Molecular Characteristics of Enterovirus B83 Strain Isolated from a Patient with Acute Viral Myocarditis and Global Transmission Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Viral Isolation, and Primary Identification
2.2. Viral RNA Extraction and Reverse Transcription
2.3. Full-Length Genome Amplification
2.4. Nucleotide Sequencing
2.5. EV-B83 Dataset Construction
2.6. Bayesian Temporal Dynamics Analysis
2.7. Recombination Analysis
2.8. Bayesian Phylogeographic Analysis
2.9. Nucleotide Sequence Accession Number
3. Results
3.1. Whole-Genome Sequences Analysis of Strain S17
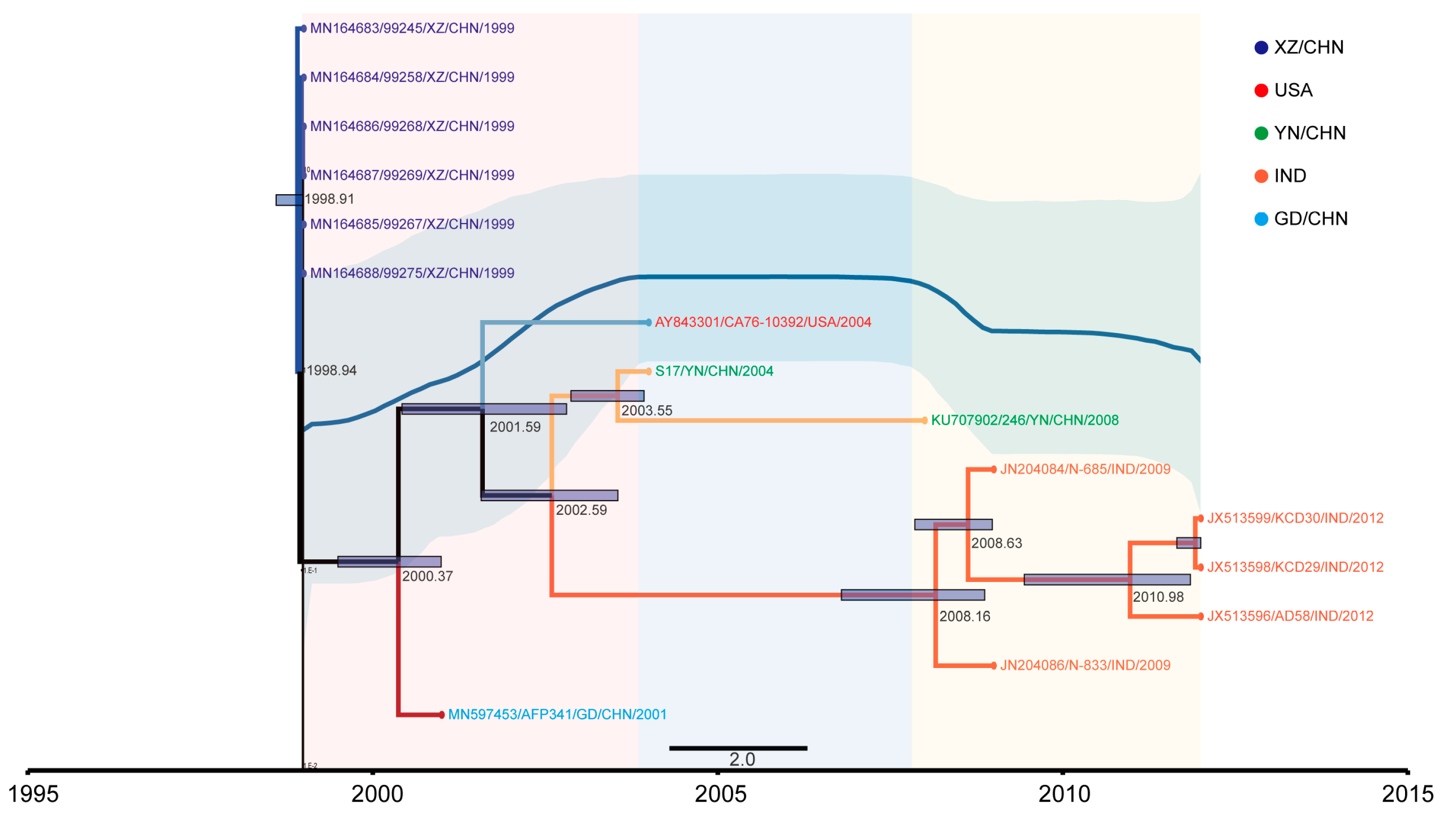
3.2. The Evolutionary Dynamics of EV-B83
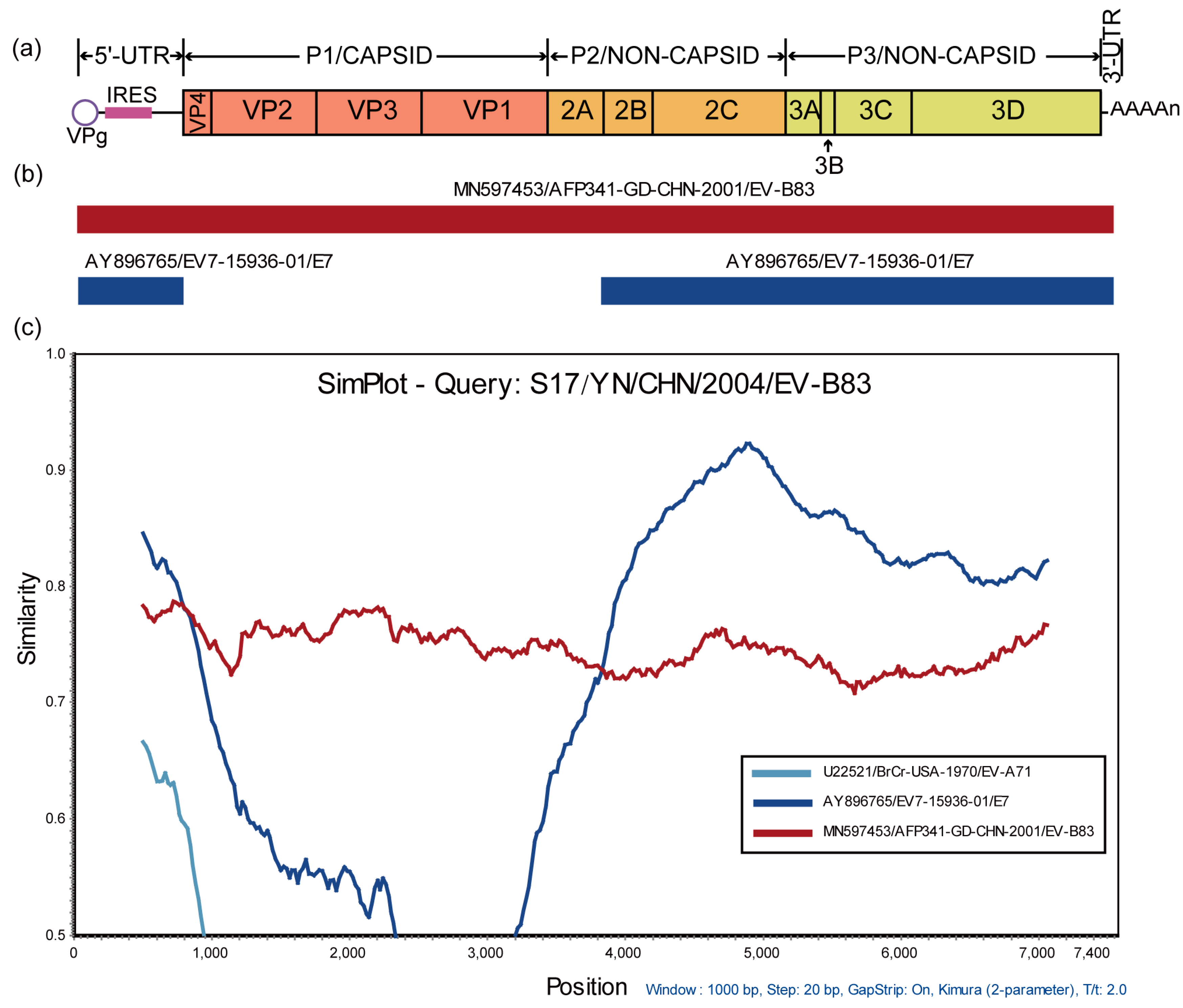
3.3. Recombination Analysis of S17
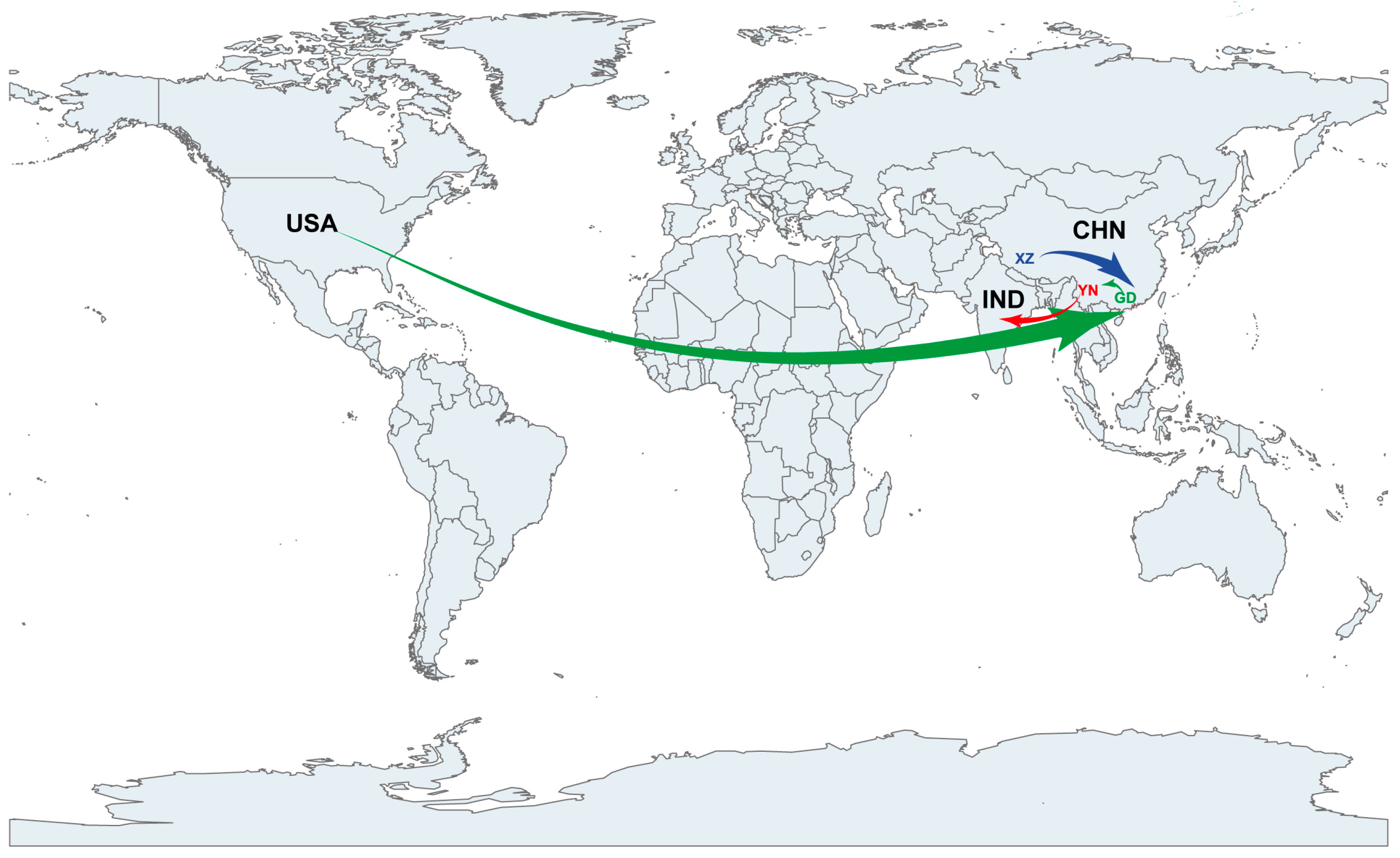
3.4. EV-B83 Phylogeographic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knowles, N.J.; Hovi, T.; Hyypiä, T.; King, A.M.Q.; Lindberg, M.; Pallansch, M.A.; Palmenberg, A.C.; Simmonds, P.; Skern, T.; Stanway, G.; et al. Picornaviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 855–880. [Google Scholar]
- Arbustini, E.; Porcu, E.; Bellini, O.; Grasso, M.; Pilotto, A.; Dal Bello, B.; Morbini, P.; Diegoli, M.; Gavazzi, A.; Specchia, G.; et al. Enteroviral infection causing fatal myocarditis and subclinical myopathy. Heart 2000, 83, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.S.; Lee, H.C.; Lee, K.M.; Gong, Y.N.; Shih, S.R. Enterovirus and Encephalitis. Front. Microbiol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, N.; Preiser, W.; van Zyl, G.; Claassen, M.; Cronje, N.; Maritz, J.; Newman, H.; McCarthy, K.; Ntshoe, G.; Essel, V.; et al. Molecular characterisation and epidemiology of enterovirus-associated aseptic meningitis in the Western and Eastern Cape Provinces, South Africa 2018–2019. J. Clin. Virol. 2021, 139, 104845. [Google Scholar] [CrossRef] [PubMed]
- Hogle, J.M.; Chow, M.; Filman, D.J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science 1985, 229, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; DeSantis, E.R. Treatment of viral myocarditis caused by coxsackievirus B. Am. J. Health Syst. Pharm. 2008, 65, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, M.; Schwartz, M.; Webber, E.; Shaffer, A.; Perry, T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1591–1601. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Nix, W.A.; Michele, S.M.; Uddin, M.; Schnurr, D.; al-Busaidy, S.; Akoua-Koffi, C.; Pallansch, M.A. Molecular identification of 13 new enterovirus types, EV79-88, EV97, and EV100-101, members of the species Human Enterovirus B. Virus. Res. 2007, 128, 34–42. [Google Scholar] [CrossRef]
- Henquell, C.; Mirand, A.; Richter, J.; Schuffenecker, I.; Bottiger, B.; Diedrich, S.; Terletskaia-Ladwig, E.; Christodoulou, C.; Peigue-Lafeuille, H.; Bailly, J.L. Phylogenetic patterns of human coxsackievirus B5 arise from population dynamics between two genogroups and reveal evolutionary factors of molecular adaptation and transmission. J. Virol. 2013, 87, 12249–12259. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.C.; Reddy, H.; Sudheendra, K.; Raghavendra, A.; Varadharaj, V.; Edula, S.; Goparaju, R.; Ratnakar, B.; Srinivasa Rao, A.S.; Maiya, P.P.; et al. Non-polio enterovirus association with persistent diarrhea in children as revealed by a follow-up study of an Indian cohort during the first two years of life. J. Clin. Virol. 2014, 61, 125–131. [Google Scholar] [CrossRef]
- Rao, D.C.; Ananda Babu, M.; Raghavendra, A.; Dhananjaya, D.; Kumar, S.; Maiya, P.P. Non-polio enteroviruses and their association with acute diarrhea in children in India. Infect. Genet. Evol. 2013, 17, 153–161. [Google Scholar] [CrossRef]
- Laxmivandana, R.; Yergolkar, P.; Gopalkrishna, V.; Chitambar, S.D. Characterization of the non-polio enterovirus infections associated with acute flaccid paralysis in South-Western India. PLoS ONE 2013, 8, e61650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.D.; Yergolkar, P.; Shankarappa, K.S. Antigenic diversity of enteroviruses associated with nonpolio acute flaccid paralysis, India, 2007-2009. Emerg. Infect. Dis. 2012, 18, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.S.; Feeroz, M.M.; Maher, K.; Nix, W.A.; Engel, G.A.; Hasan, K.M.; Begum, S.; Oh, G.; Chowdhury, A.H.; Pallansch, M.A.; et al. Characterizing the picornavirus landscape among synanthropic nonhuman primates in Bangladesh, 2007 to 2008. J. Virol. 2013, 87, 558–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, V.; Mey, C.; Eloit, M.; Zhu, H.; Danet, L.; Huang, Z.; Zou, G.; Tarantola, A.; Cheval, J.; Perot, P.; et al. Molecular epidemiology of human enterovirus 71 at the origin of an epidemic of fatal hand, foot and mouth disease cases in Cambodia. Emerg. Microbes Infect. 2016, 5, e104. [Google Scholar] [CrossRef] [Green Version]
- Bingjun, T.; Yoshida, H.; Yan, W.; Lin, L.; Tsuji, T.; Shimizu, H.; Miyamura, T. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan Province, the People’s Republic of China. J. Med. Virol. 2008, 80, 670–679. [Google Scholar] [CrossRef]
- Tang, J.; Li, Q.; Tian, B.; Zhang, J.; Li, K.; Ding, Z.; Lu, L. Complete Genome Analysis of an Enterovirus EV-B83 Isolated in China. Sci. Rep. 2016, 6, 29432. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Zhang, Y.; Hong, M.; Han, Z.; Zhang, M.; Song, Y.; Yan, D.; Zhu, S.; Xu, W. Phylogenetic characteristics and molecular epidemiological analysis of novel enterovirus EV-B83 isolated from Tibet, China. Sci. Rep. 2020, 10, 6630. [Google Scholar] [CrossRef] [Green Version]
- WHO. Polio Laboratory Manual, 4th ed.; Document World Health Oraganization/IVB/04.10: Geneva, Switzerland, 2004. [Google Scholar]
- Yang, C.F.; Naguib, T.; Yang, S.J.; Nasr, E.; Jorba, J.; Ahmed, N.; Campagnoli, R.; van der Avoort, H.; Shimizu, H.; Yoneyama, T.; et al. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 2003, 77, 8366–8377. [Google Scholar] [CrossRef] [Green Version]
- Ishiko, H.; Shimada, Y.; Yonaha, M.; Hashimoto, O.; Hayashi, A.; Sakae, K.; Takeda, N. Molecular diagnosis of human enteroviruses by phylogeny-based classification by use of the VP4 sequence. J. Infect. Dis. 2002, 185, 744–754. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Kilpatrick, D.R.; Pallansch, M.A. Molecular evolution of the human enteroviruses: Correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999, 73, 1941–1948. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Lam, T.T.; Max Carvalho, L.; Pybus, O.G. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus. Evol. 2016, 2, vew007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goss, E.M.; Tabima, J.F.; Cooke, D.E.; Restrepo, S.; Fry, W.E.; Forbes, G.A.; Fieland, V.J.; Cardenas, M.; Grünwald, N.J. The Irish potato famine pathogen Phytophthora infestans originated in central Mexico rather than the Andes. Proc. Natl. Acad. Sci. USA 2014, 111, 8791–8796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus. Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [Green Version]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.; Rambaut, A.; Pybus, O.G. Correlating viral phenotypes with phylogeny: Accounting for phylogenetic uncertainty. Infect. Genet. Evol. 2008, 8, 239–246. [Google Scholar] [CrossRef]
- Lemey, P.; Rambaut, A.; Drummond, A.J.; Suchard, M.A. Bayesian phylogeography finds its roots. PLoS Comput. Biol. 2009, 5, e1000520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielejec, F.; Rambaut, A.; Suchard, M.A.; Lemey, P. SPREAD: Spatial phylogenetic reconstruction of evolutionary dynamics. Bioinformatics 2011, 27, 2910–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Liu, X.; Du, Z.; Hou, H.; Wang, X.; Wang, F.; Yang, J. Bayesian phylodynamic analysis reveals the dispersal patterns of tobacco mosaic virus in China. Virology 2019, 528, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Muslin, C.; Joffret, M.L.; Pelletier, I.; Blondel, B.; Delpeyroux, F. Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region. PLoS Pathog. 2015, 11, e1005266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muslin, C.; Mac Kain, A.; Bessaud, M.; Blondel, B.; Delpeyroux, F. Recombination in Enteroviruses, a Multi-Step Modular Evolutionary Process. Viruses 2019, 11, 859. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Zhang, Y.; Liu, Y.; Miao, Y.; Zheng, C.; Luo, D.; Sun, J.; Hu, Q.; Xu, Y.; Wang, H.; et al. A Single Mutation in the VP1 Gene of Enterovirus 71 Enhances Viral Binding to Heparan Sulfate and Impairs Viral Pathogenicity in Mice. Viruses 2020, 12, 883. [Google Scholar] [CrossRef]
- Tseligka, E.D.; Sobo, K.; Stoppini, L.; Cagno, V.; Abdul, F.; Piuz, I.; Meylan, P.; Huang, S.; Constant, S.; Tapparel, C. A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism. PLoS Pathog. 2018, 14, e1007190. [Google Scholar] [CrossRef]
- Volle, R.; Razafindratsimandresy, R.; Joffret, M.L.; Bessaud, M.; Rabemanantsoa, S.; Andriamamonjy, S.; Raharinantoanina, J.; Blondel, B.; Heraud, J.M.; Bailly, J.L.; et al. High Permissiveness for Genetic Exchanges between Enteroviruses of Species A, including Enterovirus 71, Favors Evolution through Intertypic Recombination in Madagascar. J. Virol. 2019, 93, e01667-18. [Google Scholar] [CrossRef] [Green Version]
- Lukashev, A.N.; Shumilina, E.Y.; Belalov, I.S.; Ivanova, O.E.; Eremeeva, T.P.; Reznik, V.I.; Trotsenko, O.E.; Drexler, J.F.; Drosten, C. Recombination strategies and evolutionary dynamics of the Human enterovirus A global gene pool. J. Gen. Virol. 2014, 95, 868–873. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Kilpatrick, D.R.; Flemister, M.R.; Brown, B.A.; Pallansch, M.A. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 1999, 37, 1288–1293. [Google Scholar] [CrossRef] [Green Version]
- Oberste, M.S.; Maher, K.; Flemister, M.R.; Marchetti, G.; Kilpatrick, D.R.; Pallansch, M.A. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J. Clin. Microbiol. 2000, 38, 1170–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnaji, F.G.; Bentley, K.; Pearson, A.; Woodman, A.; Moore, J.; Fox, H.; Macadam, A.J.; Evans, D.J. Generated Randomly and Selected Functionally? The Nature of Enterovirus Recombination. Viruses 2022, 14, 916. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Mimouli, K.; Kyriakopoulou, Z.; Tsimpidis, M.; Tsakogiannis, D.; Markoulatos, P.; Amoutzias, G.D. Large-scale genomic analysis reveals recurrent patterns of intertypic recombination in human enteroviruses. Virology 2019, 526, 72–80. [Google Scholar] [CrossRef]
- Annoni, G.; De Rienzo, F.; Nonini, S.; Pugni, L.; Marianeschi, S.M.; Mauri, L.; Gatelli, I.; Mauri, L.; Aresta, F.; Bramerio, M.; et al. Enterovirus fulminant myocarditis as cause of acute heart failure in a newborn. Int. J. Cardiol. Heart Vasc. 2022, 42, 101093. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Stafford, K.A.; Sung, Y.K. Update on coxsackievirus B3 myocarditis. Curr. Opin. Rheumatol. 2012, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kraft, L.; Sauter, M.; Seebohm, G.; Klingel, K. In Vitro Model Systems of Coxsackievirus B3-Induced Myocarditis: Comparison of Commonly Used Cell Lines and Characterization of CVB3-Infected iCell((R)) Cardiomyocytes. Viruses 2021, 13, 1835. [Google Scholar] [CrossRef]
- Akuzawa, N.; Harada, N.; Hatori, T.; Imai, K.; Kitahara, Y.; Sakurai, S.; Kurabayashi, M. Myocarditis, hepatitis, and pancreatitis in a patient with coxsackievirus A4 infection: A case report. Virol. J. 2014, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.A.; Thaker, H.M.; Racaniello, V.R. Transgenic mouse model for echovirus myocarditis and paralysis. Proc. Natl. Acad. Sci. USA 2003, 100, 15906–15911. [Google Scholar] [CrossRef] [Green Version]

| Primer | Nucleotide Position (nt) | Primer Sequence | Orientation | Reference |
|---|---|---|---|---|
| 0001S48 a | GGGGACAAGTTTGTACAAAAAAGCAGGCTTTAAAACAGCTCTGGGGTT | Forward | [20] | |
| EV/PCR-1 | 539–564 | ACACGGACACCCAAAGTAGTCGGTCC | Reverse | [20] |
| EVP4 | 541–560 | CTACTTTGGGTGTCCGTGTT | Forward | [21] |
| OL68-1 | 1178–1197 | GGTAAYTTCCACCACCANCC | Reverse | [21] |
| S17-936Y | 936–955 | CTACCCGCATTGAACTCACC | Forward | This study |
| S17-1716Z | 1697–1716 | GAGGCGCAATCCATTGTACT | Reverse | This study |
| S17-1586Y | 1586–1605 | CCGGTCATTACAACTTCACC | Forward | This study |
| S17-2567Z | 2548–2567 | ATGGTGTCGCTGGGAACTAC | Reverse | This study |
| 008 | 2411–2430 | GCRTGCAATGAYTTCTCWGT | Forward | [22] |
| 011 | 3389–3408 | GCICCIGAYTGITGICCRAA | Reverse | [22] |
| S17-3167Y | 3167–3186 | GCCCACCACGTCTCTGTAAT | Forward | This study |
| S17-4245Z | 4226–4245 | TGAGGGTGCACTCTGCTCTA | Reverse | This study |
| S17-3981Y | 3981–4000 | GCGTTGGCTCAAACAAAAAG | Forward | This study |
| S17-4970Z | 4951–4970 | AACATTTCGGTCACGAGCAT | Reverse | This study |
| S17-4437Y | 4437–4456 | TGGAAAATCAGTGGCAACAA | Forward | This study |
| S17-5423Z | 5404–5423 | TATTCGGTTTTCACGGTGCT | Reverse | This study |
| S17-5277Y | 5277–5296 | GTTTGCAGGTTTCCAAGGAG | Forward | This study |
| S17-6348Z | 6329–6348 | TTTGTCCATGCACTCCTTCA | Reverse | This study |
| S17-6219Y | 6219–6238 | TGAAGGCCTAGAAGCACTGG | Forward | This study |
| S17-7353Z | 7334–7353 | GTTCGGTGAGTGTGGTAGGG | Reverse | This study |
| S17-6777Y | 6777–6796 | GTGTTCTGGGACCAGCATTT | Forward | This study |
| 7500A a | GGGGACCACTTTGTACAAGAAAGCTGGG(T)24 | Reverse | [20] |
| Genome Region | Position (nt) | Length (nt) | S17 vs. CA76-10392 (%) | S17 vs. Other EV-B Strains (%) | Note a | ||
|---|---|---|---|---|---|---|---|
| Nucleotide | Amino Acid | Nucleotide | Amino Acid | ||||
| 5′-UTR | 1–743 | 743 | 83.3 | / | 67.7–89.0 | / | One Deletion, Two Insertions |
| VP4 | 744–950 | 207 | 77.7 | 91.3 | 70.5–77.2 | 73.9–94.2 | |
| VP2 | 951–1727 | 777 | 79.9 | 98.0 | 66.2–71.7 | 73.6–84.2 | |
| VP3 | 1728–2438 | 711 | 78.8 | 97.4 | 63.2–72.8 | 65.8–82.7 | |
| VP1 | 2439–3290 | 852 | 74.9 | 94.0 | 52.7–65.8 | 54.6–69.3 | |
| 2A | 3291–3740 | 450 | 79.7 | 95.3 | 74.8–82.8 | 90.0–96.0 | |
| 2B | 3741–4037 | 297 | 81.4 | 96.9 | 74.4–87.2 | 91.9–96.9 | |
| 2C | 4038–5024 | 987 | 80.8 | 96.9 | 79.0–86.6 | 96.3–98.4 | |
| 3A | 5025–5291 | 267 | 77.5 | 96.6 | 74.1–87.2 | 92.1–100.0 | |
| 3B | 5292–5357 | 66 | 75.7 | 90.9 | 68.1–86.3 | 81.8–95.4 | |
| 3C | 5358–5906 | 549 | 79.5 | 95.6 | 75.5–86.1 | 92.8–98.9 | |
| 3D | 5907–7292 | 1386 | 78.6 | 95.0 | 76.8–86.3 | 94.1–98.0 | |
| 3′-UTR | 7296–7396 | 101 | 88.1 | / | 79.2–97.0 | / | one insertion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Lu, H.; Ma, L.; Zhu, S.; Yan, D.; Han, J.; Zhang, Y. Molecular Characteristics of Enterovirus B83 Strain Isolated from a Patient with Acute Viral Myocarditis and Global Transmission Dynamics. Viruses 2023, 15, 1360. https://doi.org/10.3390/v15061360
Song J, Lu H, Ma L, Zhu S, Yan D, Han J, Zhang Y. Molecular Characteristics of Enterovirus B83 Strain Isolated from a Patient with Acute Viral Myocarditis and Global Transmission Dynamics. Viruses. 2023; 15(6):1360. https://doi.org/10.3390/v15061360
Chicago/Turabian StyleSong, Juan, Huanhuan Lu, Lin Ma, Shuangli Zhu, Dongmei Yan, Jun Han, and Yong Zhang. 2023. "Molecular Characteristics of Enterovirus B83 Strain Isolated from a Patient with Acute Viral Myocarditis and Global Transmission Dynamics" Viruses 15, no. 6: 1360. https://doi.org/10.3390/v15061360
APA StyleSong, J., Lu, H., Ma, L., Zhu, S., Yan, D., Han, J., & Zhang, Y. (2023). Molecular Characteristics of Enterovirus B83 Strain Isolated from a Patient with Acute Viral Myocarditis and Global Transmission Dynamics. Viruses, 15(6), 1360. https://doi.org/10.3390/v15061360







