Associations between Dengue Incidence, Ecological Factors, and Anthropogenic Factors in Singapore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Dengue Data
2.2. Exposures
2.3. Statistical Analysis
2.4. Spatial Autocorrelation Analysis
3. Results
3.1. Study Setting
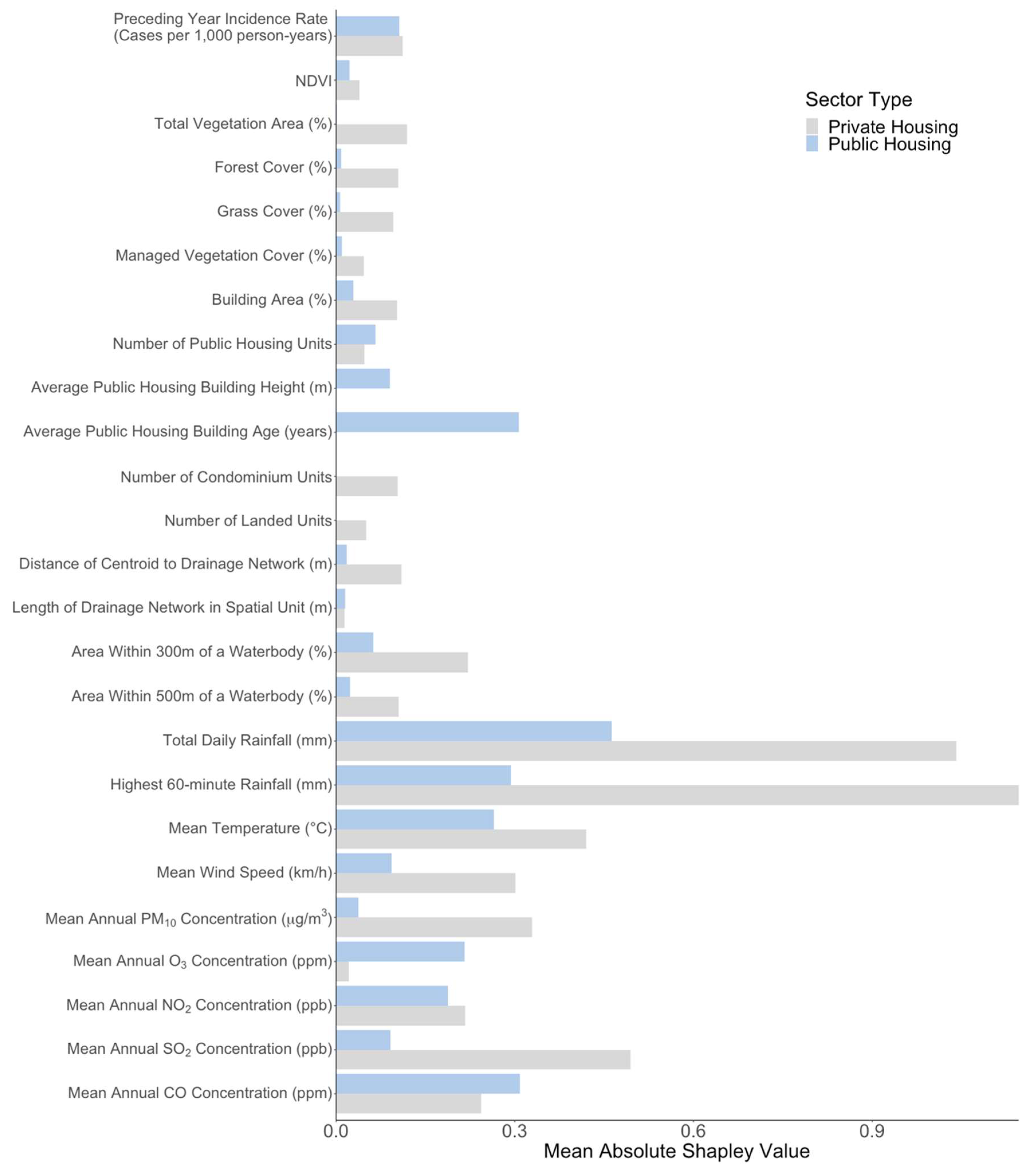
3.2. Model Assesment
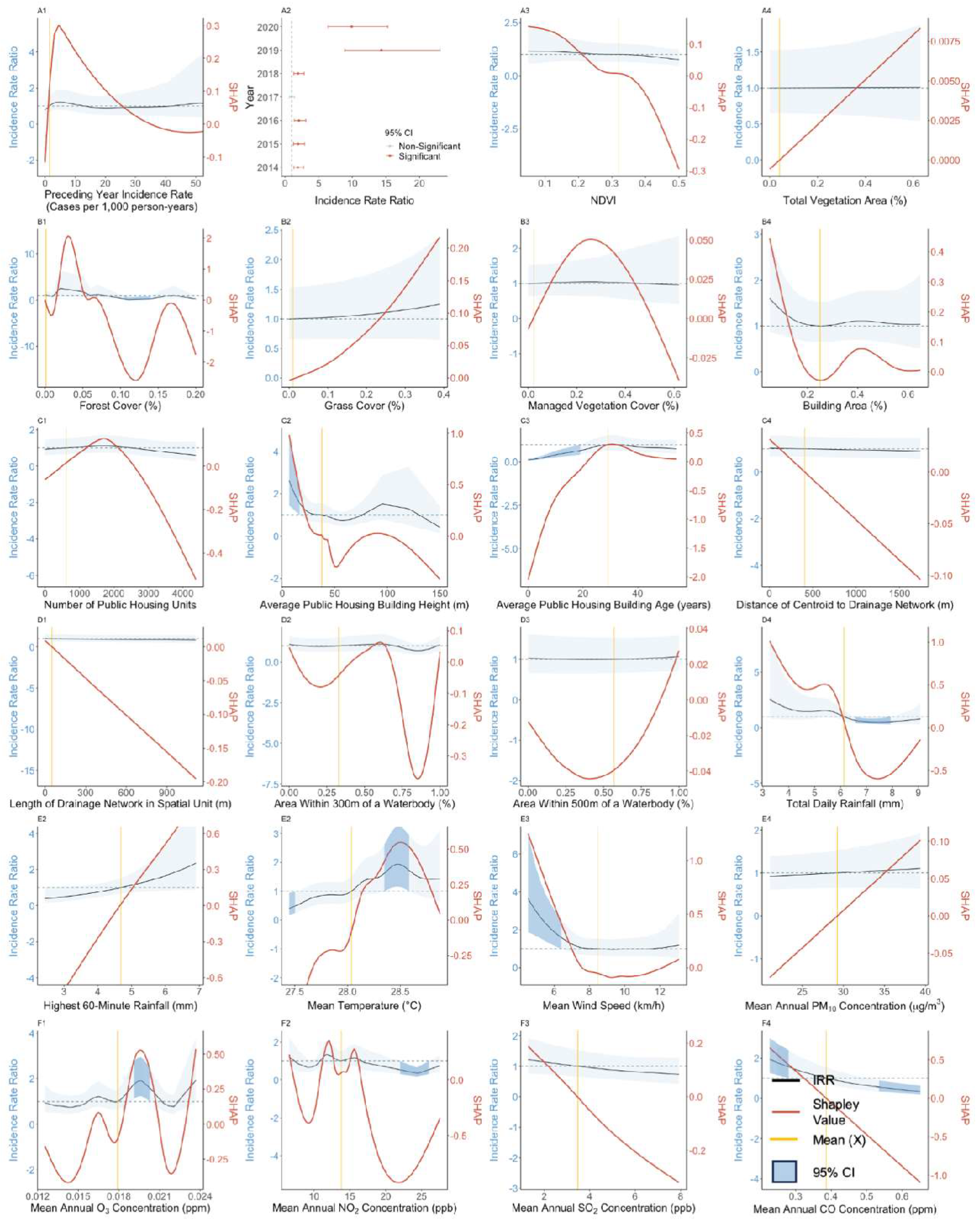
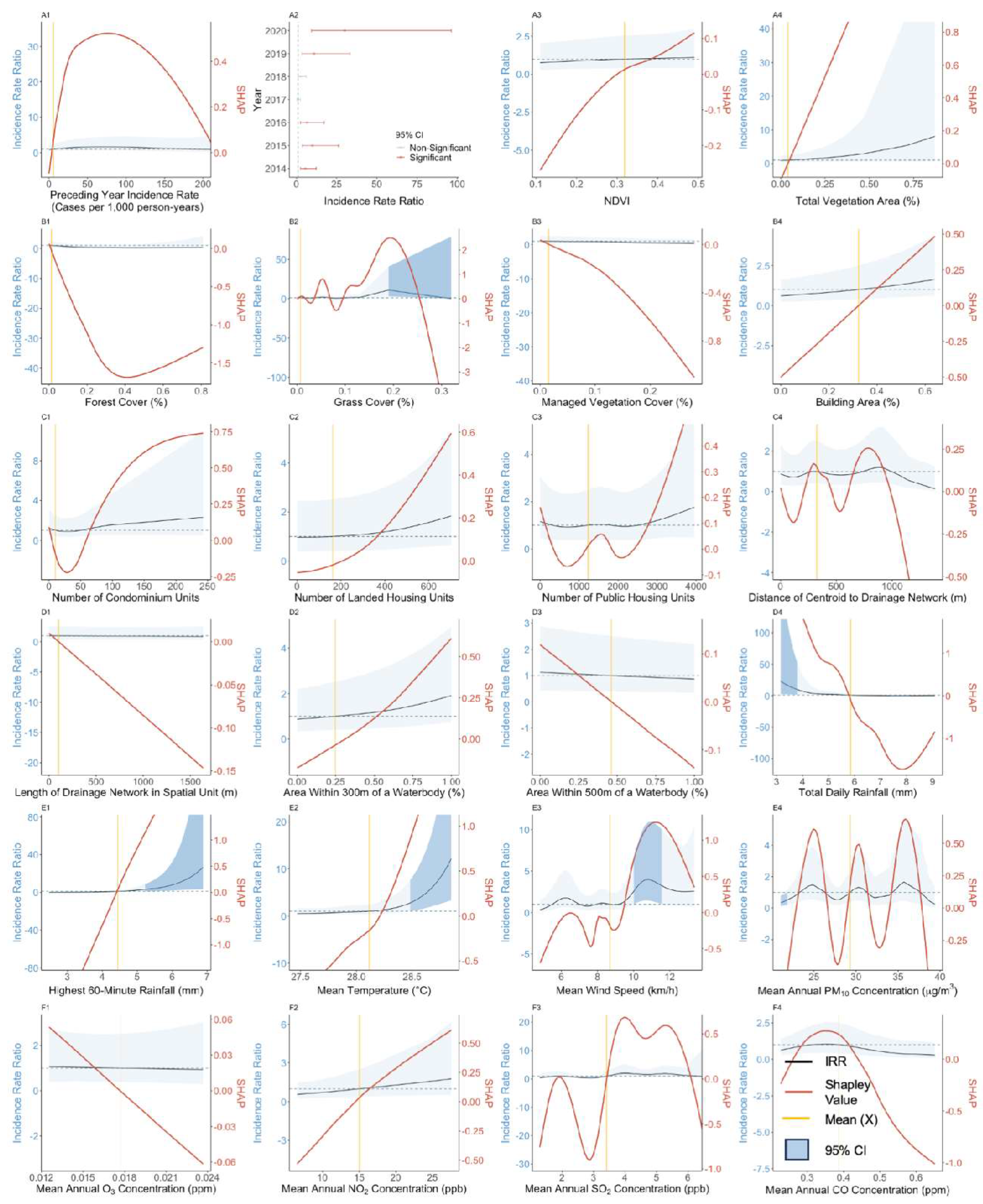
3.3. Effect of Preceding Year Incidence Rate
3.4. Effect of Vegetation-Related Exposures
3.5. Effect of Anthropogenic Exposures
3.6. Effect of Meteorological and Atmospheric Exposures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhan, J.; Chen, L.; Chen, H.; Cheng, S. Global, regional, and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. EClinicalMedicine 2021, 32, 100712. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Kolimenakis, A.; Heinz, S.; Wilson, M.L.; Winkler, V.; Yakob, L.; Michaelakis, A.; Papachristos, D.; Richardson, C.; Horstick, O. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit—A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009631. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.A.; Duguay, C.; Ost, K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: A scoping review of reviews. Glob. Health 2022, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.J.; Kotsila, P.; Mortyn, P.G.; Sarto i Monteys, V.; Brancati, C.U. Influence of socio-economic, demographic and climate factors on the regional distribution of dengue in the United States and Mexico. Int. J. Health Geogr. 2020, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Buhler, C.; Winkler, V.; Runge-Ranzinger, S.; Boyce, R.; Horstick, O. Environmental methods for dengue vector control—A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007420. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21st Century. Trop. Med. Health 2011, 39 (Suppl. 4), 3–11. [Google Scholar] [CrossRef]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020; World Health Organization: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/handle/10665/75303 (accessed on 16 May 2023).
- Redoni, M.; Yacoub, S.; Rivino, L.; Giacobbe, D.R.; Luzzati, R.; Di Bella, S. Dengue: Status of current and under-development vaccines. Rev. Med. Virol. 2020, 30, e2101. [Google Scholar] [CrossRef]
- Torres-Flores, J.M.; Reyes-Sandoval, A.; Salazar, M.I. Dengue Vaccines: An Update. BioDrugs 2022, 36, 325–336. [Google Scholar] [CrossRef]
- EMA. Qdenga. European Medicines Agency. 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/qdenga (accessed on 27 June 2023).
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Population Trends. Available online: http://www.singstat.gov.sg/publications/population/population-trends (accessed on 18 May 2023).
- Environment. Available online: http://www.singstat.gov.sg/find-data/search-by-theme/society/environment/latest-data (accessed on 18 May 2023).
- The World Bank. World Bank Open Data. Available online: https://data.worldbank.org (accessed on 18 May 2023).
- Sim, S.; Ng, L.C.; Lindsay, S.W.; Wilson, A.L. A greener vision for vector control: The example of the Singapore dengue control programme. PLoS Negl. Trop. Dis. 2020, 14, e0008428. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Lim, J.T.; Ong, J.; Hapuarachchi, H.C.; Sim, S.; Ng, L.C. Singapore’s 5 decades of dengue prevention and control—Implications for global dengue control. PLoS Negl. Trop. Dis. 2023, 17, e0011400. [Google Scholar] [CrossRef]
- Koh, B.K.; Ng, L.C.; Kita, Y.; Tang, C.S.; Ang, L.W.; Wong, K.Y.; James, L.; Goh, K.T. The 2005 dengue epidemic in Singapore: Epidemiology, prevention and control. Ann. Acad. Med. Singap. 2008, 37, 538–545. [Google Scholar] [CrossRef] [PubMed]
- MOH|Infectious Diseases Act. Available online: https://www.moh.gov.sg/policies-and-legislation/infectious-diseases-act (accessed on 28 June 2023).
- Wood, S.N. Thin plate regression splines. J. R. Stat. Soc. Ser. B Stat. Methodol. 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2010, 73, 3–36. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In 2nd International Symposium on Information Theory; Petrov, B.N., Csáki, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 267–281. [Google Scholar]
- Lundberg, S.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar] [CrossRef]
- Sun, H.; Dickens, B.L.; Richards, D.; Ong, J.; Rajarethinam, J.; Hassim, M.E.E.; Lim, J.T.; Carrasco, L.R.; Aik, J.; Yap, G.; et al. Spatio-temporal analysis of the main dengue vector populations in Singapore. Parasites Vectors 2021, 14, 41. [Google Scholar] [CrossRef]
- Soh, S.; Ho, S.H.; Seah, A.; Ong, J.; Richards, D.R.; Gaw, L.Y.-F.; Dickens, B.S.; Tan, K.W.; Koo, J.R.; Cook, A.R.; et al. Spatial Methods for Inferring Extremes in Dengue Outbreak Risk in Singapore. Viruses 2022, 14, 2450. [Google Scholar] [CrossRef]
- Ong, J.; Soh, S.; Ho, S.H.; Seah, A.; Dickens, B.S.; Tan, K.W.; Koo, J.R.; Cook, A.R.; Richards, D.R.; Gaw, L.Y.-F.; et al. Fine-scale estimation of effective reproduction numbers for dengue surveillance. PLoS Comput. Biol. 2022, 18, e1009791. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.T.; Han, Y.; Dickens, B.S.L.; Choo, E.L.W.; Chew, L.Z.X.; Cook, A.R. Revealing two dynamic dengue epidemic clusters in Thailand. BMC Infect. Dis. 2020, 20, 927. [Google Scholar] [CrossRef] [PubMed]
- da Consolação Magalhães Cunha, M.; Ju, Y.; Morais, M.H.F.; Dronova, I.; Ribeiro, S.P.; Bruhn, F.R.P.; Lima, L.L.; Sales, D.M.; Schultes, O.L.; Rodriguez, D.A.; et al. Disentangling associations between vegetation greenness and dengue in a Latin American city: Findings and challenges. Landsc. Urban Plan. 2021, 216, 104255. [Google Scholar]
- Sari, S.Y.I.; Adelwin, Y.; Rinawan, F.R. Land Use Changes and Cluster Identification of Dengue Hemorrhagic Fever Cases in Bandung, Indonesia. Trop. Med. Infect. Dis. 2020, 5, 70. [Google Scholar] [CrossRef]
- Huang, C.-C.; Tam, T.Y.T.; Chern, Y.-R.; Lung, S.-C.C.; Chen, N.-T.; Wu, C.-D. Spatial Clustering of Dengue Fever Incidence and Its Association with Surrounding Greenness. Int. J. Environ. Res. Public Health 2018, 15, 1869. [Google Scholar] [CrossRef]
- Asmare, Y.; Hill, S.R.; Hopkins, R.J.; Tekie, H.; Ignell, R. The role of grass volatiles on oviposition site selection by Anopheles arabiensis and Anopheles coluzzii. Malar. J. 2017, 16, 65. [Google Scholar] [CrossRef]
- Seidahmed, O.M.E.; Lu, D.; Chong, C.S.; Ng, L.C.; Eltahir, E.A.B. Patterns of Urban Housing Shape Dengue Distribution in Singapore at Neighborhood and Country Scales. GeoHealth 2018, 2, 54–67. [Google Scholar] [CrossRef]
- Montgomery, B.L.; Ritchie, S.A.; Hart, A.J.; Long, S.A.; Walsh, I.D. Subsoil drain sumps are a key container for Aedes aegypti in Cairns, Australia. J. Am. Mosq. Control Assoc. 2004, 20, 365–369. [Google Scholar]
- Paploski, I.A.D.; Rodrigues, M.S.; Mugabe, V.A.; Kikuti, M.; Tavares, A.S.; Reis, M.G.; Kitron, U.; Ribeiro, G.S. Storm drains as larval development and adult resting sites for Aedes aegypti and Aedes albopictus in Salvador, Brazil. Parasites Vectors 2016, 9, 419. [Google Scholar] [CrossRef]
- Fernandez, S.A.; Sun, H.; Dickens, B.L.; Ng, L.C.; Cook, A.R.; Lim, J.T. Features of the urban environment associated with Aedes aegypti abundance in high-rise public apartments in Singapore: An environmental case-control study. PLoS Negl. Trop. Dis. 2023, 17, e0011075. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M.; Besse-Lototskaya, A.A. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 2014, 45, 69–79. [Google Scholar] [CrossRef]
- Francisco, M.E.; Carvajal, T.M.; Ryo, M.; Nukazawa, K.; Amalin, D.M.; Watanabe, K. Dengue disease dynamics are modulated by the combined influences of precipitation and landscape: A machine learning approach. Sci. Total Environ. 2021, 792, 148406. [Google Scholar] [CrossRef] [PubMed]
- Polwiang, S. The time series seasonal patterns of dengue fever and associated weather variables in Bangkok (2003–2017). BMC Infect. Dis. 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Struchiner, C.J.; Rocklöv, J.; Wilder-Smith, A.; Massad, E. Increasing Dengue Incidence in Singapore over the Past 40 Years: Population Growth, Climate and Mobility. PLoS ONE 2015, 10, e0136286. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Fu, X.; Lee, L.K.H.; Ma, S.; Goh, K.T.; Wong, J.; Habibullah, M.S.; Lee, G.K.K.; Lim, T.K.; Tambyah, P.A.; et al. Statistical Modeling Reveals the Effect of Absolute Humidity on Dengue in Singapore. PLoS Negl. Trop. Dis. 2014, 8, e2805. [Google Scholar] [CrossRef]
- Lu, L.; Lin, H.; Tian, L.; Yang, W.; Sun, J.; Liu, Q. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009, 9, 395. [Google Scholar] [CrossRef]
- Cheong, Y.L.; Burkart, K.; Leitão, P.J.; Lakes, T. Assessing Weather Effects on Dengue Disease in Malaysia. Int. J. Environ. Res. Public Health 2013, 10, 6319–6334. [Google Scholar] [CrossRef]
- Hoffmann, E.J.; Miller, J.R. Reduction of mosquito (Diptera: Culicidae) attacks on a human subject by combination of wind and vapor-phase DEET repellent. J. Med. Èntomol. 2002, 39, 935–938. [Google Scholar] [CrossRef]
- Ehelepola, N.D.B.; Ariyaratne, K.; Buddhadasa, W.M.N.P.; Ratnayake, S.; Wickramasinghe, M. A study of the correlation between dengue and weather in Kandy City, Sri Lanka (2003–2012) and lessons learned. Infect. Dis. Poverty 2015, 4, 42. [Google Scholar] [CrossRef]
- Goto, K.; Kumarendran, B.; Mettananda, S.; Gunasekara, D.; Fujii, Y.; Kaneko, S. Analysis of Effects of Meteorological Factors on Dengue Incidence in Sri Lanka Using Time Series Data. PLoS ONE 2013, 8, e63717. [Google Scholar] [CrossRef]
- Fan, J.; Lin, H.; Wang, C.; Bai, L.; Yang, S.; Chu, C.; Yang, W.; Liu, Q. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong Province, China from 2005 to 2011. Epidemiol. Infect. 2013, 142, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dou, Q.; Lu, Y.; Xiang, H.; Yu, X.; Liu, S. Effects of ambient temperature and precipitation on the risk of dengue fever: A systematic review and updated meta-analysis. Environ. Res. 2020, 191, 110043. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Coelho, M.; Oliver, L.; Massad, E. The influence of climate variables on dengue in Singapore. Int. J. Environ. Health Res. 2011, 21, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Benedum, C.M.; Seidahmed, O.M.E.; Eltahir, E.A.B.; Markuzon, N. Statistical modeling of the effect of rainfall flushing on dengue transmission in Singapore. PLoS Negl. Trop. Dis. 2018, 12, e0006935. [Google Scholar] [CrossRef] [PubMed]
- Feldhaar, H.; Otti, O. Pollutants and Their Interaction with Diseases of Social Hymenoptera. Insects 2020, 11, 153. [Google Scholar] [CrossRef]
- Phanitchat, T.; Ampawong, S.; Yawootti, A.; Denpetkul, T.; Wadmanee, N.; Sompornrattanaphan, M.; Sivakorn, C. Dose-Dependent Blood-Feeding Activity and Ovarian Alterations to PM2.5 in Aedes aegypti. Insects 2021, 12, 948. [Google Scholar] [CrossRef]
- Soh, S.; Ho, S.H.; Seah, A.; Ong, J.; Dickens, B.S.; Tan, K.W.; Koo, J.R.; Cook, A.R.; Tan, K.B.; Sim, S.; et al. Economic impact of dengue in Singapore from 2010 to 2020 and the cost-effectiveness of Wolbachia interventions. PLoS Glob. Public Health 2021, 1, e0000024. [Google Scholar] [CrossRef]
- Gaw, L.Y.-F.; Yee, A.T.K.; Richards, D.R. A high-resolution map of singapore’s terrestrial ecosystems. Data 2019, 4, 116. [Google Scholar] [CrossRef]
- USGS. 2023. Available online: https://www.usgs.gov/landsat-missions/landsat-normalized-difference-vegetation-index (accessed on 18 May 2023).
- OneMap. Onemap Api. 2023. Available online: https://www.onemap.gov.sg/main/v2/ (accessed on 18 May 2023).
- Technical Assistance Document for the Reporting of Daily Air Quality—The Air Quality Index (AQI). Available online: https://www.airnow.gov/sites/default/files/2020-05/aqi-technical-assistance-document-sept2018.pdf (accessed on 18 May 2023).
- ECMWF. Resale Flat Prices. 2023. Available online: https://www.ecmwf.int/ (accessed on 18 May 2023).
- DoS. Singapore Department of Statistics (dos). 2023. Available online: https://www.singstat.gov.sg/ (accessed on 18 May 2023).
| Mean (SD) | ||||
|---|---|---|---|---|
| Exposure | Description | Public Housing Spatial Units | Private Housing Spatial Units | |
| Current Year Incidence Rate (Cases per 1000 person-years) | 1.57 (4.54) | 7.43 (19.57) | ||
| Population | 5103.62 (2661.30) | 949.00 (852.25) | ||
| Preceding Year Incidence Rate (Cases per 1000 person-years) | 1.45 (4.41) | 5.86 (18.11) | ||
| Vegetation-Related | NDVI | NDVI serves as an alternative measure of vegetation cover. | 0.32 (0.06) | 0.32 (0.06) |
| Total Vegetation Area (%) | Percentage cover of vegetation exposures, with areas classified across multiple vegetation types including grass, forest, and managed vegetation. Managed Vegetation was defined as vegetation found in park areas. | 0.04 (0.08) | 0.04 (0.09) | |
| Forest Cover (%) | 0.001 (0.01) | 0.01 (0.07) | ||
| Grass Cover (%) | 0.01 (0.04) | 0.01 (0.03) | ||
| Managed Vegetation Cover (%) | 0.02 (0.05) | 0.02 (0.03) | ||
| Anthropogenic | Building Area (%) | The percentage cover of built area was calculated by summing the areas of all commercial, industrial, and residential buildings, and serves as a measure of urbanicity. | 0.25 (0.07) | 0.32 (0.09) |
| Number of Public Housing Units | Data on locations of public housing estates, representing the residences of most of the population. | 609.77 (719.96) | 1229.55 (677.14) | |
| Average Public Housing Building Height (m) | Average public housing building height was determined based on the number of floors and an assumed average height of 3 m per floor. | 37.56 (12.32) | ||
| Average Public Housing Building Age (years) | Average age of buildings within each spatial unit was collected through residential location and resale data. | 29.10 (11.98) | ||
| Number of Condominium Units | The number of condominiums and landed properties was collected for each spatial unit. | 10.00 (19.43) | ||
| Number of Landed Housing Units | 161.67 (122.21) | |||
| Distance of Centroid to Drainage Network (m) | Data on the major open drainage network in Singapore were collected from the Public Utilities Board, and were used to measure the distance between each HDB block and a drain, as well as the length of the drainage network present within the spatial unit. | 407.20 (289.54) | 325.61 (226.01) | |
| Length of Drainage Network in Spatial Unit (m) | 50.47 (130.61) | 103.26 (237.07) | ||
| Area Within 300 m of a Waterbody (%) | Area within spatial units that fall within 300 m and 500 m radius buffers around waterbodies. | 0.33 (0.40) | 0.25 (0.36) | |
| Area Within 500 m of a Waterbody (%) | 0.57 (0.44) | 0.46 (0.44) | ||
| Meteorological | Total Daily Rainfall (mm) | Meteorological exposures that have been well established to influence DENV transmission were obtained from 21 local weather stations. As these variables were collected on a daily timescale, the values were harmonized to the annual timescale of the dengue incidence rates by taking the annual mean of respective exposure within each spatial unit. | 6.13 (1.16) | 5.85 (1.27) |
| Highest 60-Minute Rainfall (mm) | 4.68 (0.88) | 4.44 (0.95) | ||
| Mean Temperature (°C) | 28.04 (0.26) | 28.11 (0.26) | ||
| Mean Wind Speed (km/h) | 8.47 (1.20) | 8.68 (1.10) | ||
| Atmospheric | Mean Annual PM10 Concentration (µg/m3) | Surface concentrations of ambient air pollutants were obtained from the Air Quality Open Data Platform, which compiles values provided by Singapore’s National Environment Agency (NEA). These values were also aggregated at an annual level to correspond with DENV data. | 29.27 (4.46) | 29.30 (4.50) |
| Mean Annual O3 Concentration (ppm) | 0.02 (0.002) | 0.02 (0.002) | ||
| Mean Annual NO2 Concentration (ppb) | 13.81 (4.60) | 15.02 (5.33) | ||
| Mean Annual SO2 Concentration (ppb) | 3.48 (1.34) | 3.41 (1.22) | ||
| Mean Annual CO Concentration (ppm) | 0.39 (0.09) | 0.39 (0.10) | ||
| Observations | 5611 | 2828 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewari, P.; Guo, P.; Dickens, B.; Ma, P.; Bansal, S.; Lim, J.T. Associations between Dengue Incidence, Ecological Factors, and Anthropogenic Factors in Singapore. Viruses 2023, 15, 1917. https://doi.org/10.3390/v15091917
Tewari P, Guo P, Dickens B, Ma P, Bansal S, Lim JT. Associations between Dengue Incidence, Ecological Factors, and Anthropogenic Factors in Singapore. Viruses. 2023; 15(9):1917. https://doi.org/10.3390/v15091917
Chicago/Turabian StyleTewari, Pranav, Peihong Guo, Borame Dickens, Pei Ma, Somya Bansal, and Jue Tao Lim. 2023. "Associations between Dengue Incidence, Ecological Factors, and Anthropogenic Factors in Singapore" Viruses 15, no. 9: 1917. https://doi.org/10.3390/v15091917






