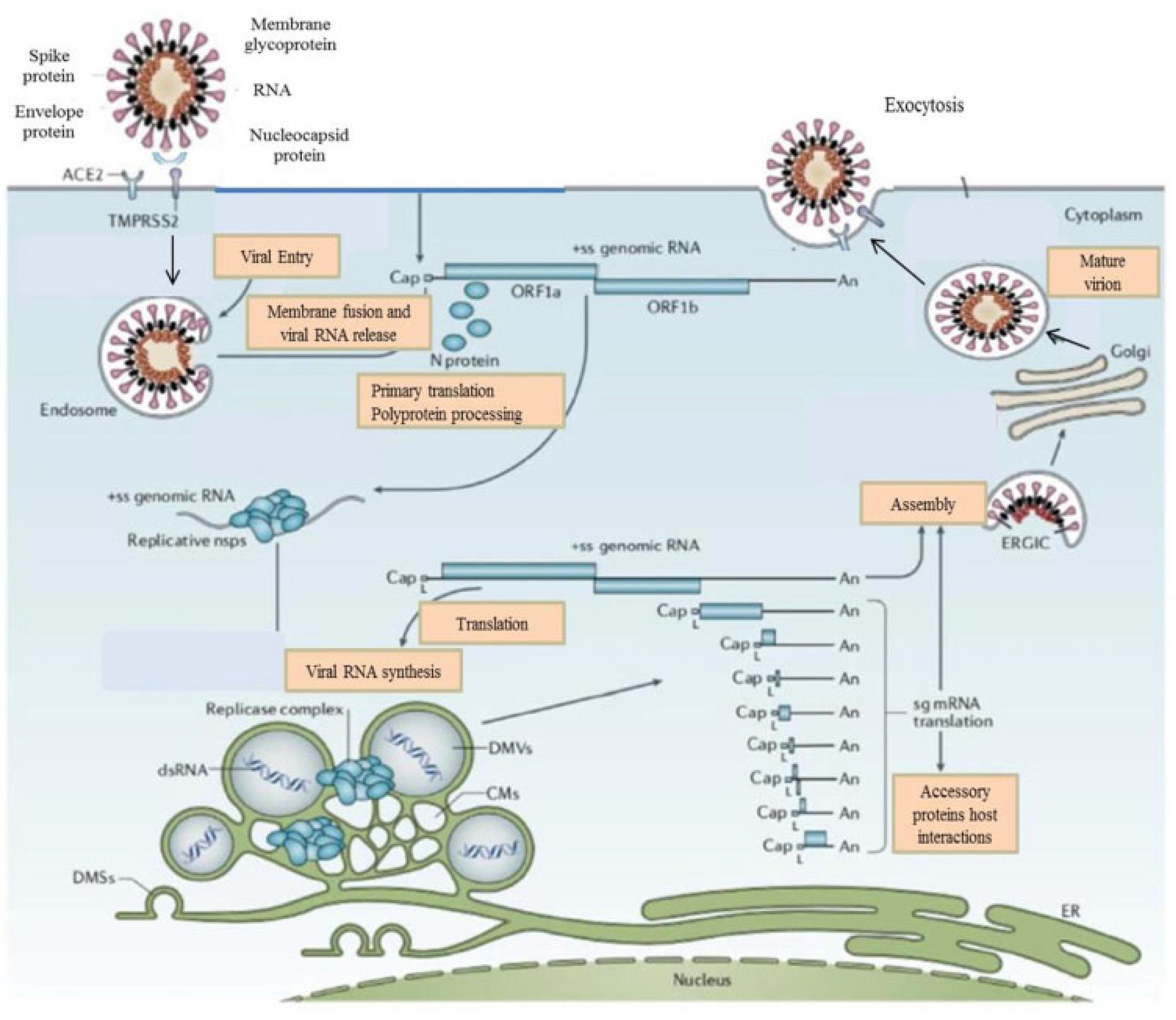
:1. Introduction
2. Mechanisms Involved in Liver Injury in COVID-19
2.1. Direct Cytotoxicity
2.2. Vascular Alterations following COVID-19 in Liver
2.3. The Impact of Immunological and Inflammatory Processes of COVID-19 on the Liver
2.4. Liver Injury following COVID-19 Vaccination
2.5. Drug-Induced Liver Injury
3. SARS-CoV-2 Infection in Special Populations with CLD
3.1. Cirrhosis
3.2. Non-Alcoholic Fatty Liver Disease
3.3. Chronic Viral Hepatitis
3.4. Liver Transplantation
3.5. Hepatocellular Carcinoma
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.; Kanneganti, T.-D. Innate immunity: The first line defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.D. The “cytokine Storm”: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases 0f 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with Novel Coronavirus-infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Akkız, H. Implications of the Novel Mutations in the SARS-CoV-2 Genome for Transmission, Disease Severity, and the Vaccine Development. Front. Med. 2021, 6, 636532. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar]
- Anderson, K.G.; Rombout, A.; Lipkin, W.I.; Holmes, E.C.; Gerry, R.E. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–455. [Google Scholar] [CrossRef]
- V’kavski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020, 19, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Akkız, H. The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Concern. Front. Med. 2022, 9, 849217. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fisher, W.M.; Gnanakaran, S.; Yoon, H.; Thelier, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking changes in SARS-CoV-2 spike: Evidencee that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhol, J.; Breugem, T.L.; Ravelli, R.B.G.; Schayck, J.P.V.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 1126, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Brandi, N.; Ciccorese, F.; Rimondi, M.R.; Balacchi, C.; Modolon, C.; Sportoletti, C.; Renzulli, M.; Coppola, F.; Golfieri, R. An Imaging Overview of COVID-19 ARDS in ICU Patients and Its Complications: A Pictorial Review. Diagnostics 2022, 12, 846. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Muti, N.D.; Finocchi, F.; Tovetta, G.; Salvio, G.; Cutini, M.; Marzioni, D.; Balercia, G. Could SARS-CoV-2 infection affect male fertility and sexuality? APMIS 2022, 130, 243–252. [Google Scholar] [CrossRef]
- Kumar, M.P.; Miskra, S.; Jha, D.K.; Shukla, J.; Choudhury, A.; Mohindra, R.; Mandavdhare, H.S.; Dutta, U.; Sharma, V. Coronavirus disease 19 (COVID-19) and the Liver: A comprehensive systematic review and meta-analysis. Hepatol. Int. 2020, 14, 711–722. [Google Scholar] [CrossRef]
- Marjot, T.; Eberhardt, C.S.; Boettler, T.; Belli, L.S.; Berenguer, M.; Buti, M.; Jalan, R.; Mondelli, M.U.; Moreau, R.; Shouval, D.; et al. Impact of COVID-19 on the liver and the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: An updated EASL position paper. J. Hepatol. 2022, 77, 1161–1197. [Google Scholar]
- Li, D.; Ding, X.; Xie, M.; Tian, D.; Xia, L. COVID-19-associated liver injury: From bedside to bench. J. Gastroenterol. 2021, 56, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-K.; Yu, X.-L.; Zhou, L.-Y.; Si, H.-M.; Hui, J.-F.; Hou, D.-Y.; Li, W.-P.; Yang, J.-S. COVID-19 and liver dysfunction: What nutritionists need to know. World J. Gastroenterol. 2022, 28, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Aghemo, A.; Forner, A.; Valenti, L. COVID-19 and liver disease. Liver Int. 2020, 40, 1278–1281. [Google Scholar] [CrossRef]
- Saviano, A.; Wrensch, F.; Ghany, M.G.; Baumert, T.F. Liver Disease and Coronavirus Disease 2019, from Pathogenesis to Clinical Care. Hepatology 2021, 74, 1088–1100. [Google Scholar] [CrossRef]
- Luo, M.; Ballester, M.P.; Soffientini, U.; Jalan, R.; Mehta, G. SARS-CoV-2 infection and liver involvement. Hepatol. Int. 2022, 16, 755–774. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Huang, G.; Thuluvath, P.J.; Satapathy, S.K. Elevated Liver Biochemistries in Hospitalized Chinese Patients with Sever COVID-19: Systemic Review and Meta-analysis. Hepatology 2021, 73, 1521–1530. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef]
- Yaugel-Novoa, M.; Bourlet, T.; Paul, S. Role of the humoral immune response during COVID-19: Guilty or not guilty. Nat. Immunol. 2022, 15, 1170–1180. [Google Scholar] [CrossRef]
- Kanneganti, T.D. Intracellular innate immune receptors: Life inside the cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Imura, I.; Uriu, K.; Fukushi, M.; Irie, T.; Koyanagi, Y.; Sauter, D.; Gifford, R.J.; Nakagawa, S.; Sato, K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020, 32, 108–185. [Google Scholar] [CrossRef]
- Li, J.; Liao, C.H.; Wang, Q.; Tan, Y.J.; Luo, R.; Qiu, Y.; Ge, X.Y. The ORF6, ORF8, and nucleocapsid proteins of SARS-CoV-2 inhibit the type 2 interferon signaling pathway. Virus Res. 2020, 286, 198074. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives the development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type 1 interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, R.; Bai, T.; Han, P.; He, Q.; Jing, M.; Xiong, X.; Zhao, X.; Quan, R.; Chen, C.; et al. Clinical Features of Patients Infected with Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study. Hepatology 2021, 73, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.M.; Barraza, L.H.; LaSota, E.D.; Sobieszczyk, M.E.; Pereira, M.R.; Zheng, E.X.; Fox, A.N.; Zucker, J.; Verna, E.C. Acute liver injury in COVID-19: Prevalence Association with Clinical Outcomes in a large, U.S. Cohort. Hepatology 2020, 72, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Jathimoni, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Heucke, N.; Keitel, V. COVID-19-associated c3holangiopathy: What is left after the virus has gone? Hepatology 2022, 76, 1560–1562. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Labenz, C.; Wörns, M.A.; Menge, P.; Weinmann, A.; Galle, P.R.; Sprinzl, M.F. Patterns of liver injury—A German case series. United Eur. Gastroenterol. J. 2020, 8, 814–819. [Google Scholar] [CrossRef]
- Lenti, M.V.; Borrelli de Andreis, F.; Pellegrino, I.; Klersy, C.; Merli, S.; Micelli, E.; Aronico, N.; Mengoli, C.; Di Stefano, M.; Cococcia, S.; et al. Impact of COVID-19 on liver function: Results from an internal medicine unit in Northern Italy. Intern. Emerg. Med. 2020, 15, 1399–1407. [Google Scholar] [CrossRef]
- Mantovani, A.; Beatrice, G.; Dalbeni, A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020, 40, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Haslinger, K.; Angerer, M.; Semmier, G.; Schneeweiss-Gleixner, M.; Jachs, M.; Simbrunner, B.; Bauer, D.J.M.; Eigenbauer, E.; Strassl, R.; et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology 2022, 76, 1563–1575. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, D.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Li, Z.; Zhou, G.; Gou, J.; Qu, J.; et al. COVID-19: Abnormal liver function tests. J. Hepatol. 2020, 73, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.F.; Marjot, T.; Becchetti, C.; Tilg, H. COVID-19, and liver disease. Gut 2021, 71, 326792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ni, C.; Gao, R.; Wang, Y.; Yang, L.; Wei, J.; Lv, T.; Liang, J.; Zhang, Q.; Xu, W.; et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020, 11, 771–775. [Google Scholar] [CrossRef]
- Lui, V.C.; Hui, K.P.; Babu, R.O.; Yue, H.; Chung, P.H.; Tam, P.K.; Chan, M.C.; Wong, K.K. Human liver organoid-derived intra-hepatic bile duct cells support SARS-CoV-2 infection and replication. Sci. Rep. 2022, 12, 5375. [Google Scholar] [CrossRef]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021, 41, 20–32. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Bain, W.; Naqvi, A.; Staines, B.; Castanha, P.M.S.; Yang, H.; Boltz, V.F.; Barratt-Boyes, S.; Marques, E.T.A.; Mitchell, S.L.; et al. Severe acute respiratory syndrome coronavirus 2 viremias are associated with coronavirus disease 2019 severity and predict clinical outcomes. Clin. Infect. Dis. 2022, 74, 1525–1533. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020, 27, 125–136e7. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.; Yuen, T.T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Bangash, M.N.; Patel, J.M.; Parekh, D.; Murphy, N.; Brown, R.M.; Elsharkawy, A.M.; Mehta, G.; Armstrong, M.J.; Neil, D. SARS-CoV-2: Is the liver merely a bystander to severe disease? J. Hepatol. 2020, 73, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Bruin, S.; Groen, A.K.; Nieuwdorp, M.; Herrema, H. Increased expression of key SARS-CoV-2 entry points in multiple tissues in individuals with NAFLD. J. Hepatol. 2021, 74, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Paizis, G.; Tikellis, C.; Cooper, M.E.; Schembri, J.M.; Lew, R.A.; Smith, A.I.; Shaw, T.; Warner, F.J.; Zuilli, A.; Burrell, L.M.; et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin-converting enzyme 2. Gut 2005, 54, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Warner, F.J.; Rajapaksha, H.; Shackel, N.; Herath, C.B. ACE2: From the protection of liver disease to the propagation of COVID-19. Clin. Sci. 2020, 134, 3137–3158. [Google Scholar]
- McCarron, S.; Bathon, B.; Conlon, D.M.; Abbey, D.; Rader, D.J.; Gawronski, K.; Brown, C.D.; Olthoff, K.M.; Shaked, A.; Raabe, T.D. Functional characterization of organoids from the irreversibly damaged liver of patients with NASH. Hepatology 2021, 74, 1825–1844. [Google Scholar] [CrossRef]
- Papic, N.; Pangercic, A.; Vargovic, M.; Vince, A.; Kuzman, I. Liver involvement during influenza infection: Prospective on the 2009 influenza pandemic. Influenza Other Respir. Viruses 2012, 6, e2–e5. [Google Scholar] [CrossRef] [PubMed]
- Shafran, N.; Issachar, A.; Shochat, T.; Shafran, I.H.; Bursztyn, M.; Shlomai, A. Abnormal liver tests in patients with SARS-CoV-2 and influenza-prognostic similarities and temporal disparities. JHEP Rep. 2021, 3, 100258. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2020, 18, 194–209. [Google Scholar]
- McConnell, M.J.; Kawaguchi, N.; Kondo, R.; Sonzogni, A.; Licini, L.; Valle, C.; Bonaffini, P.A.; Sironi, S.; Alessio, M.G.; Previtali, G.; et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced enthoeliopathy. J. Hepatol. 2021, 75, 647–658. [Google Scholar] [CrossRef]
- Sonzogni, A.; Previtali, G.; Seghezzi, M.; Alessio, G.; Gianatti, A.; Licini, L.; Morotti, D.; Zerbi, P.; Carsana, L.; Rossi, R.; et al. Liver histopathology in severe COVID-19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020, 40, 2110–2116. [Google Scholar] [CrossRef]
- Falasca, I.; Nardacci, R.; Colombo, D.; Lalle, E.; Di Caro, A.; Nicastri, E.; Antinori, A.; Petrosillo, N.; Marchioni, L.; Biava, G.; et al. Postmortem findings in Italian patients with COVID-19: A descriptive full autopsy study of cases with and without comorbidities. J. Infect. Dis. 2020, 222, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.; Baumert, T.F. Unraveling the role of liver sinusoidal endothelial cells in COVID-19 liver injury. J. Hepatol. 2021, 75, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-center, cross-sectional study. Lancet 2020, 7, 575–582. [Google Scholar] [CrossRef]
- Chornenkyy, Y.; Mejia-Bautista, M.; Brucal, M.; Blanke, T.; Dittmann, D.; Yeldandi, A.; Boike, J.R.; Lomasney, J.W.; Nayar, R.; Jennings, L.J.; et al. Liver pathology and SARS-CoV-2 detection in formalin-fixed tissue of patients with COVID-19. Am. J. Clin. Pathol. 2021, 155, 802–814. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Eng. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar]
- Schimidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar]
- Nishimoto, N.; Terao, K.; Mima, T.; Nakahara, H.; Takagi, N.; Kakehi, T. Mechanisms and pathologic significances in the increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008, 112, 3959–3964. [Google Scholar] [CrossRef]
- Patra, T.; Meyer, K.; Geerling, L.; Isbell, T.S.; Hoft, D.F.; Brien, J.; Pinto, A.K.; Ray, R.B.; Ray, R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling on epithelial cells. PLoS Pathog. 2020, 16, 1009128. [Google Scholar] [CrossRef]
- Zarbock, A.; Ley, K. Neutrophil adhesion and activation under flow. Microcirculation 2009, 16, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Wang, I.J.; Li, H.; Yuan, L.T.; Gale, R.P.; Liang, Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): A meta-analysis. Leukemia 2021, 35, 2616–2620. [Google Scholar] [CrossRef] [PubMed]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Moon, C. Fighting COVID-19 exhausts T cells. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Notarbartolo, S.; Ranzani, V.; Bandera, A.; Gruarin, P.; Bevilacqua, V.; Putignano, A.R.; Gobbini, A.; Galeota, E.; Manara, C.; Bombaci, M.; et al. Integrated longitudinal immunotherapeutic, transcriptional, and repertoire analysis delineate immune responses in COVID-19 patients. Sci. Immunol. 2021, 6, eabg5021. [Google Scholar] [CrossRef]
- Graham, M.B.; Braciale, V.L.; Braciale, T.J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 1994, 180, 1273–1282. [Google Scholar] [CrossRef]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multomics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell 2020, 183, 1479–1495. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analysis reveals immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Cai, S.; Zhuang, Z.; Zhao, Z.; Jin, S.; Xie, W.; Zhou, L.; Zhang, L.; Zhao, J.; et al. RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-κB hyper-activation and inflammation. Signal Trnsduct. Target Ther. 2021, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Lonati, P.; Grossi, C.; Borghi, M.O.; Novembrino, C.; et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2021, 116, 102560. [Google Scholar] [CrossRef]
- Gimpel, A.K.; Maccataio, A.; Unterweger, H.; Sokolova, M.V.; Schett, G.; Steffen, U. IgA complexes induce neutrophil extracellular trap formation more potently than IgG complexes. Front. Immunol. 2022, 12, 761816. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.D.; Golubeva, D.; Posca, A.; Ang, J.C.; Novakowski, K.E.; Zahoor, M.A.; Kaushic, C.; Cairns, E.; Bowdish, D.M.E.; Mullarkey, C.E.; et al. IgA potentiates NETosis in response to viral infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101497118. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19, and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artendi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef]
- Sgamato, C.; Rocco, A.; Compare, D.; Minieri, S.; Marchitto, S.A.M.; Maurea, S.; Nardone, G. Autoimmune liver diseases and SARS-CoV-2. World J. Gastroenterol. 2023, 29, 1838–1849. [Google Scholar] [CrossRef]
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.-A. SARS-CoV-2 triggering autoimmune diseases. Cytokine 2022, 154, 155873. [Google Scholar] [CrossRef]
- Angileri, F.; Legare, S.; Marino Gammazza, A.; Conway de Macario, E.; Macario, J.L.; Cappello, F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020, 19, 10259. [Google Scholar] [CrossRef] [PubMed]
- Marino Gammazza, A.; Légaré, S.; Lo Bosco, G.; Fucarino, A.; Angileri, F.; Conway de Macario, E.; Macario, A.J.; Cappello, F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes capable of eliciting autoimmunity against endothelial cells: Possible role of molecular mimicry in COVID-19. Cell Stress Chaperones 2020, 28, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respirat. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, T.; Xu, Y.; Lu, X.; Sang, X. Autoimmune hepatitis after COVID-19 vaccination. Front. Immunol. 2022, 13, 1035073. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Efficacy and safety of the mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszezyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Teijero, J.R.; Forber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Dhanasekaran, R.; Lammert, C.; Ebik, C.; Higuera-de la Tijera, F.; Aloman, C.; Calışkan, A.R.; Peralta, M.; Gerussi, A.; Massoumi, H.; et al. Outcome of COVID-19 in Patients with Autoimmune Hepatitis: An International Multicenter Study. Hepatology 2021, 73, 2099–2109. [Google Scholar] [CrossRef]
- Durazo, F.A.; Kristbaum, K.; Miller, J.; Sacian, K.; Selim, M.; Hong, J.C. De novo Autoimmune Hepatitis after COVID-19 Infection in an Unvaccinated Patient. Case Rep. Hepatol. 2022, 12, 8409269. [Google Scholar] [CrossRef]
- Bril, F.; Al Diffalha, S.; Dean, M.; Fetting, D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J. Hepatol. 2021, 75, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Tzivaki, I.; Marangos, M. COVID-19 vaccine, and autoimmunity: Awakening the sleeping drogan. Clin. Immunol. 2021, 226, 108721. [Google Scholar] [CrossRef]
- Londono, M.C.; Gratacos-Gines, J.; Saez-Panataro, J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination-still casualty. J. Hepatol. 2021, 75, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Zin Tun, G.S.; Gleeson, D.; Al-Joudeh, A.; Dube, A. Immone-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 2022, 76, 747–749. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Kumar, P.; Tevethia, H.V.; Premkumar, M.; Arab, J.B.; Candia, R.; Talukdar, R.; Sharma, M.; Qi, X.; Rao, P.N.; et al. Systematic review with meta-analysis: Liver manifestations and outcomes in COVID-19. Aliment. Pharmacol. Ther. 2020, 52, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.; Ramersdorfer, C.; Palitzsch, K.D.; Schölmerich, J.; Lock, G. Fatal liver failure after corticosteroid treatment of a hepatitis B carrier. Desch. Med. Microbe 1999, 124, 687–690. [Google Scholar]
- Shiota, G.; Harada, K.; Oyama, K.; Udagawa, A.; Nomi, T.; Tanaka, K.; Tsutsumi, A.; Noguchi, N.; Kishimoto, Y.; Horie, Y.; et al. Severe exacerbation of hepatitis after short-term corticosteroid therapy in patients with “latent” chronic hepatitis B. Liver 2000, 20, 415–420. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19-Final report. N. Eng. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Brandi, N.; Spinelli, D.; Granito, A.; Tovoli, F.; Piscaglia, F.; Golfieri, R.; Renzulli, M. COVID-19: Has the liver Been Spared? Int. J. Mol. Sci. 2023, 24, 1091. [Google Scholar] [CrossRef]
- Schütte, A.; Ciesek, S.; Wedemeyer, H.; Lange, C.M. Influenza virus infection is the precipitating event of acute-on-chronic liver failure. J. Hepatol. 2019, 70, 797–799. [Google Scholar] [CrossRef]
- Moon, A.M.; Webb, G.J.; Aloman, C.; Armstrong, M.J.; Cargill, T.; Dhanasekaran, R.; Genescà, J.; Gill, U.S.; James, T.W.; Jones, P.D.; et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J. Hepatol. 2020, 73, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Pose, E.; Brenner, E.J.; Cargill, T.; Catana, M.A.; et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J. Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khan, A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: A multicenter research network study. Gastroenterology 2020, 159, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Choudhury, A.; Lau, G.K.; Zheng, M.H.; Ji, D.; Abd-Elsalam, S.; Hwang, J.; Qi, X.; Cua, I.H.; Suh, J.I.; et al. Pre-existing liver disease is associated with poor outcomes in patients with SARS-CoV-2 infection; The APCOLIS Study (APASL, COVID-19 liver Injury Spectrum Study). Hepatol. Intern. 2020, 14, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Liang, P.S.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Fan, V.S.; et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infections in US veterans: Risk of infection, hospitalization, ventilation, and mortality. Hepatology 2021, 74, 322–335. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation of the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Szabo, G.; Csak, T. Inflammasomes in liver diseases. J. Hepatol. 2012, 57, 642–654. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; Lewandrowski, M.; Ingber, J.; de Lacerda, L.B.; Parry, B.; Ravid, S.; Clark, S.; Ho, F.; et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis, and cytokine release. Res. Sq. 2021, 3, 153628. [Google Scholar]
- Mao, R.; Qiu, Y.; He, J.-S.; Tan, J.-Y.; Li, X.-H.; Liang, J.; Shen, J.; Zhu, L.-R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Hashemi, N.; Viveiros, K.; Redd, W.D.; Zhou, J.C.; McCarty, T.R.; Bazarbashi, A.N.; Hathorn, K.; Wong, D.; Njie, C.; Shen, L.; et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicenter United States experience. Liver Int. 2020, 40, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zheng, K.I.; Wang, X.B.; Yan, H.D.; Sun, Q.F.; Pan, K.H.; Wang, T.-Y.; Ma, H.-L.; Chen, Y.-P.; George, J.; et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J. Hepatol. 2020, 73, 719–721. [Google Scholar] [CrossRef]
- Fondevila, M.F.; Mercado-Gómez, M.; Rodríguez, A.; Gonzalez-Rellan, M.J.; Iruzubieta, P.; Valentí, V.; Escalada, J.; Schwaninger, M.; Prevot, V.; Dieguez, C.; et al. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J. Hepatol. 2021, 74, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.C.; Wong, V.W.; Lui, G.C.; Chow, V.C.; Tse, Y.; Hui, V.W.; Liang, L.Y.; Chan, H.L.; Hui, D.S.; Wong, G.L. Current and past infections of HBV do not increase mortality in patients with COVID-19. Hepatology 2021, 74, 1750–1765. [Google Scholar] [CrossRef]
- Kang, S.H.; Cho, D.-H.; Choi, J.; Balk, S.K.; Gwon, J.G.; Kim, M.Y. Association between chronic hepatitis B infection and COVID-19 outcomes: A Korean nationwide cohort study. PLoS ONE 2021, 16, e258229. [Google Scholar] [CrossRef] [PubMed]
- Lens, S.; Miquel, M.; Mateos-Munoz, B.; Garcia-Samaniego, J.; Forns, X. SARS-CoV-2 in patients with on antiviral HBV and HCV therapy in Spain. J. Hepatol. 2020, 73, 1262–1263. [Google Scholar] [CrossRef]
- But, A.A.; Yan, P.; Chotani, R.A.; Shaikh, O.S. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021, 41, 1824–1831. [Google Scholar] [CrossRef]
- Ronderos, D.; Omar, A.M.S.; Abbas, H.; Makker, J.; Baiomi, A.; Sun, H.; Mantri, N.; Choi, Y.; Fortuzi, K.; Shin, D.; et al. Chronic hepatitis c infection in COVID-19 patients is associated with increased hospital mortality. World J. Clin. Cases 2021, 9, 8749–8762. [Google Scholar] [CrossRef]
- Cerbu, B.; Pantea, S.; Bratosin, F.; Vidican, I.; Turaiche, M.; Frent, S.; Borsi, E.; Marincu, I. Liver impairment and hematological changes in patients with chronic hepatitis C and COVID-19: A retrospective study after one year of pandemic. Medicina 2021, 57, 597. [Google Scholar] [CrossRef]
- Calmenero, J.; Rodriguez-Peralvarez, M.; Salcedo, M.; Arias-Milla, A.; Muñoz-Serrano, A.; Graus, J.; Nuño, J.; Gastaca, M.; Bustamante-Schneider, J.; Cachero, A.; et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J. Heaptol. 2021, 74, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bhoori, S.; Rossi, R.E.; Cittero, D.; Mazzaferro, V. COVID-19 long-term liver transplant patients: Preliminary experience from an Italian transplant center in Lombardy. Lancet Gastroenterol. Hepatol. 2020, 5, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Adeniji, N.; Latt, N.; Kumar, S.; Bloom, P.P.; Aby, E.S.; Perumalswami, P.; Roytman, M.; Li, M.; Vogel, A.S.; et al. Predictors and outcomes of COVID-19 in patients with chronic liver disease: US multicenter study. Clin. Gastroenterol. Hepatol. 2021, 18, 1469–1479.e19. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkiz, H. Unraveling the Molecular and Cellular Pathogenesis of COVID-19-Associated Liver Injury. Viruses 2023, 15, 1287. https://doi.org/10.3390/v15061287
Akkiz H. Unraveling the Molecular and Cellular Pathogenesis of COVID-19-Associated Liver Injury. Viruses. 2023; 15(6):1287. https://doi.org/10.3390/v15061287
Chicago/Turabian StyleAkkiz, Hikmet. 2023. "Unraveling the Molecular and Cellular Pathogenesis of COVID-19-Associated Liver Injury" Viruses 15, no. 6: 1287. https://doi.org/10.3390/v15061287



