Innate Immune Response to Viral Vectors in Gene Therapy
Abstract
:1. Introduction
| Date | Drugs | Regulatory Approval | Application | Vector |
|---|---|---|---|---|
| 2003 | Gendicine (recombinant human p53 adenovirus) [15] | China Food and Drug Administration (CFDA) | Head and neck squamous cell carcinoma (HNSCC) | Ad-p53 |
| 2006 | Oncorine [16] | Chinese SFDA |
| H101 |
| 2012 | Alipogene tiparvovec (Glybera) (it was withdrawn from the market in 2017) [17] | EMA |
| AAV1-LPL |
| 2017 | Luxturna [18] | FDA | Leber congenital amaurosis caused by RPE65 mutations | AAV2-RPE65 |
| 2017 | Kymriah (tisagenlecleucel) [19] | FDA |
| LV-CD19 |
| 2019 | Zolgensma [20] | FDA |
| scAAV9-SMN1 |
| 2020 | Libmeldy [21] | EU | Metachromatic leukodystrophy (MLD) | SIN LV vector |
| 2021 | Elivaldogene autotemcel (Skysona, eli-cel) [22] | EU | Cerebral adrenoleukodystrophy (CALD) | LV. ABCD1 |
| 2021 | Breyanzi (lisocabtagene maraleucel) [23] | FDA |
| LV-CD19 |
| 2021 | Abecma (idecabtagene vicleucel, ide-cel) [23] | FDA | Relapsed or refractory multiple myeloma (R/R MM) | LV-CD19 |
| 2022 | Eladocagene exuparvovec (Upstaza) [24] | EMA |
| rAAV2-hAADC |
| 2022 | Roctavian (valoctocogene roxaparvovec) [25] | EMA |
| BMN 270: AAV5 -hFVIII-SQ |
| 2022 | Adstiladrin [26] | FDA | Patients with NMIBC who do not respond to BCG | rAd-IFNα/Syn3 |
| 2022 | Zynteglo (betibeglogene autotemcel, beti-cel) [27] | FDA | β-thalassemia patients | BB305 LV vector |
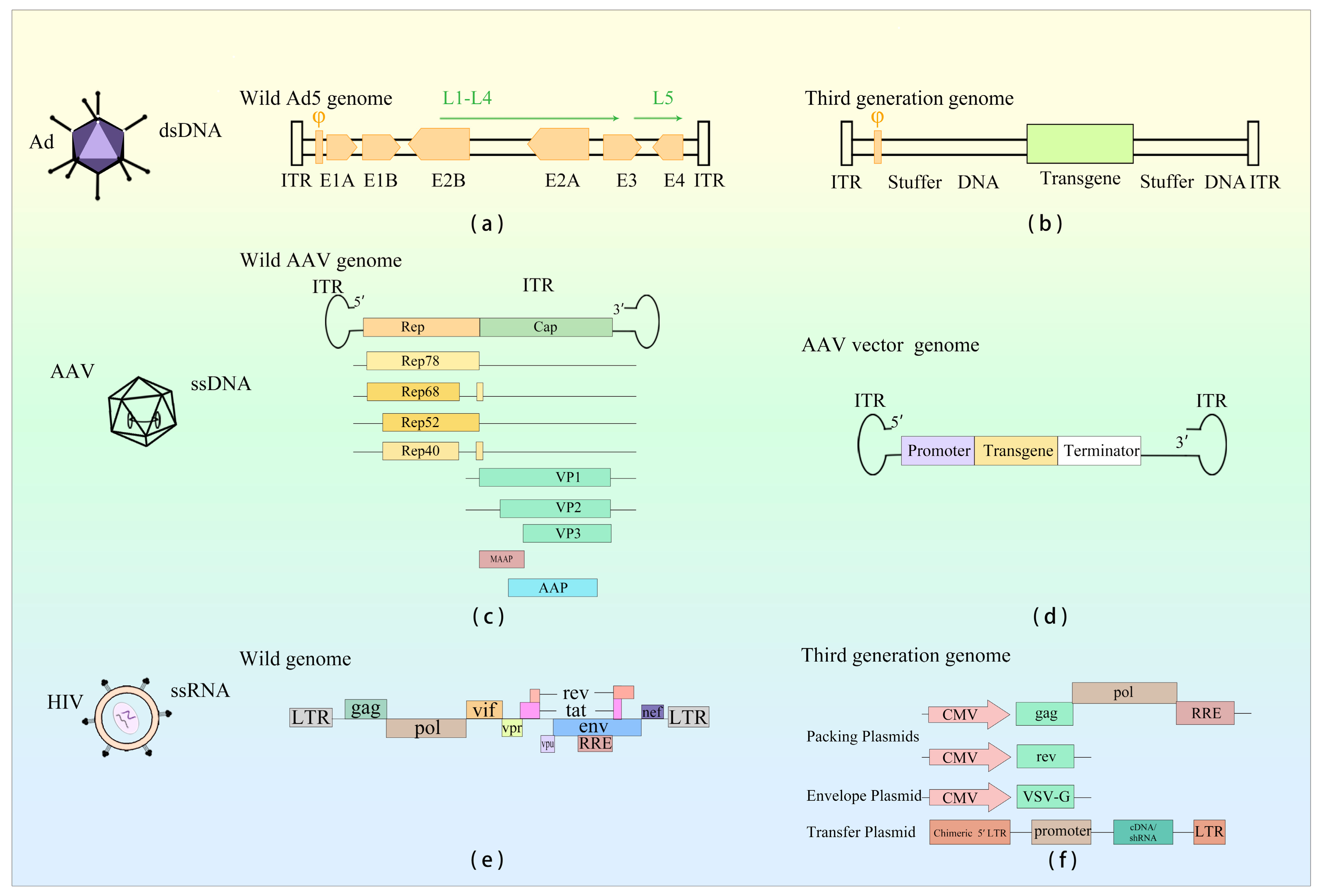
| Virus Type | Size | Genome Structure | Genome Type | Immunogenicity | Integration | |
|---|---|---|---|---|---|---|
| Ad | Wild-type Virus | 26–45 kb | ITR, φ, E1A, E1B, E2, E3, E4, L1–L5 | dsDNA | High | Rarely |
| Viral Vector | OAd: 3 kb; HDAd: 34 kb | ITR, φ, transgene | ||||
| AAV | Wild-type Virus | 4.7 kb | ITR, Rep, Cap, AAP, MAAP | ssDNA | Low | Rarely |
| Viral Vector | 4.7 kb | ITR, transgene | ||||
| LV | Wild-type Virus | 8–9 kb | LTR, gag, pol, env, rev, tat, vpr, vpu, vif, nef | ssRNA | Moderate | Random |
| Viral Vector | <5 kb | 1: gag, pol, rre, transgene; 2: rev, transgene | ||||
2. Adenovirus Vector Therapy
2.1. Introduction to Adenovirus Vectors
2.1.1. Principle of Gene Therapy with Adenoviral Vectors
2.1.2. Application of Adenoviral Vectors in Gene Therapy
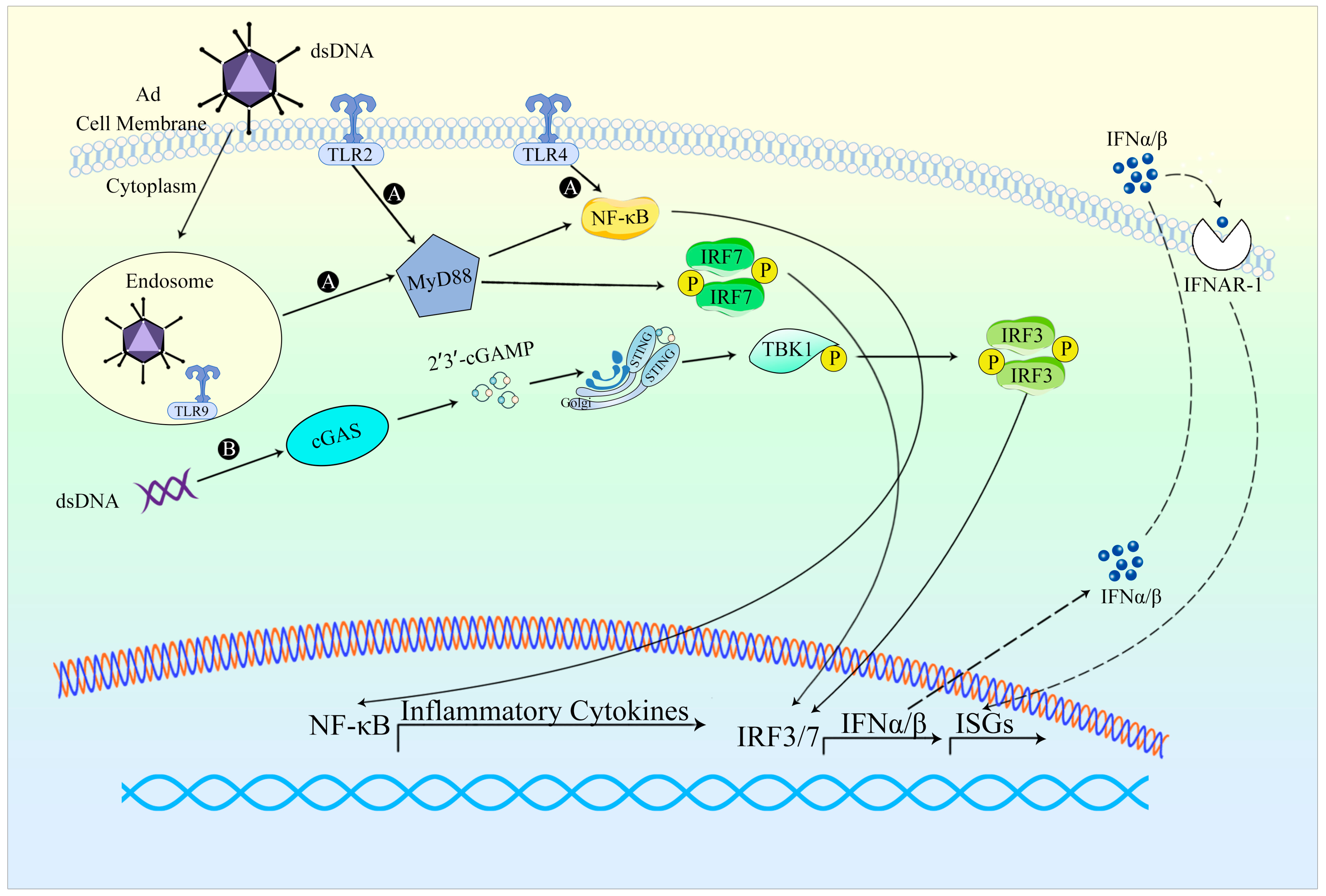
2.2. Innate Immune Responses against Adenovirus Vectors
2.2.1. Occurrence of Innate Immune Response
2.2.2. Evasion of Innate Immune Response
3. AAV Vector Therapy
3.1. Introduction to AAV Vector
3.1.1. Principle of Action of AAV Vector
3.1.2. Application of AAV Vectors in Gene Therapy
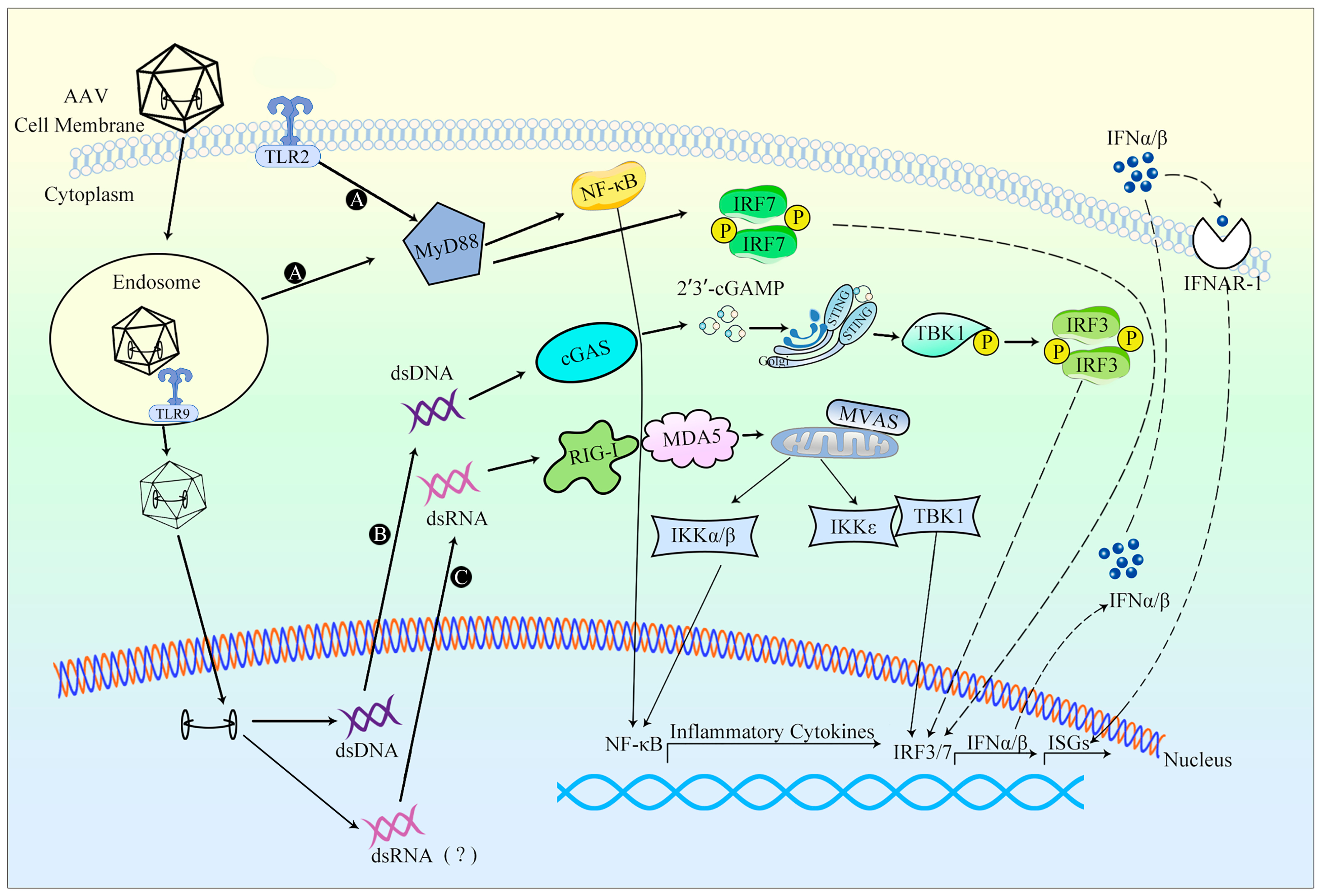
3.2. Innate Immune Responses against AAV Vectors
3.2.1. Occurrence of Innate Immune Response
3.2.2. Evasion of Innate Immune Response
4. Lentiviral Vector Therapy
4.1. Introduction to Lentiviral Vectors
4.1.1. Principle of Action of Lentiviral Vector
4.1.2. Application of Lentiviral Vectors in Gene Therapy
4.2. Innate Immune Responses against Lentiviral Vectors
4.2.1. Occurrence of Innate Immune Response
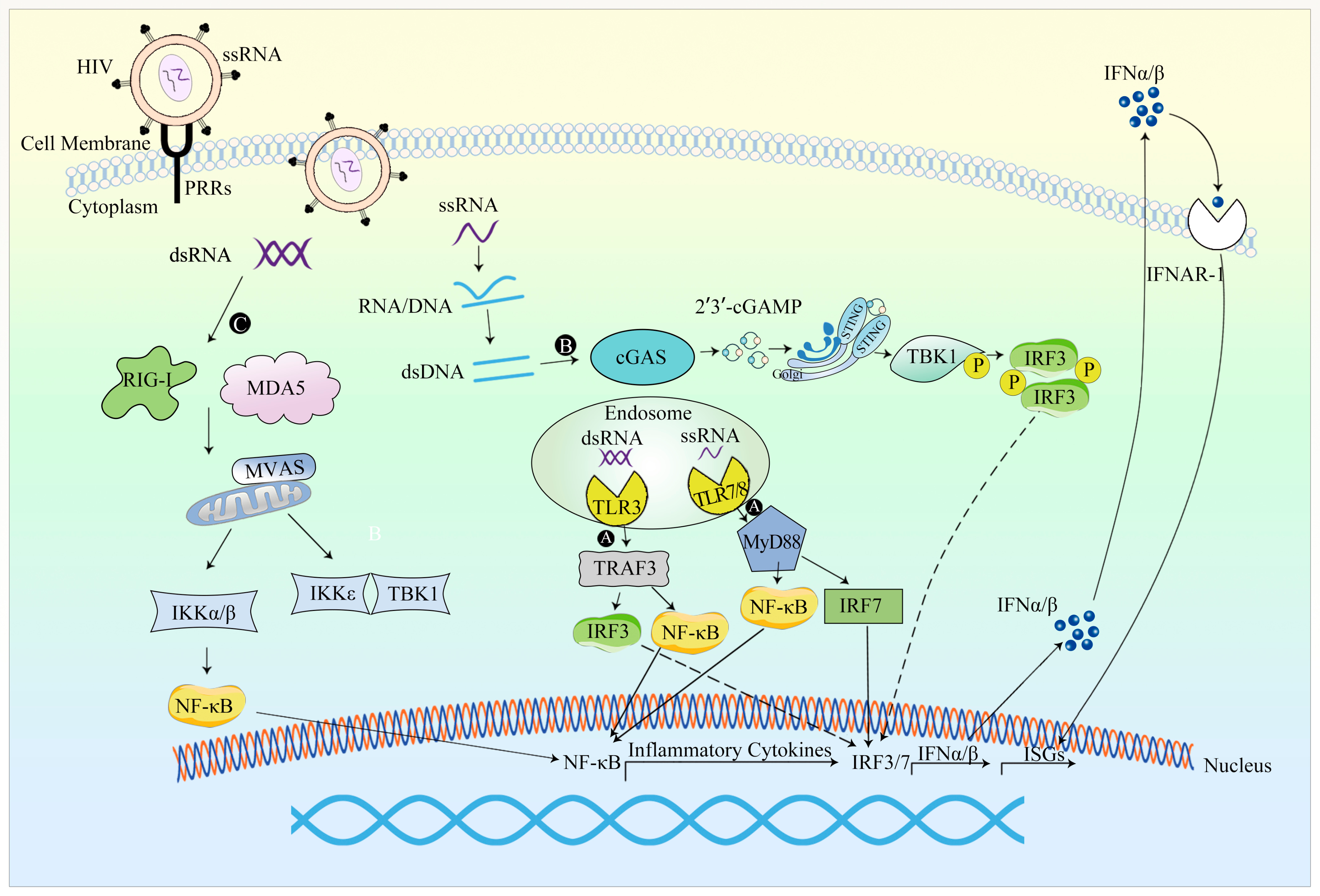
4.2.2. Evasion of the Innate Immune Response
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sato-Dahlman, M.; LaRocca, C.J.; Yanagiba, C.; Yamamoto, M. Adenovirus and Immunotherapy: Advancing Cancer Treatment by Combination. Cancers 2020, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G.P. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Hensley, S.E.; Cun, A.S.; Giles-Davis, W.; Li, Y.; Xiang, Z.Q.; Lasaro, M.O.; Williams, B.R.G.; Silverman, R.H.; Ertl, H.C.J. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol. Ther. 2007, 15, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Sack, B.K.; Herzog, R.W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr. Opin. Mol. Ther. 2009, 11, 493–503. [Google Scholar] [PubMed]
- Rogers, G.L.; Martino, A.T.; Aslanidi, G.V.; Jayandharan, G.R.; Srivastava, A.; Herzog, R.W. Innate Immune Responses to AAV Vectors. Front. Microbiol. 2011, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Rabourdin-Combe, C. Innate immunity modulation in virus entry. Curr. Opin. Virol. 2011, 1, 6–12. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Atasheva, S.; Shayakhmetov, D.M. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses 2022, 14, 888. [Google Scholar] [CrossRef]
- Sohn, S.Y.; Hearing, P. Adenoviral strategies to overcome innate cellular responses to infection. FEBS Lett. 2019, 593, 3484–3495. [Google Scholar] [CrossRef]
- Vachon, V.K.; Conn, G.L. Adenovirus VA RNA: An essential pro-viral non-coding RNA. Virus Res. 2016, 212, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.; Chan, Y.K.; Samulski, R.J. Adeno-associated Virus (AAV) versus Immune Response. Viruses 2019, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Muhuri, M.; Maeda, Y.; Ma, H.; Ram, S.; Fitzgerald, K.A.; Tai, P.W.; Gao, G. Overcoming innate immune barriers that impede AAV gene therapy vectors. J. Clin. Investig. 2021, 131, e143780. [Google Scholar] [CrossRef] [PubMed]
- Bergantz, L.; Subra, F.; Deprez, E.; Delelis, O.; Richetta, C. Interplay between Intrinsic and Innate Immunity during HIV Infection. Cells 2019, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Gendicine: The first commercial gene therapy product. Hum. Gene Ther. 2005, 16, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.A.; Shalaev, E.; Karami, T.K.; Cunningham, J.; Slater, N.K.H.; Rivers, H.M. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm. Res. 2018, 36, 29. [Google Scholar] [CrossRef]
- Ali, S.; Kjeken, R.; Niederlaender, C.; Markey, G.; Saunders, T.S.; Opsata, M.; Moltu, K.; Bremnes, B.; Grønevik, E.; Muusse, M.; et al. The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the Treatment of Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma. Oncologist 2020, 25, e321–e327. [Google Scholar] [CrossRef]
- Day, J.W.; Mendell, J.R.; Mercuri, E.; Finkel, R.S.; Strauss, K.A.; Kleyn, A.; Tauscher-Wisniewski, S.; Tukov, F.F.; Reyna, S.P.; Chand, D.H. Clinical Trial and Postmarketing Safety of Onasemnogene Abeparvovec Therapy. Drug Saf. 2021, 44, 1109–1119. [Google Scholar] [CrossRef]
- Jensen, T.L.; Gøtzsche, C.R.; Woldbye, D.P.D. Current and Future Prospects for Gene Therapy for Rare Genetic Diseases Affecting the Brain and Spinal Cord. Front. Mol. Neurosci. 2021, 14, 695937. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Elivaldogene Autotemcel: First Approval. Mol. Diagn. Ther. 2021, 25, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Eladocagene Exuparvovec: First Approval. Drugs 2022, 82, 1427–1432. [Google Scholar] [CrossRef]
- Blair, H.A. Valoctocogene Roxaparvovec: First Approval. Drugs 2022, 82, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Correction to: Nadofaragene Firadenovec: First Approval. Drugs 2023, 83, 951. [Google Scholar] [CrossRef] [PubMed]
- Schuessler-Lenz, M.; Enzmann, H.; Vamvakas, S. Regulators’ Advice Can Make a Difference: European Medicines Agency Approval of Zynteglo for Beta Thalassemia. Clin. Pharmacol. Ther. 2020, 107, 492–494. [Google Scholar] [CrossRef]
- Watanabe, M.; Nishikawaji, Y.; Kawakami, H.; Kosai, K.I. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses 2021, 13, 2502. [Google Scholar] [CrossRef]
- Athanasopoulos, T.; Munye, M.M.; Yanez-Munoz, R.J. Nonintegrating Gene Therapy Vectors. Hematol. Oncol. Clin. N. Am. 2017, 31, 753–770. [Google Scholar] [CrossRef]
- Fang, B.; Lai, J.; Liu, Y.; Yu, T.T.; Yu, X.; Li, X.; Dong, L.; Zhang, X.; Yang, W.; Yan, Q.; et al. Genetic characterization of human adenoviruses in patients using metagenomic next-generation sequencing in Hubei, China, from 2018 to 2019. Front. Microbiol. 2023, 14, 1153728. [Google Scholar] [CrossRef]
- Wu, C.; Wei, F.K.; Xu, Z.Y.; Wen, R.M.; Chen, J.C.; Wang, J.Q.; Mao, L.J. Tropism and transduction of oncolytic adenovirus vectors in prostate cancer therapy. Front. Biosci. 2021, 26, 866–872. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Kulanayake, S.; Tikoo, S.K. Adenovirus Core Proteins: Structure and Function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Rosewell Shaw, A.; Porter, C.; Biegert, G.; Jatta, L.; Suzuki, M. HydrAd: A Helper-Dependent Adenovirus Targeting Multiple Immune Pathways for Cancer Immunotherapy. Cancers 2022, 14, 2769. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Sun, W. Applications of Recombinant Adenovirus-p53 Gene Therapy for Cancers in the Clinic in China. Curr. Gene Ther. 2020, 20, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, S.; Luo, Z. Oncolytic Adenovirus, a New Treatment Strategy for Prostate Cancer. Biomedicines 2022, 10, 3262. [Google Scholar] [CrossRef]
- The Medical Letter, Inc. In brief: Adstiladrin—A gene therapy for bladder cancer. Med. Lett. Drugs Ther. 2023, 65, e40–e41. [Google Scholar] [CrossRef]
- Muhammad, T.; Sakhawat, A.; Khan, A.A.; Ma, L.; Gjerset, R.A.; Huang, Y. Mesenchymal stem cell-mediated delivery of therapeutic adenoviral vectors to prostate cancer. Stem Cell Res. Ther. 2019, 10, 190. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Crosby, C.M.; Heller, G.J.; Mendel, Z.I.; Barry, M.E.; Barry, M.A. Oncolytic adenovirus Ad657 for systemic virotherapy against prostate cancer. Oncolytic Virother. 2018, 7, 43–51. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, J.G.; Du, S.T.; Li, N. The effect of gene therapy on postoperative recurrence of small hepatocellular carcinoma (less than 5cm). Cancer Gene Ther. 2019, 26, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhao, R.; Zhang, Q.; Iqbal, M.Z.; Lu, J.; Zhao, Q.; Luo, D.; Feng, C.; Zhang, K.; Liu, X.; et al. Biosilicified oncolytic adenovirus for cancer viral gene therapy. Biomater. Sci. 2020, 8, 5317–5328. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Zhang, P.B.; Feng, Q.; Pan, X.Y.; Song, S.L.; Cui, J.; Yang, J.L. Cytokine-induced killer cells carrying recombinant oncolytic adenovirus expressing p21Ras scFv inhibited liver cancer. J. Cancer 2021, 12, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liu, Z.; Deng, R.; Li, C.; Fu, S.; Chen, G.; Zhang, X.; Ke, F.; Ke, S.; Yu, X.; et al. Aptamer-mediated gene therapy enhanced antitumor activity against human hepatocellular carcinoma in vitro and in vivo. J. Control Release 2017, 258, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Barretina-Ginesta, M.P.; García-Donas, J.; Jayson, G.C.; Roxburgh, P.; Vázquez, R.M.; Michael, A.; Antón-Torres, A.; Brown, R.; Krige, D.; et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: A phase 1 clinical trial. J. Immunother. Cancer 2021, 9, e003645. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Monk, B.J.; Herzog, T.J.; Moore, K.N.; Shapira-Frommer, R.; Ledermann, J.A.; Tewari, K.S.; Secord, A.A.; Minei, T.R.; Freedman, L.S.; et al. Utilizing an interim futility analysis of the OVAL study (VB-111-701/GOG 3018) for potential reduction of risk: A phase III, double blind, randomized controlled trial of ofranergene obadenovec (VB-111) and weekly paclitaxel in patients with platinum resistant ovarian cancer. Gynecol. Oncol. 2021, 161, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.B.; Li, J.; Lv, L.J.; Nie, B.J.; Lu, P.; Xue, F.; Zhang, Z.M. The effects of interleukin 2 and rAd-p53 as a treatment for glioblastoma. Mol. Med. Rep. 2018, 17, 4853–4859. [Google Scholar] [CrossRef]
- Xia, Y.; Du, Z.H.; Wang, X.Y.; Li, X.Q. Treatment of Uterine Sarcoma with rAd-p53 (Gendicine) Followed by Chemotherapy: Clinical Study of TP53 Gene Therapy. Hum. Gene Ther. 2018, 29, 242–250. [Google Scholar] [CrossRef]
- Tan, T.J.; Ang, W.X.G.; Wang, W.W.; Chong, H.S.; Tan, S.H.; Cheong, R.; Chia, J.W.; Syn, N.L.; Shuen, W.H.; Ba, R.; et al. A phase I study of an adenoviral vector delivering a MUC1/CD40-ligand fusion protein in patients with advanced adenocarcinoma. Nat. Commun. 2022, 13, 6453. [Google Scholar] [CrossRef]
- Sterman, D.H.; Alley, E.; Stevenson, J.P.; Friedberg, J.; Metzger, S.; Recio, A.; Moon, E.K.; Haas, A.R.; Vachani, A.; Katz, S.I.; et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNα Combined with Chemotherapy. Clin. Cancer Res. 2016, 22, 3791–3800. [Google Scholar] [CrossRef]
- Ben-Gary, H.; McKinney, R.L.; Rosengart, T.; Lesser, M.L.; Crystal, R.G. Systemic interleukin-6 responses following administration of adenovirus gene transfer vectors to humans by different routes. Mol. Ther. 2002, 6, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Yao, J.; Shayakhmetov, D.M. Innate immunity to adenovirus: Lessons from mice. FEBS Lett. 2019, 593, 3461–3483. [Google Scholar] [CrossRef] [PubMed]
- Fejer, G.; Freudenberg, M.; Greber, U.F.; Gyory, I. Adenovirus-triggered innate signalling pathways. Eur. J. Microbiol. Immunol. 2011, 1, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Bottermann, M.; Foss, S.; van Tienen, L.M.; Vaysburd, M.; Cruickshank, J.; O’Connell, K.; Clark, J.; Mayes, K.; Higginson, K.; Hirst, J.C.; et al. TRIM21 mediates antibody inhibition of adenovirus-based gene delivery and vaccination. Proc. Natl. Acad. Sci. USA 2018, 115, 10440–10445. [Google Scholar] [CrossRef] [PubMed]
- Minamitani, T.; Iwakiri, D.; Takada, K. Adenovirus virus-associated RNAs induce type I interferon expression through a RIG-I-mediated pathway. J. Virol. 2011, 85, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kawabata, K.; Kouyama, E.; Ishii, K.J.; Katayama, K.; Suzuki, T.; Kurachi, S.; Sakurai, F.; Akira, S.; Mizuguchi, H. Induction of type I interferon by adenovirus-encoded small RNAs. Proc. Natl. Acad. Sci. USA 2010, 107, 17286–17291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Clise-Dwyer, K.; Ruisaard, K.E.; Fan, X.; Tian, W.; Gumin, J.; Lamfers, M.L.; Kleijn, A.; Lang, F.F.; Yung, W.K.; et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE 2014, 9, e97407. [Google Scholar] [CrossRef]
- Hoare, J.I.; Osmani, B.; O’Sullivan, E.A.; Browne, A.; Campbell, N.; Metcalf, S.; Nicolini, F.; Saxena, J.; Martin, S.A.; Lockley, M. Carvedilol targets β-arrestins to rewire innate immunity and improve oncolytic adenoviral therapy. Commun. Biol. 2022, 5, 106. [Google Scholar] [CrossRef]
- Wang, W.C.; Sayedahmed, E.E.; Mittal, S.K. Significance of Preexisting Vector Immunity and Activation of Innate Responses for Adenoviral Vector-Based Therapy. Viruses 2022, 14, 2727. [Google Scholar] [CrossRef]
- Tian, M.; Liu, W.; Zhang, Q.; Huang, Y.; Li, W.; Wang, W.; Zhao, P.; Huang, S.; Song, Y.; Shereen, M.A.; et al. MYSM1 Represses Innate Immunity and Autoimmunity through Suppressing the cGAS-STING Pathway. Cell Rep. 2020, 33, 108297. [Google Scholar] [CrossRef]
- Kreppel, F.; Hagedorn, C. Capsid and Genome Modification Strategies to Reduce the Immunogenicity of Adenoviral Vectors. Int. J. Mol. Sci. 2021, 22, 2417. [Google Scholar] [CrossRef] [PubMed]
- Gabal, Y.; Ramsey, J.D. Surface Modification of Adenovirus Vector to Improve Immunogenicity and Tropism. Methods Mol. Biol. 2021, 2183, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.M.; Borovjagin, A.V.; Richter, J.R.; Jani, A.H.; Wu, H.; Zinn, K.R.; Warram, J.M. Genetic strategy to decrease complement activation with adenoviral therapies. PLoS ONE 2019, 14, e0215226. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Emerson, C.C.; Yao, J.; Young, C.; Stewart, P.L.; Shayakhmetov, D.M. Systemic cancer therapy with engineered adenovirus that evades innate immunity. Sci. Transl. Med. 2020, 12, eabc6659. [Google Scholar] [CrossRef] [PubMed]
- Thaci, B.; Ulasov, I.V.; Wainwright, D.A.; Lesniak, M.S. The challenge for gene therapy: Innate immune response to adenoviruses. Oncotarget 2011, 2, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Liu, X.; Chen, X.; Liu, C.; Zhang, Y.; Chu, C.; Wang, J.; Wang, X.; Chen, X.; Liu, G. Genetically Engineered Cell Membrane Nanovesicles for Oncolytic Adenovirus Delivery: A Versatile Platform for Cancer Virotherapy. Nano Lett. 2019, 19, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef]
- Laredj, L.N.; Beard, P. Adeno-associated virus activates an innate immune response in normal human cells but not in osteosarcoma cells. J. Virol. 2011, 85, 13133–13143. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Hareendran, S.; Balakrishnan, B.; Sen, D.; Kumar, S.; Srivastava, A.; Jayandharan, G.R. Adeno-associated virus (AAV) vectors in gene therapy: Immune challenges and strategies to circumvent them. Rev. Med. Virol. 2013, 23, 399–413. [Google Scholar] [CrossRef]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009, 199, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.M.; Flotte, T.R. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef]
- Prado, D.A.; Acosta-Acero, M.; Maldonado, R.S. Gene therapy beyond luxturna: A new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol. 2020, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- High, K.A.; Roncarolo, M.G. Gene Therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Ragni, M.V.; Rasko, J.E.J.; Raffini, L.J.; Samelson-Jones, B.J.; Ozelo, M.; Hazbon, M.; Runowski, A.R.; Wellman, J.A.; Wachtel, K.; et al. Long-Term Follow-Up of the First in Human Intravascular Delivery of AAV for Gene Transfer: AAV2-hFIX16 for Severe Hemophilia B. Mol. Ther. 2020, 28, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Monahan, P.E.; Eyster, M.E.; Sullivan, S.K.; Ragni, M.V.; Croteau, S.E.; Rasko, J.E.J.; Recht, M.; Samelson-Jones, B.J.; MacDougall, A.; et al. Multiyear Factor VIII Expression after AAV Gene Transfer for Hemophilia A. N. Engl. J. Med. 2021, 385, 1961–1973. [Google Scholar] [CrossRef]
- Miesbach, W.; Meijer, K.; Coppens, M.; Kampmann, P.; Klamroth, R.; Schutgens, R.; Tangelder, M.; Castaman, G.; Schwäble, J.; Bonig, H.; et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018, 131, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Pasi, K.J.; Rangarajan, S.; Mitchell, N.; Lester, W.; Symington, E.; Madan, B.; Laffan, M.; Russell, C.B.; Li, M.; Pierce, G.F.; et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N. Engl. J. Med. 2020, 382, 29–40. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef]
- Sands, M.S. AAV-mediated liver-directed gene therapy. Methods Mol. Biol. 2011, 807, 141–157. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N., Jr.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Weleber, R.G.; Yang, P.; Whitebirch, C.; Thean, B.; Flotte, T.R.; Humphries, M.; Chegarnov, E.; Beasley, K.N.; Stout, J.T.; et al. Results at 5 Years After Gene Therapy for RPE65-Deficient Retinal Dystrophy. Hum. Gene Ther. 2018, 29, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: An open label, phase I trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, L.; Chtarto, A.; Lehtonen, E.; Velu, T.; Brotchi, J.; Levivier, M. Recombinant AAV-mediated gene delivery to the central nervous system. J. Gene Med. 2004, 6 (Suppl. 1), S212–S222. [Google Scholar] [CrossRef]
- Castle, M.J.; Baltanas, F.C.; Kovacs, I.; Nagahara, A.H.; Barba, D.; Tuszynski, M.H. Postmortem Analysis in a Clinical Trial of AAV2-NGF Gene Therapy for Alzheimer’s Disease Identifies a Need for Improved Vector Delivery. Hum. Gene Ther. 2020, 31, 415–422. [Google Scholar] [CrossRef]
- Pearson, T.S.; Gupta, N.; San Sebastian, W.; Imamura-Ching, J.; Viehoever, A.; Grijalvo-Perez, A.; Fay, A.J.; Seth, N.; Lundy, S.M.; Seo, Y.; et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021, 12, 4251. [Google Scholar] [CrossRef]
- Spronck, E.A.; Brouwers, C.C.; Valles, A.; de Haan, M.; Petry, H.; van Deventer, S.J.; Konstantinova, P.; Evers, M.M. AAV5-miHTT Gene Therapy Demonstrates Sustained Huntingtin Lowering and Functional Improvement in Huntington Disease Mouse Models. Mol. Ther. Methods Clin. Dev. 2019, 13, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Martier, R.; Sogorb-Gonzalez, M.; Stricker-Shaver, J.; Hubener-Schmid, J.; Keskin, S.; Klima, J.; Toonen, L.J.; Juhas, S.; Juhasova, J.; Ellederova, Z.; et al. Development of an AAV-Based MicroRNA Gene Therapy to Treat Machado-Joseph Disease. Mol. Ther. Methods Clin. Dev. 2019, 15, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, J.; Nobre, R.J.; Pereira de Almeida, L. Gene therapy for the CNS using AAVs: The impact of systemic delivery by AAV9. J. Control Release 2016, 241, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Kaifer, K.A.; Villalon, E.; Smith, C.E.; Simon, M.E.; Marquez, J.; Hopkins, A.E.; Morcos, T.I.; Lorson, C.L. AAV9-DOK7 gene therapy reduces disease severity in Smn(2B/-) SMA model mice. Biochem. Biophys. Res. Commun. 2020, 530, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Sahenk, Z.; Lehman, K.; Nease, C.; Lowes, L.P.; Miller, N.F.; Iammarino, M.A.; Alfano, L.N.; Nicholl, A.; Al-Zaidy, S.; et al. Assessment of Systemic Delivery of rAAVrh74.MHCK7.micro-dystrophin in Children with Duchenne Muscular Dystrophy: A Nonrandomized Controlled Trial. JAMA Neurol. 2020, 77, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Annoussamy, M.; Lilien, C.; Gidaro, T.; Gargaun, E.; Che, V.; Schara, U.; Gangfuss, A.; D’Amico, A.; Dowling, J.J.; Darras, B.T.; et al. X-linked myotubular myopathy: A prospective international natural history study. Neurology 2019, 92, e1852–e1867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, Z.; Wang, X.; Jiang, H.; Neng, L.; Zhang, Y.; Yu, Q.; Burwood, G.; Song, J.; Auer, M.; et al. VEGFA165 gene therapy ameliorates blood-labyrinth barrier breakdown and hearing loss. JCI Insight 2021, 6, e143285. [Google Scholar] [CrossRef]
- Gyorgy, B.; Sage, C.; Indzhykulian, A.A.; Scheffer, D.I.; Brisson, A.R.; Tan, S.; Wu, X.D.; Volak, A.; Mu, D.K.; Tamvakologos, P.I.; et al. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 2017, 25, 379–391. [Google Scholar] [CrossRef]
- György, B.; Meijer, E.J.; Ivanchenko, M.V.; Tenneson, K.; Emond, F.; Hanlon, K.S.; Indzhykulian, A.A.; Volak, A.; Karavitaki, K.D.; Tamvakologos, P.I.; et al. Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate. Mol. Ther. Methods Clin. Dev. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Costa Verdera, H.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J., Jr.; et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13, eabd3438. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Di Paolo, N.C.; Mossman, K.L. Recognition of virus infection and innate host responses to viral gene therapy vectors. Mol. Ther. 2010, 18, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Yang, Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Investig. 2009, 119, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.T.; Suzuki, M.; Markusic, D.M.; Zolotukhin, I.; Ryals, R.C.; Moghimi, B.; Ertl, H.C.; Muruve, D.A.; Lee, B.; Herzog, R.W. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 2011, 117, 6459–6468. [Google Scholar] [CrossRef] [PubMed]
- Kessler, N.; Viehmann, S.F.; Krollmann, C.; Mai, K.; Kirschner, K.M.; Luksch, H.; Kotagiri, P.; Böhner, A.M.C.; Huugen, D.; de Oliveira Mann, C.C.; et al. Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing. J. Exp. Med. 2022, 219, e20220759. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Earley, L.F.; Chai, Z.; Chen, X.; Sun, J.; He, T.; Deng, M.; Hirsch, M.L.; Ting, J.; Samulski, R.J.; et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight 2018, 3, e120474. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Gross, D.A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Seregin, S.S.; Appledorn, D.M.; McBride, A.J.; Schuldt, N.J.; Aldhamen, Y.A.; Voss, T.; Wei, J.; Bujold, M.; Nance, W.; Godbehere, S.; et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther. 2009, 17, 685–696. [Google Scholar] [CrossRef]
- Dauletbekov, D.L.; Pfromm, J.K.; Fritz, A.K.; Fischer, M.D. Innate Immune Response Following AAV Administration. Adv. Exp. Med. Biol. 2019, 1185, 165–168. [Google Scholar] [CrossRef]
- Maheshri, N.; Koerber, J.T.; Kaspar, B.K.; Schaffer, D.V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 2006, 24, 198–204. [Google Scholar] [CrossRef]
- Bertolini, T.B.; Shirley, J.L.; Zolotukhin, I.; Li, X.; Kaisho, T.; Xiao, W.; Kumar, S.R.P.; Herzog, R.W. Effect of CpG Depletion of Vector Genome on CD8(+) T Cell Responses in AAV Gene Therapy. Front. Immunol. 2021, 12, 672449. [Google Scholar] [CrossRef] [PubMed]
- Munis, A.M. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses 2020, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- de Pablo-Maiso, L.; Domenech, A.; Echeverria, I.; Gomez-Arrebola, C.; de Andres, D.; Rosati, S.; Gomez-Lucia, E.; Reina, R. Prospects in Innate Immune Responses as Potential Control Strategies against Non-Primate Lentiviruses. Viruses 2018, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Rayat, A. Lentiviral Vector Bioprocessing. Viruses 2021, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.B.; Sumner, R.P.; Rodriguez-Plata, M.T.; Rasaiyaah, J.; Milne, R.S.; Thrasher, A.J.; Qasim, W.; Towers, G.J. Lentiviral Vector Production Titer Is Not Limited in HEK293T by Induced Intracellular Innate Immunity. Mol. Ther. Methods Clin. Dev. 2020, 17, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B.; Booth, C.; Kang, E.M.; Pai, S.Y.; Shaw, K.L.; Santilli, G.; Armant, M.; Buckland, K.F.; Choi, U.; De Ravin, S.S.; et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat. Med. 2020, 26, 200–206. [Google Scholar] [CrossRef]
- Thompson, A.A.; Walters, M.C.; Kwiatkowski, J.; Rasko, J.E.J.; Ribeil, J.A.; Hongeng, S.; Magrin, E.; Schiller, G.J.; Payen, E.; Semeraro, M.; et al. Gene Therapy in Patients with Transfusion-Dependent beta-Thalassemia. N. Engl. J. Med. 2018, 378, 1479–1493. [Google Scholar] [CrossRef]
- Magrin, E.; Semeraro, M.; Hebert, N.; Joseph, L.; Magnani, A.; Chalumeau, A.; Gabrion, A.; Roudaut, C.; Marouene, J.; Lefrere, F.; et al. Long-term outcomes of lentiviral gene therapy for the β-hemoglobinopathies: The HGB-205 trial. Nat. Med. 2022, 28, 81–88. [Google Scholar] [CrossRef]
- Mamcarz, E.; Zhou, S.; Lockey, T.; Abdelsamed, H.; Cross, S.J.; Kang, G.; Ma, Z.J.; Condori, J.; Dowdy, J.; Triplett, B.; et al. Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1. N. Engl. J. Med. 2019, 380, 1525–1534. [Google Scholar] [CrossRef]
- Cowan, M.J.; Yu, J.S.; Facchino, J.; Fraser-Browne, C.; Sanford, U.; Kawahara, M.; Dara, J.; Long-Boyle, J.; Oh, J.; Chan, W.Y.; et al. Lentiviral Gene Therapy for Artemis-Deficient SCID. N. Engl. J. Med. 2022, 387, 2344–2355. [Google Scholar] [CrossRef]
- Eichler, F.; Duncan, C.; Musolino, P.L.; Orchard, P.J.; De Oliveira, S.; Thrasher, A.J.; Armant, M.; Dansereau, C.; Lund, T.C.; Miller, W.P.; et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2017, 377, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Gentner, B.; Tucci, F.; Galimberti, S.; Fumagalli, F.; De Pellegrin, M.; Silvani, P.; Camesasca, C.; Pontesilli, S.; Darin, S.; Ciotti, F.; et al. Hematopoietic Stem- and Progenitor-Cell Gene Therapy for Hurler Syndrome. N. Engl. J. Med. 2021, 385, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Lorioli, L.; Fumagalli, F.; Acquati, S.; Redaelli, D.; Baldoli, C.; Canale, S.; Lopez, I.D.; Morena, F.; Calabria, A.; et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: An ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 2016, 388, 476–487. [Google Scholar] [CrossRef]
- Liu, S.; Deng, B.; Yin, Z.; Lin, Y.; An, L.; Liu, D.; Pan, J.; Yu, X.; Chen, B.; Wu, T.; et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am. J. Hematol. 2021, 96, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef]
- Bryson, P.D.; Han, X.; Truong, N.; Wang, P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine 2017, 35, 5842–5849. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.Y.; Ahn, H.J. Systemic delivery of CRISPR/Cas9 to hepatic tumors for cancer treatment using altered tropism of lentiviral vector. Biomaterials 2021, 272, 120793. [Google Scholar] [CrossRef]
- Matsunaga, W.; Ichikawa, M.; Nakamura, A.; Ishikawa, T.; Gotoh, A. Lentiviral Vector-mediated Gene Transfer in Human Bladder Cancer Cell Lines. Anticancer Res. 2018, 38, 2015–2020. [Google Scholar] [CrossRef]
- Russell, A.L.; Prince, C.; Lundgren, T.S.; Knight, K.A.; Denning, G.; Alexander, J.S.; Zoine, J.T.; Spencer, H.T.; Chandrakasan, S.; Doering, C.B. Non-genotoxic conditioning facilitates hematopoietic stem cell gene therapy for hemophilia A using bioengineered factor VIII. Mol. Ther. Methods Clin. Dev. 2021, 21, 710–727. [Google Scholar] [CrossRef]
- Olgasi, C.; Borsotti, C.; Merlin, S.; Bergmann, T.; Bittorf, P.; Adewoye, A.B.; Wragg, N.; Patterson, K.; Calabria, A.; Benedicenti, F.; et al. Efficient and safe correction of hemophilia A by lentiviral vector-transduced BOECs in an implantable device. Mol. Ther. Methods Clin. Dev. 2021, 23, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Canepari, C.; Liu, T.; Biffi, M.; Russo, F.; Plati, T.; Curto, R.; Patarroyo-White, S.; Drager, D.; Visigalli, I.; et al. Liver-directed lentiviral gene therapy corrects hemophilia A mice and achieves normal-range factor VIII activity in non-human primates. Nat. Commun. 2022, 13, 2454. [Google Scholar] [CrossRef]
- Chen, Y.; Schroeder, J.A.; Gao, C.; Li, J.; Hu, J.; Shi, Q. In vivo enrichment of genetically manipulated platelets for murine hemophilia B gene therapy. J. Cell Physiol. 2021, 236, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Tan, V.T.Y.; Thompson, N.; Guevremont, D.; Mockett, B.G.; Tate, W.P.; Abraham, W.C.; Hughes, S.M.; Williams, J. Lentivirus-Mediated Expression of Human Secreted Amyloid Precursor Protein-Alpha Promotes Long-Term Induction of Neuroprotective Genes and Pathways in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2021, 79, 1075–1090. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Yang, T.T.; Zhou, H.J.; Zhao, Y.; Kuang, X.; Duan, W.; Du, J.R. Lentiviral vector-mediated overexpression of Klotho in the brain improves Alzheimer’s disease-like pathology and cognitive deficits in mice. Neurobiol. Aging 2019, 78, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, J.M.; Kay, D.G. Progranulin gene delivery reduces plaque burden and synaptic atrophy in a mouse model of Alzheimer’s disease. PLoS ONE 2017, 12, e0182896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Wang, Y.; Lv, H.Y.; Han, Q.W.; Fan, H.; Guo, B.; Wang, L.L.; Han, W.D. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015, 23, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.D.; Sitia, G.; Annoni, A.; Hauben, E.; Sergi, L.S.; Zingale, A.; Roncarolo, M.G.; Guidotti, L.G.; Naldini, L. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood 2007, 109, 2797–2805. [Google Scholar] [CrossRef]
- Chintala, K.; Mohareer, K.; Banerjee, S. Dodging the Host Interferon-Stimulated Gene Mediated Innate Immunity by HIV-1: A Brief Update on Intrinsic Mechanisms and Counter-Mechanisms. Front. Immunol. 2021, 12, 716927. [Google Scholar] [CrossRef]
- Piras, F.; Riba, M.; Petrillo, C.; Lazarevic, D.; Cuccovillo, I.; Bartolaccini, S.; Stupka, E.; Gentner, B.; Cittaro, D.; Naldini, L.; et al. Lentiviral vectors escape innate sensing but trigger p53 in human hematopoietic stem and progenitor cells. EMBO Mol. Med. 2017, 9, 1198–1211. [Google Scholar] [CrossRef]
- Kajaste-Rudnitski, A.; Naldini, L. Cellular innate immunity and restriction of viral infection: Implications for lentiviral gene therapy in human hematopoietic cells. Hum. Gene Ther. 2015, 26, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Coroadinha, A.S. Host Cell Restriction Factors Blocking Efficient Vector Transduction: Challenges in Lentiviral and Adeno-Associated Vector Based Gene Therapies. Cells 2023, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Annoni, A.; Gregori, S.; Naldini, L.; Cantore, A. Modulation of immune responses in lentiviral vector-mediated gene transfer. Cell Immunol. 2019, 342, 103802. [Google Scholar] [CrossRef] [PubMed]
- He, T.S.; Dang, L.L.; Zhang, J.H.; Zhang, J.Q.; Wang, G.P.; Wang, E.N.; Xia, H.; Zhou, W.H.; Wu, S.; Liu, X.Q. The Hippo signaling component LATS2 enhances innate immunity to inhibit HIV-1 infection through PQBP1-cGAS pathway. Cell Death Differ. 2022, 29, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Guo, D.; Chen, S. Application of CRISPR/Cas9-Based Gene Editing in HIV-1/AIDS Therapy. Front. Cell Infect. Microbiol. 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Annoni, A.; Moalli, F.; Liu, T.; Cesana, D.; Calabria, A.; Bartolaccini, S.; Biffi, M.; Russo, F.; Visigalli, I.; et al. Phagocytosis-shielded lentiviral vectors improve liver gene therapy in nonhuman primates. Sci. Transl. Med. 2019, 11, eaav7325. [Google Scholar] [CrossRef] [PubMed]
- Alyami, E.M.; Tarar, A.; Peng, C.A. Less phagocytosis of viral vectors by tethering with CD47 ectodomain. J. Mater. Chem. B 2021, 10, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Sutlu, T.; Nystrom, S.; Gilljam, M.; Stellan, B.; Applequist, S.E.; Alici, E. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: Implications for gene therapy. Hum. Gene Ther. 2012, 23, 1090–1100. [Google Scholar] [CrossRef]
- Petrillo, C.; Thorne, L.G.; Unali, G.; Schiroli, G.; Giordano, A.M.S.; Piras, F.; Cuccovillo, I.; Petit, S.J.; Ahsan, F.; Noursadeghi, M.; et al. Cyclosporine H Overcomes Innate Immune Restrictions to Improve Lentiviral Transduction and Gene Editing in Human Hematopoietic Stem Cells. Cell Stem Cell 2018, 23, 820–832.e9. [Google Scholar] [CrossRef]
- Agudo, J.; Ruzo, A.; Kitur, K.; Sachidanandam, R.; Blander, J.M.; Brown, B.D. A TLR and non-TLR mediated innate response to lentiviruses restricts hepatocyte entry and can be ameliorated by pharmacological blockade. Mol. Ther. 2012, 20, 2257–2267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shao, W. Innate Immune Response to Viral Vectors in Gene Therapy. Viruses 2023, 15, 1801. https://doi.org/10.3390/v15091801
Wang Y, Shao W. Innate Immune Response to Viral Vectors in Gene Therapy. Viruses. 2023; 15(9):1801. https://doi.org/10.3390/v15091801
Chicago/Turabian StyleWang, Yixuan, and Wenwei Shao. 2023. "Innate Immune Response to Viral Vectors in Gene Therapy" Viruses 15, no. 9: 1801. https://doi.org/10.3390/v15091801
APA StyleWang, Y., & Shao, W. (2023). Innate Immune Response to Viral Vectors in Gene Therapy. Viruses, 15(9), 1801. https://doi.org/10.3390/v15091801






