Seasonal Occurrence of African Swine Fever in Wild Boar and Domestic Pigs in EU Member States
Abstract
:1. Introduction
2. Materials and Methods
2.1. African Swine Fever Surveillance Data
2.2. Data Analyses
3. Results
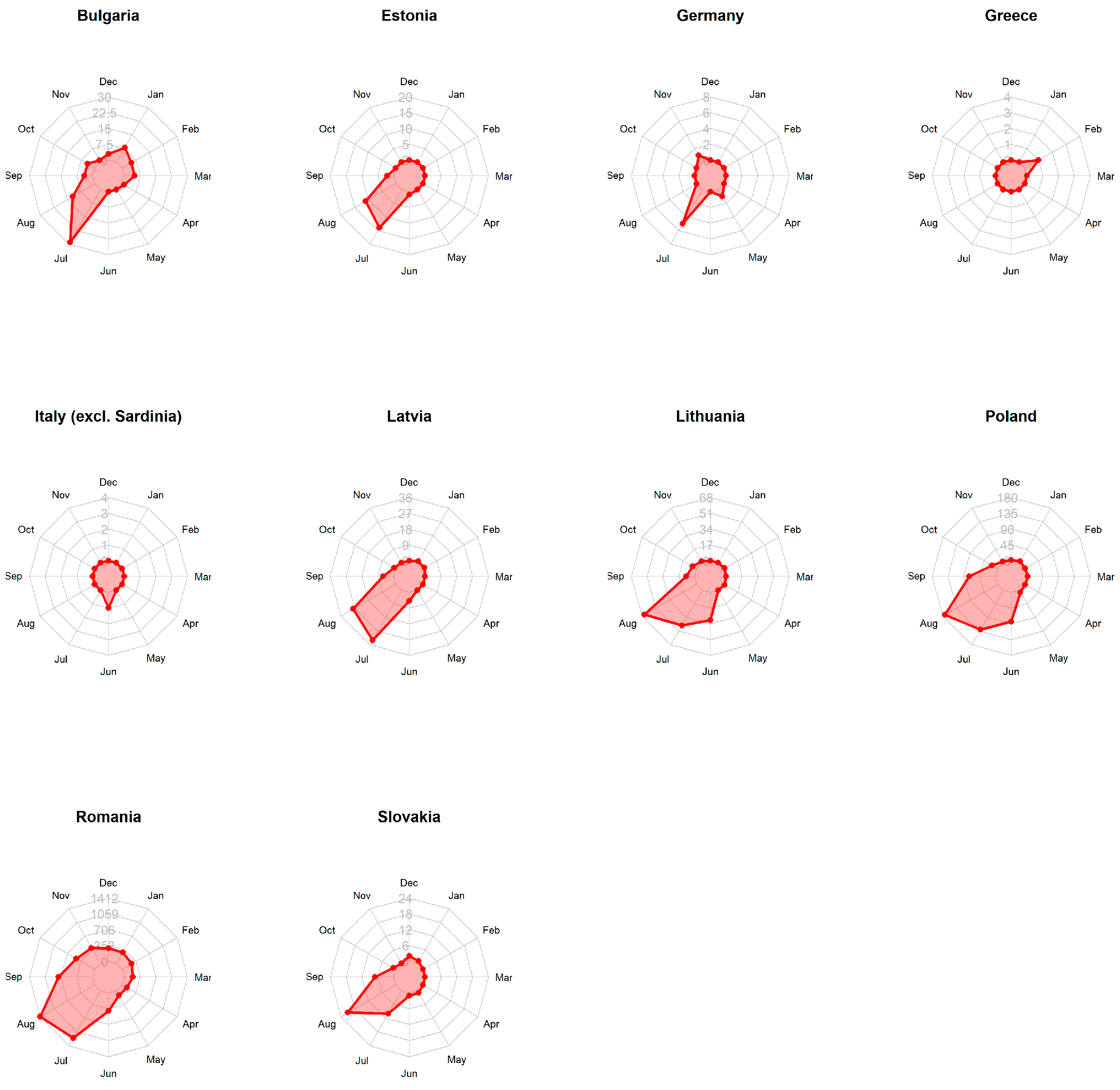
3.1. Domestic Pigs
3.2. Wild Boar
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Country | Test Statistic | p-Value | Year of First Occurrence | Year of Last Occurrence |
|---|---|---|---|---|
| Bulgaria | 10.31 | 0.503 | 2018 | 2022 |
| Estonia | 5.51 | 0.904 | 2015 | 2021 |
| Germany | 7.77 | 0.734 | 2021 | 2022 |
| Greece | n.a. | n.a. | 2020 | 2020 |
| Italy | n.a. | n.a. | 2022 | 2022 |
| Latvia | 21.12 | 0.032 | 2014 | 2022 |
| Lithuania | 21.84 | 0.026 | 2014 | 2022 |
| Poland | 27.75 | 0.004 | 2014 | 2022 |
| Romania | 27.83 | 0.003 | 2017 | 2022 |
| Slovakia | 11.10 | 0.435 | 2019 | 2022 |

| Country | Test Statistic | p-Value | Year of First Occurrence | Year of Last Occurrence |
|---|---|---|---|---|
| Belgium | 10.92 | 0.450 | 2018 | 2019 |
| Bulgaria | 24.26 | 0.012 | 2018 | 2022 |
| Czech Republic | 2.55 | 0.995 | 2017 | 2022 |
| Estonia | 38.12 | <0.001 | 2014 | 2022 |
| Germany | 17.92 | 0.083 | 2020 | 2022 |
| Hungary | 24.95 | 0.009 | 2018 | 2022 |
| Italy | n.a. | n.a. | 2022 | 2022 |
| Latvia | 46.81 | <0.001 | 2014 | 2022 |
| Lithuania | 22.87 | 0.018 | 2014 | 2022 |
| Poland | 30.61 | 0.001 | 2014 | 2022 |
| Romania | 27.98 | 0.003 | 2018 | 2022 |
| Slovakia | 12.84 | 0.304 | 2019 | 2022 |
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Gabriel, C.; Dietze, K.; Breithaupt, A.; Beer, M. High virulence of African swine fever virus caucasus isolate in European wild boars of all ages. Emerg. Infect. Dis. 2012, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- Nurmoja, I.; Schulz, K.; Staubach, C.; Sauter-Louis, C.; Depner, K.; Conraths, F.J.; Viltrop, A. Development of African swine fever epidemic among wild boar in Estonia—Two different areas in the epidemiological focus. Sci. Rep. 2017, 7, 12562. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Guinat, C.; Beer, M.; Pronin, V.; Tauscher, K.; Petrov, A.; Keil, G.; Blome, S. Course and transmission characteristics of oral low-dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Arch. Virol. 2015, 160, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Vial, L.; Penrith, M.L.; Pérez-Sánchez, R.; Etter, E.; Albina, E.; Michaud, V.; Roger, F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 2013, 173, 212–227. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare. Scientific Opinion on African Swine Fever. EFSA J. 2010, 8, 1556. [Google Scholar] [CrossRef]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The persistence of African swine fever virus in field-infected Ornithodoros erraticus during the ASF endemic period in Portugal. PLoS ONE 2011, 6, e20383. [Google Scholar] [CrossRef]
- Franzoni, G.; Dei Giudici, S.; Loi, F.; Sanna, D.; Floris, M.; Fiori, M.; Sanna, M.L.; Madrau, P.; Scarpa, F.; Zinellu, S.; et al. African Swine Fever Circulation among Free-Ranging Pigs in Sardinia: Data from the Eradication Program. Vaccines 2020, 8, 549. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare. Scientific Opinion on African swine fever. EFSA J. 2014, 12, 3628. [Google Scholar] [CrossRef]
- Mačiulskis, P.; Masiulis, M.; Pridotkas, G.; Buitkuvienė, J.; Jurgelevičius, V.; Jacevičienė, I.; Zagrabskaitė, R.; Zani, L.; Pilevičienė, S. The African Swine Fever Epidemic in Wild Boar (Sus scrofa) in Lithuania (2014–2018). Vet. Sci. 2020, 7, 15. [Google Scholar] [CrossRef]
- Oļševskis, E.; Guberti, V.; Seržants, M.; Westergaard, J.; Gallardo, C.; Rodze, I.; Depner, K. African swine fever virus introduction into the EU in 2014: Experience of Latvia. Res. Vet. Sci. 2016, 105, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Woźniakowski, G.; Kozak, E.; Kowalczyk, A.; Łyjak, M.; Pomorska-Mól, M.; Niemczuk, K.; Pejsak, Z. Current status of African swine fever virus in a population of wild boar in eastern Poland (2014–2015). Arch. Virol. 2016, 161, 189–195. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Boklund, A.; Cay, B.; Depner, K.; Földi, Z.; Guberti, V.; Masiulis, M.; Miteva, A.; More, S.; Oļševskis, E.; et al. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA J. 2018, 16, e05494. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Boklund, A.; Bøtner, A.; Chesnoiu Vasile, T.; Depner, K.; Desmecht, D.; Guberti, V.; Helyes, G.; Korytarova, D.; Linden, A.; et al. Scientific report on the epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA J. 2020, 18, e05996. [Google Scholar] [CrossRef]
- European Food Safety Authority; Baños, J.V.; Boklund, A.; Gogin, A.; Gortázar, C.; Guberti, V.; Helyes, G.; Kantere, M.; Korytarova, D.; Linden, A.; et al. Scientific report on the epidemiological analyses of African swine fever in the European Union. EFSA J. 2022, 20, e07290. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Göldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2021, 68, 1744–1752. [Google Scholar] [CrossRef]
- Iscaro, C.; Dondo, A.; Ruocco, L.; Masoero, L.; Giammarioli, M.; Zoppi, S.; Guberti, V.; Feliziani, F. January 2022: Index case of new African Swine Fever incursion in mainland Italy. Transbound. Emerg. Dis. 2022, 69, 1707–1711. [Google Scholar] [CrossRef]
- World Organisation of Animal Health (OIE). Self-Declaration of the Recovery of Freedom from African Fwine Fever in All Suids by the Czech Republic; OIE: Prague, Czech Republic, 2019; Available online: https://www.woah.org/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Self-declarations/2019_05_CzechRep_ASF_ANG.pdf (accessed on 13 September 2023).
- World Organisation of Animal Health (OIE). Self-Declaration of Belgium’s African Swine Fever-Free Status in All Swine Species. 2020. Available online: https://www.fasfc.be/sites/default/files/content/explorer/Animals/ASF/OIE/2020_12_Belgium_ASF_self-declaration_ENG.pdf (accessed on 13 September 2023).
- Chenais, E.; Ståhl, K.; Guberti, V.; Depner, K. Identification of Wild Boar-Habitat Epidemiologic Cycle in African Swine Fever Epizootic. Emerg. Infect. Dis. 2018, 24, 810–812. [Google Scholar] [CrossRef]
- Boklund, A.; Dhollander, S.; Chesnoiu Vasile, T.; Abrahantes, J.C.; Bøtner, A.; Gogin, A.; González Villeta, L.C.; Gortázar, C.; More, S.; Papanikolaou, A.; et al. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Sci. Rep. 2020, 10, 10215. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porcine Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef]
- Pautienius, A.; Grigas, J.; Pilevičienė, S.; Zagrabskaitė, R.; Buitkuvienė, J.; Pridotkas, G.; Stankevicius, R.; Streimikyte, Z.; Salomskas, A.; Zienius, D.; et al. Prevalence and spatiotemporal distribution of African swine fever in Lithuania, 2014–2017. Virol. J. 2018, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Pautienius, A.; Schulz, K.; Staubach, C.; Grigas, J.; Zagrabskaitė, R.; Buitkuvienė, J.; Stankevicius, R.; Streimikyte, Z.; Oberauskas, V.; Zienius, D.; et al. African swine fever in the Lithuanian wild boar population in 2018: A snapshot. Virol. J. 2020, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Frant, M.P.; Łyjak, M.; Bocian, Ł.; Barszcz, A.; Niemczuk, K.; Woźniakowski, G. African swine fever virus (ASFV) in Poland: Prevalence in a wild boar population (2017–2018). Vet. Med. 2020, 65, 143–158. [Google Scholar] [CrossRef]
- Schulz, K.; Oļševskis, E.; Viltrop, A.; Masiulis, M.; Staubach, C.; Nurmoja, I.; Lamberga, K.; Seržants, M.; Malakauskas, A.; Conraths, F.J.; et al. Eight Years of African Swine Fever in the Baltic States: Epidemiological Reflections. Pathogens 2022, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- The R Foundation for Statistical Computing. R Studio 4.0.3. Available online: https://www.R-project.org/ (accessed on 9 June 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Nakazawa, M. fmsb: Functions for Medical Statistics Book with Some Demographic Data. Available online: https://CRAN.R-project.org/package=fmsb (accessed on 26 July 2022).
- R Core Team. The R Stats Package. 2021. Available online: https://rdocumentation.org/packages/stats (accessed on 9 June 2023).
- Kendall, M.G.; Ord, J.K.; Stuart, A. The Advanced Theory of Statistics; Design and Analysis and Time-Series; Wiley: Hoboken, NJ, USA, 1985; Volume 3. [Google Scholar]
- Ollech, D. Seastests: Seasonality Tests. 2022. Available online: https://cran.r-project.org/web/packages/seastests/seastests.pdf (accessed on 21 July 2023).
- Friedman, M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Petrov, A.; Forth, J.H.; Zani, L.; Beer, M.; Blome, S. No evidence for long-term carrier status of pigs after African swine fever virus infection. Transbound. Emerg. Dis. 2018, 65, 1318–1328. [Google Scholar] [CrossRef]
- Eblé, P.L.; Hagenaars, T.J.; Weesendorp, E.; Quak, S.; Moonen-Leusen, H.W.; Loeffen, W.L.A. Transmission of African Swine Fever Virus via carrier (survivor) pigs does occur. Vet. Microbiol. 2019, 237, 108345. [Google Scholar] [CrossRef]
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals 2021, 11, 2692. [Google Scholar] [CrossRef]
- Probst, C.; Gethmann, J.; Amendt, J.; Lutz, L.; Teifke, J.P.; Conraths, F.J. Estimating the Postmortem Interval of Wild Boar Carcasses. Vet. Sci. 2020, 7, 6. [Google Scholar] [CrossRef]
- Keuling, O.; Lauterbach, K.; Stier, N.; Roth, M. Hunter feedback of individually marked wild boar Sus scrofa L.: Dispersal and efficiency of hunting in northeastern Germany. Eur. J. Wildl. Res. 2010, 56, 159–167. [Google Scholar] [CrossRef]
- Quirós-Fernández, F.; Marcos, J.; Acevedo, P.; Gortázar, C. Hunters serving the ecosystem: The contribution of recreational hunting to wild boar population control. Eur. J. Wildl. Res. 2017, 63, 57. [Google Scholar] [CrossRef]
- Probst, C.; Gethmann, J.; Amler, S.; Globig, A.; Knoll, B.; Conraths, F.J. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 2019, 9, 11450. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Globig, A.; Knoll, B.; Conraths, F.J.; Depner, K. Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. R. Soc. Open Sci. 2017, 4, 170054. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Golnar, A.J.; Abdo, Z.; Podgórski, T. Ecological drivers of African swine fever virus persistence in wild boar populations: Insight for control. Ecol. Evol. 2020, 10, 2846–2859. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A. Short time window for transmissibility of African swine fever virus from a contaminated environment. Transbound. Emerg. Dis. 2018, 65, 1024–1032. [Google Scholar] [CrossRef]
- Petrini, S.; Feliziani, F.; Casciari, C.; Giammarioli, M.; Torresi, C.; de Mia, G.M. Survival of African swine fever virus (ASFV) in various traditional Italian dry-cured meat products. Prev. Vet. Med. 2019, 162, 126–130. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Woźniakowski, G. Natural inactivation of African swine fever virus in tissues: Influence of temperature and environmental conditions on virus survival. Vet. Microbiol. 2020, 242, 108609. [Google Scholar] [CrossRef]
- Śmietanka, K.; Woźniakowski, G.; Kozak, E.; Niemczuk, K.; Frączyk, M.; Bocian, Ł.; Kowalczyk, A.; Pejsak, Z. African Swine Fever Epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef]
- Podgórski, T.; Borowik, T.; Łyjak, M.; Woźniakowski, G. Spatial epidemiology of African swine fever: Host, landscape and anthropogenic drivers of disease occurrence in wild boar. Prev. Vet. Med. 2020, 177, 104691. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, X.; Chen, J.; Wang, J.; Chen, Q.; Niu, B. Risk analysis of African swine fever in Poland based on spatio-temporal pattern and Latin hypercube sampling, 2014–2017. BMC Vet. Res. 2019, 15, 160. [Google Scholar] [CrossRef]
- Podgórski, T.; Apollonio, M.; Keuling, O. Contact rates in wild boar populations: Implications for disease transmission. J. Wildl. Manag. 2018, 82, 1210–1218. [Google Scholar] [CrossRef]
- Stokstad, E. Deadly virus threatens European pigs and boar: African swine fever outbreak alarms wildlife biologists and veterinarians. Science 2017, 358, 1516–1517. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Schulz, K.; Richter, M.; Staubach, C.; Mettenleiter, T.C.; Conraths, F.J. African swine fever: Why the situation in Germany is not comparable to that in the Czech Republic or Belgium. Transbound. Emerg. Dis. 2022, 69, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Šatrán, P.; Jarosil, T.; Semerád, Z. African swine fever in wild boar in the Czech Republic. In Book of Abstracts, Proceedings of the 12th International Symposium on Wild Boar and Other Suids, Láznĕ Bĕlohrad, Czech Republic, 4–7 September 2018; Drimaj, J., Kamler, J., Eds.; Láznĕ Bĕlohrad, Czech Republic, 2018; pp. 75–76. ISBN 978-80-7509-565-7. [Google Scholar]
- Nurmoja, I.; Mõtus, K.; Kristian, M.; Niine, T.; Schulz, K.; Depner, K.; Viltrop, A. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Prev. Vet. Med. 2020, 181, 104556. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, S.; Beltrán-Alcrudo, D.; Rozstalnyy, A.; Gogin, A.; Kolbasov, D.; Pinto, J.; Lubroth, J.; Martin, V. African swine fever in the Russian Federation: Risk factors for Europe and beyond. Empres Watch 2013, 28, 1–14. [Google Scholar]
- Oganesyan, A.S.; Petrova, O.N.; Korennoy, F.I.; Bardina, N.S.; Gogin, A.; Dudnikov, S.A. African swine fever in the Russian Federation: Spatio-temporal analysis and epidemiological overview. Virus Res. 2013, 173, 204–211. [Google Scholar] [CrossRef]
- Blokhin, A.; Toropova, N.; Burova, O.; Sevskikh, T.; Gogin, A.; Debeljak, Z.; Zakharova, O. Spatio-Temporal Analysis of the Spread of ASF in the Russian Federation in 2017–2019. Acta Vet. 2020, 70, 194–206. [Google Scholar] [CrossRef]
- Loi, F.; Cappai, S.; Laddomada, A.; Feliziani, F.; Oggiano, A.; Franzoni, G.; Rolesu, S.; Guberti, V. Mathematical Approach to Estimating the Main Epidemiological Parameters of African Swine Fever in Wild Boar. Vaccines 2020, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Woźniakowski, G.; Pejsak, Z.; Jabłoński, A. Emergence of African Swine Fever in Poland (2014–2021). Successes and Failures in Disease Eradication. Agriculture 2021, 11, 738. [Google Scholar] [CrossRef]
- Diaz, A.V.; Netherton, C.L.; Dixon, L.K.; Wilson, A.J. African swine fever virus strain Georgia 2007/1 in Ornithodoros erraticus ticks. Emerg. Infect. Dis. 2012, 18, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Ferreira, H.C.; Tudela Zúquete, S.; Wijnveld, M.; Weesendorp, E.; Jongejan, F.; Stegeman, A.; Loeffen, W.L.A. No evidence of African swine fever virus replication in hard ticks. Ticks Tick Borne Dis. 2014, 5, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Herm, R.; Kirik, H.; Vilem, A.; Zani, L.; Forth, J.H.; Müller, A.; Michelitsch, A.; Wernike, K.; Werner, D.; Tummeleht, L.; et al. No evidence for African swine fever virus DNA in haematophagous arthropods collected at wild boar baiting sites in Estonia. Transbound. Emerg. Dis. 2021, 68, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Olesen, A.S.; Lohse, L.; Hansen, M.F.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A.; Bødker, R. Infection of pigs with African swine fever virus via ingestion of stable flies (Stomoxys calcitrans). Transbound. Emerg. Dis. 2018, 65, 1152–1157. [Google Scholar] [CrossRef]
- Plowright, W.; Parker, J.; Peirce, M.A. African swine fever virus in ticks (Ornithodoros moubata, murray) collected from animal burrows in Tanzania. Nature 1969, 221, 1071–1073. [Google Scholar] [CrossRef]
- Mellor, P.S.; Kitching, R.P.; Wilkinson, P.J. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Res. Vet. Sci. 1987, 43, 109–112. [Google Scholar] [CrossRef]





| Country | Domestic Pigs | Wild Boar | ||
|---|---|---|---|---|
| First Date | # Records | First Date | # Records | |
| Belgium | --- | 0 | 13 September 2018 | 648 |
| Bulgaria | 31 August 2018 | 72 | 23 October 2018 | 1453 |
| Czech Republic | --- | 0 | 26 June 2017 | 231 |
| Estonia | 21 July 2015 | 28 | 8 September 2014 | 2956 |
| Germany | 15 July 2021 | 7 | 10 September 2020 | 4554 |
| Greece | 5 February 2020 | 1 | --- | 0 |
| Hungary | --- | 0 | 21 April 2018 | 8899 |
| Italy (excl. Sardinia) | 9 June 2022 | 1 | 7 January 2022 | 268 |
| Latvia | 26 June 2014 | 75 | 26 June 2014 | 5367 |
| Lithuania | 24 July 2014 | 157 | 24 January 2014 | 4475 |
| Poland | 23 July 2014 | 502 | 17 February 2014 | 15,306 |
| Romania | 31 July 2017 | 5941 | 29 May 2018 | 3267 |
| Slovakia | 24 July 2019 | 44 | 8 August 2019 | 2634 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogoll, L.; Güttner, A.-K.; Schulz, K.; Bergmann, H.; Staubach, C.; Conraths, F.J.; Sauter-Louis, C. Seasonal Occurrence of African Swine Fever in Wild Boar and Domestic Pigs in EU Member States. Viruses 2023, 15, 1955. https://doi.org/10.3390/v15091955
Rogoll L, Güttner A-K, Schulz K, Bergmann H, Staubach C, Conraths FJ, Sauter-Louis C. Seasonal Occurrence of African Swine Fever in Wild Boar and Domestic Pigs in EU Member States. Viruses. 2023; 15(9):1955. https://doi.org/10.3390/v15091955
Chicago/Turabian StyleRogoll, Lisa, Ann-Kathrin Güttner, Katja Schulz, Hannes Bergmann, Christoph Staubach, Franz J. Conraths, and Carola Sauter-Louis. 2023. "Seasonal Occurrence of African Swine Fever in Wild Boar and Domestic Pigs in EU Member States" Viruses 15, no. 9: 1955. https://doi.org/10.3390/v15091955
APA StyleRogoll, L., Güttner, A.-K., Schulz, K., Bergmann, H., Staubach, C., Conraths, F. J., & Sauter-Louis, C. (2023). Seasonal Occurrence of African Swine Fever in Wild Boar and Domestic Pigs in EU Member States. Viruses, 15(9), 1955. https://doi.org/10.3390/v15091955






